Abstract
The subcutaneous (SC) route is of growing interest for the administration of biotherapeutics. Key products on the biotherapeutic market such as insulins, but also several immunoglobulins or Fc-fusion proteins, are administered SC. Despite the importance of the SC route, the available knowledge about the processes involved in the SC absorption of biotherapeutics is limited. This review summarizes available information on the physiology of the SC tissue and on the pharmacokinetic processes after SC administration including “first pass catabolism” at the administration site as well as transport in the extracellular matrix of the SC tissue, followed by absorption into the blood circulation or the lymphatic system. Both monoclonal antibodies and other biotherapeutics are discussed. Determinants of absorption after SC administration are summarized including compound properties such as charge or molecular weight. Scale-up of animal data to humans is discussed, including the current shortcomings of empirical scaling approaches and the lack of suitable mechanistic approaches.
KEY WORDS: bioavailability, hypodermis, lymphatic uptake, subcutaneous, therapeutic proteins
INTRODUCTION
Proteins and other biotherapeutics with molecular weights >2 kDa are currently being developed as potential therapeutic agents for a multitude of diseases and disorders, and it is predicted that the overall market for biotechnology products will grow further in the coming years (1). Subcutaneous (SC) administration represents a convenient method of administration compared with intravenous (IV) administration and has been approved for the administration of therapeutic proteins including insulin and human growth hormone and for six monoclonal antibodies (adalimumab, canakinumab, efalizumab, golimumab, omalizumab, and ustekinumab) (2). In some cases of proteins with shorter elimination half-lives, SC administration has been demonstrated to provide prolonged exposure to these proteins by maintaining high plasma concentrations for longer periods. SC administration may be better tolerated compared to IV administration, as it was demonstrated for alemtuzumab (3). Limitations of this route of administration include the possibility of incomplete bioavailability and potentially higher immunogenicity to the protein (1,4), though the frequently reported view of higher immunogenicity after SC dosing might be an overgeneralization (5).
Drugs administered by the SC route can reach the systemic circulation either by uptake by blood capillaries or by lymphatic vessels. Compounds with molecular weights (MW) less than or equal to 16 kDa can reach the systemic circulation via blood capillaries. Protein therapeutics with higher MWs exhibit limited transport into the blood capillaries and enter the systemic circulation via an indirect route, through the lymphatics (6). Almost a century ago, Lewis reported in 1921 that horse serum, injected SC to dogs, reached the thoracic lymph duct in 40 min but reached the blood after 3.5 h (7), and the absorption after SC administration occurred primarily by the lymphatic vessels (8). In 1984, it was first reported that a monoclonal antibody (mAb) was taken up by lymphatic capillaries and lymph nodes following SC injection and eventually entered the blood circulation via the thoracic duct (9).
There are very few studies that have examined the relationship between MW and other physicochemical properties of proteins and the extent of lymphatic absorption following SC administration. Physiological factors including blood flow at the site of SC injection, depth and volume of the injection, and the use of massage or heat at the injection site, as well as binding interactions and catabolism in subcutaneous tissues, can also impact absorption after SC administration. In this review, we have summarized relevant literature on factors that may affect the absorption of therapeutic proteins from subcutaneous sites. Additionally, the clinical relevance of animal models for the study of absorption and bioavailability of therapeutic proteins and the potential for scale-up from animals to humans will be discussed.
THE SUBCUTANEOUS SPACE
SC administration delivers the biologic into the interstitial space of the hypodermis, also referred to as subcutaneous tissue. Thus, when considering the physiology of SC administration, focus needs to be put on the hypodermis, rather than on the skin (dermis and epidermis). The hypodermis is located between the skin and the deep fascia covering the muscle tissue (Fig. 1). The structure of the hypodermis differs across species (Fig. 2). This section of the review will focus on the hypodermis in humans, while the differences in hypodermis tissue present in various animals are discussed in the section on “ANIMAL MODELS—SCALE-UP TO HUMANS.” The thickness of the hypodermis in humans differs across various sites of the body and from person to person. For example, it increases with body mass index (BMI) and decreases with age. Females tend to have a thicker hypodermis as compared to males of the same BMI and age (10).
Fig. 1.

Sagittal magnetic resonance image of human skin: thigh of a woman with low BMI, showing, from right to left, skin (epidermis/dermis), hypodermis with subcutaneous fat lobules separated by dark fibrous septa (thickness of hypodermis 11.3 mm), and muscle. Reproduced with permission from (John Wiley and Sons) (11)
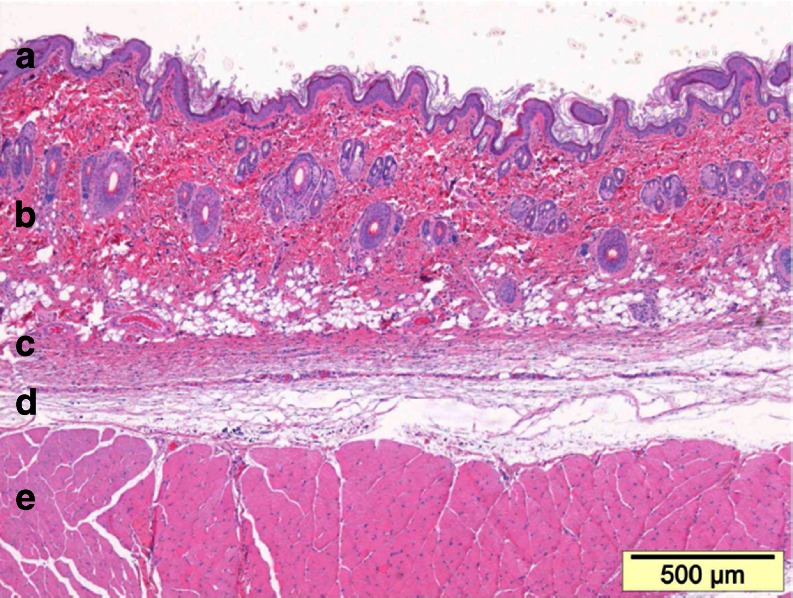
Fig. 2.
Sagittal section of skin–muscle tissue taken from the scapular region of a rat (body weight ca. 200 g) showing, from top to bottom, a epidermis, b dermis, c skin (panniculus) muscle, d subcutaneous connective tissue, and e skeletal muscle of trunk. Hematoxylin-eosin staining. Bar = 0.5 mm
The hypodermis consists mainly of adipose tissue. The adipose tissue is not homogeneous. Rather, it is separated into lobules by a fibrovascular network of connective tissue septa, which link the dermis to the deep fascia (11). The size and appearance of the fat lobules are not homogenous within the hypodermis. In the thigh, next to the dermis are fat lobules that are separated by radially running septa (Fig. 1). In the lower part of the hypodermis, fat lobules are flatter and septa run tangential to the fascia. It is of note that the size and shape of fat lobules is also dependent on gender and BMI (11). A similar structure has been reported for the abdomen, another commonly used SC administration site (12). A superficial adipose layer next to the dermis is comprised of polygonal–oval fat lobules. A continuous membranous layer, about 0.85-mm thick and rich in elastic fibers, separates the superficial adipose layer and the deep adipose tissue. The membranous layer consists of various sub-layers of fibrous tissue. Similar to the thigh, the deep adipose layer consists of large flat lobules. Below the deep adipose tissue, a fascial layer (deep fascia) covers the abdominal wall muscle. The thickness of the superficial and deep adipose layers varies with BMI. For the abdomen, Lanceretto et al. (12) reported average values for the superficial and deep adipose layers of 17 and 18 mm in obese and 3.7 and 3.1 mm in slim subjects. The thickness of the hypodermis may need to be considered for the selection of needle length and injection angle to avoid intramuscular administration, as reported for insulin SC administration in diabetic patients (10). However, the influence of the various hypodermis layers on spreading and absorption of SC administered biologics, is poorly understood.
Cellular components of the hypodermis include mainly adipocytes and, to a lesser extent, fibroblasts and macrophages. Adipocytes can be found in the adipose tissue lobules, while fibroblasts are present in connective tissue septa. Fibroblasts synthesize components of the extracellular matrix (ECM) such as collagen or glycosaminoglycans (13). Dendritic cells, an important part of the immune system, appear to be present mainly in the dermis rather than in the hypodermis. Another antigen-presenting cell type, the Langerhans cells, is present mainly in the epidermis (14). The presence of these professional antigen-presenting cells in the dermis and epidermis rather than in the hypodermis may decrease the immunogenicity of biologics after SC administration compared with intradermal administration (5).
The connective tissue septa represent the majority of the ECM and consist of areolar (i.e., loose) connective tissue. The ECM is a physiological barrier to drug delivery after SC administration (15). Thus, an understanding of the ECM components is crucial to understand absorption processes after SC administration. The ECM and the interstitial–lymphatic interface has been the subject of several reviews (13,15–18), which interested readers may consult for in-depth information on the topic. We will provide a high-level overview to provide a basis for discussion of drug transport in the hypodermis. The ECM determines the mechanical properties of the hypodermis, including strength, hydration, and hydraulic conductivity (16). The structure of the connective tissue is mainly provided by collagen (15,16). Collagen fibers link the dermis to the deep fascia. Elastin as a highly extensible fibrous protein provides elasticity (18). Collagen is positively charged at physiological pH, though with a relatively low charge on a molar basis (13). The gel-like phase of the ECM is formed by glycosaminoglycans (GAGs) and proteoglycans. GAGs are highly negatively charged polysaccharides, consisting of repeating disaccharide units of N-substituted hexosamine and uronic acid (15). Hyaluronic acid (also referred to as hyaluronan) is the most common GAG in the hypodermis, and its disaccharide units are composed of N-acetylglucosamine and glucuronic acid. Other GAGs, e.g., heparin sulfate, heparin, and chondroitin 4- and 6-sulfate, attach to protein cores to form proteoglycans.
The strongly negatively charged GAGs, particularly hyaluronan, control interstitial fluid content and hydraulic conductivity in the ECM (13,16). Due to the limited hydraulic conductivity of the hypodermis, SC administration volumes are usually limited to 1–2 mL in humans (2,15). Co-administration of hyaluronidase allows the administration of larger volumes by transient cleavage of hyaluronan (15,19). Due to the rapid turnover of hyaluronan (usually 30% turnover each day), the effect of hyaluronidase treatment was found to be fully reversible within 24–48 h. Hyaluronan contributes also to the reduction in the interstitial fluid volume available for the distribution of administered therapeutic proteins. Such interstitial volume exclusion is due to the fact that two macromolecules cannot occupy the same volume space simultaneously (13). The volume fraction not accessible for both IgG and albumin was reported to be about 50% of the total interstitial fluid volume in both dermis and skeletal muscle. The main contributor to interstitial volume exclusion, however, is collagen. On a milliliter H20/milligram basis, hyaluronan occupies a 10-fold higher exclusion volume than collagen (19), but the collagen content in, e.g., rat skin (in milligrams per gram skin), is about 100-fold higher than that of hyaluronan (20). The contributions of collagen and hyaluronan to the volume exclusion of albumin in rat skin were estimated at two thirds and one third, respectively (20).
As mentioned before, collagen and GAGs, as the major components of the ECM, have opposite charges. In the hypodermis of rats, the positive and negative charges from collagen and hyaluronan appear to be of similar magnitude (20). However, the presence of additional negative charges from proteoglycans may lead to a net negative interstitial charge (13). The net negative charge influences drug transport in the ECM, with a more rapid transport of negatively charged molecules compared with positively charged molecules (21).
The interstitial fluid derives from leakage of plasma water out of capillaries. The majority of the exudate is re-absorbed in post-capillary venules (16). The remainder of the exudate forms the interstitial fluid and is finally taken up by the lymphatic system, which leads to a small net fluid flux from the vasculature to the lymphatics. As the interstitial fluid derives from plasma water, it is not surprising that its composition is similar to that of plasma water, except that the endogenous protein content is only about 40% of that in plasma (16). It is reasonable to assume that the exudate from the capillaries contains also proteases and their inhibitors present in the plasma. However, little is known about the activity of blood-derived proteases in the ECM and their role in the catabolism of SC-administered biologics (22).
Blood and lymph vessels are unevenly distributed within the hypodermis. Arteries and veins that supply the skin traverse the hypodermis via the interlobular septa. In fat lobules, arterioles and venules are few (23). Capillaries in fat lobules differ from those in the dermis. Capillary walls in fat lobules are markedly thinner than those in upper dermal capillaries (0.1–0.3 vs. 2–3 μm) (23). For the absorption of high molecular weight biotherapeutics, the lymphatics play an important role (6). The lymphatic vascular system starts with blind-ending lymphatic capillaries. Such initial lymphatics are present in a plexus at the dermal/subcutaneous junction (24). From the plexus, lymph drains into large lymphatic trunks. These trunks pass through the fibrous septa of the hypodermis (24). From the trunks, lymph enters lymphatic collectors that run through the hypodermis to the first draining lymph node (25). Fat lobules are obviously devoid of lymphatics. Lymphatic capillaries are open ended. Their endothelial cells have no tight junctions and overlap in a roof tile-like manner; thus, they can give way for entry of large molecules (26). Lymphatic endothelial cells are attached via anchoring filaments to the collagen/elastin fibers of the ECM (27). This link between endothelial cells and fibers controls fluid uptake by the lymphatic system. When interstitial pressure in the ECM does not exceed the pressure in the lymphatic vessel, lymphatic capillaries and their intercellular clefts are collapsed, and no fluid uptake occurs (27). When interstitial pressure increases, the volume expansion moves fibers in the ECM. This leads to an opening of the intercellular clefts by the anchoring filaments that connect lymphatic capillaries and extracellular fibers. Opening of the intercellular cleft allows influx of interstitial fluid and solutes in the lymphatic capillary.
TRANSPORT FROM THE SC INJECTION SITE
After SC administration, protein drugs can be transported to the systemic circulation directly via blood capillaries or indirectly via the lymphatics, both of which contribute to the absorption of protein drugs from the SC interstitial region (28). Absorption is also affected by transport through ECM and presystemic elimination. Small peptides and proteins (<16 kDa) primarily leave the SC site after injection by diffusion into blood capillaries. Transport of larger proteins from the SC site involves travel through the interstitium (the space between capillary walls and cells) and into the lymphatic system. The driving forces for the interstitial and lymphatic flows are the hydrostatic and osmotic differences that occur among blood, interstitium, and the lymphatics. Interstitial fluid pressure is dependent on factors including ECM composition, cell density, blood pressure, tissue metabolism, hydration, and exercise. Both diffusion and convection are important in the transport of molecules through the ECM. For diffusion, this is inversely related to molecular size and therefore is limited for large proteins; for convection, although not limited by molecular size until molecules are so large that they are entrapped in the ECM, steric hindrance and charge interactions are important (18,29). Compared with blood capillaries, the lymphatic capillaries have a higher permeability to large molecules due to the absence of a well-defined basement membrane and the presence of clefts between the endothelial cells, as previously described above (16). Also, in the lymphatics, the resistance to fluid flow is less than that in the ECM (16). Therefore, after SC injection, proteins must be transported through the interstitial space by diffusion and convection mechanisms before uptake by the lymphatic capillaries draining the injection site; from the lymphatic capillaries, they pass into the lymphatic vessels supplying local lymph nodes. Efferent lymph vessels generally leave the local node and transport lymph to the larger collecting lymphatic vessels and finally to the thoracic and other lymph ducts and the systemic circulation (28). Either process, interstitial fluid transport or lymphatic transport, may be rate limiting in this process and results in delayed absorption rates. Further research is necessary in this area to examine the importance of these two processes in the overall absorption of proteins.
For proteins with a relatively rapid elimination, the rate of absorption can be slower than the rate of elimination, leading to longer apparent half-lives (flip–flop kinetics) and prolonged exposure compared to IV administration, which can result in reduced dosing frequency (4). In humans, the absorption rate constant (ka) values for monoclonal antibodies are generally smaller than 0.5 days−1, with an absorption half-life of about 1.4 days. The SC route is the accepted route of administration for human growth hormone (hGH) in children with growth hormone deficiency, as improved efficacy was reported following SC administration compared with the intramuscular route of injection (30). For recombinant human erythropoietin (rHuEPO), a smaller dose can be administered SC, compared to IV, which translates to decreased treatment costs (31). One additional important benefit of SC administration is that drug therapy can be targeted to lymph nodes and the lymphatic system.
However, there are challenges associated with SC administration of proteins (including mAbs), which include the potential for immunogenicity and incomplete bioavailability. Further characterization of absorption after SC administration of proteins and other macromolecules is needed to improve protein targeting to the lymphatics, to improve the bioavailability of administered proteins, and to better understand the pharmacokinetics and pharmacodynamics of proteins after SC administration.
DETERMINANTS OF ABSORPTION AFTER SC ADMINISTRATION
Site of SC Administration
The site of SC injection may represent an important determinant in the lymphatic absorption and lymph node uptake of proteins (30,32); however, the results reported are controversial. In humans, the influence of SC administration site on the rate and extent of absorption has been reported for insulin, hGH, rHuEPO, interferon, and several other proteins (30,32–34). It was generally found that the extent of absorption does not differ significantly, but the rate of absorption may vary, based on the site of injection. A higher absorption rate was reported when insulin, insulin lispro (32), or hGH (30) was injected in the abdomen compared to the thigh or upper arm. Lymphatic absorption of insulin (35) and hGH (30) from the abdomen was faster than that from the thigh. Recently, a report of the bioavailability of golimumab, an antitumor necrosis factor alpha human IgG1kappa monoclonal antibody, after administration at three SC sites (upper arm, abdomen, and thigh) reported no differences in absorption from the three different sites (36). Differences in SC uptake are generally attributed to differences of blood flow to those areas and/or to regional variations in lymph flow (4).
In the majority of studies conducted in sheep, an animal model extensively used to study SC administration, protein was injected in the interdigital space of the hind limb (6,28,37–39). This site was chosen for a practical reason, as it allowed collection of peripheral lymph from the popliteal lymph vessel, which drains from the site of injection. The site of SC administration is an important factor because the pressure gradient between lymphatics and interstitium plays an important role in lymphatic uptake. It is speculated that when the drug is introduced into a narrow space (such as the interdigital space in the sheep model), it can lead to increased hydrostatic pressure, which may result in higher lymphatic drainage (40). Kota et al. (41) studied darbepoetin α (DA) in sheep after SC administration at different sites, namely into the interdigital space, abdomen, and shoulder. The amount of drug reaching the systemic circulation was expressed as percent of dose administered and was 92%, 83%, and 99%, administered into the interdigital space, abdomen, and shoulder. The fraction of dose taken up by the lymphatics was 90% for interdigital and 67% for abdominal injection. Calculation of the lymphatic absorption from the shoulder site was not possible as it drained directly into the jugular vein and therefore was not present in thoracic lymph. The authors concluded that the site of administration had very little effect on lymphatic uptake of DA or on the extent of absorption. However, the rate of absorption was different based on the site of administration. These differences were attributed to variable blood flow and lymph flow as well as body movements. Lymph movement is promoted by motion or activity, and therefore, a slower rate of absorption from the abdomen could be due to less movement compared to absorption from an interdigital region, an area with higher movement (16,41).
Only a few studies have examined SC administration at different sites in rodents. Bioavailability of rituximab (10 mg/kg) in rats after SC administration in the abdomen (43.7%) was significantly higher than in the back (31.2%). The absorption for the low dose (1 mg/kg) was faster when SC injections were made in the foot compared to the abdomen or back (42).
Physicochemical Properties of Proteins
Molecular Size and Weight of Proteins
Molecular size is one of the most important factors affecting uptake from the SC site. Molecules smaller than 10 nm are absorbed by blood capillaries. Optimal molecular size for lymphatic uptake is 10–100 nm (16). Although there is no strict upper size limit for lymphatic uptake, molecules above 100 nm have been found to be trapped at the site of administration for longer durations resulting in a reduced extent of uptake (43). Therefore, MW or molecular size of a protein represents an important determinant of lymphatic uptake. In general, diffusion of large proteins present in lymphatic vessels to the venous circulation is considered negligible (44).
Previous studies have reported higher lymphatic uptake of proteins with increasing MW with many of the studies performed in a sheep animal model. Some of these proteins were included in a review article by Porter and Charman (4). Lymphatic absorption of hGH (MW 22 kDa) after SC administration was monitored, and it was reported that ∼62% of hGH was absorbed by the lymphatics (38). More than 75% of a SC-administered dose of rHuEPO (MW, 30 kDa) was recovered in the lymphatics (37). A linear correlation between MW of proteins and lymphatic absorption was observed. The erythropoeitic drug darbepoetin α has a higher MW (37 kDa) than its analog rHuEPO (30 kDa). Both are preferentially absorbed through the lymphatics in the sheep model after SC administration (99% for DA and 96% for rHuEPO) (37,45). The predominant pathway for absorption of IL-2 in pigs after SC administration is also through the lymphatics (46). After intralymphatic injection of 131I albumin to dogs, albumin entered the venous circulation almost in its entirety via the thoracic duct, and less than 3% of the injected dose appeared in the systemic circulation by non-lymphatic routes (47).
Overall, the literature suggests the possibility of species differences in the lymphatic uptake of proteins. One comparison of lymphatic absorption by a number of species has been published for INF-α. Supersaxo et al. (48) reported that IFN-α was primarily absorbed by lymphatics in popliteal lymph duct-cannulated sheep. The amount of IFN-α recovered in the peripheral lymph of sheep was about 60% after SC or intradermal injection. Conversely, Bocci et al. (49) reported that less than 0.1% of IFN-α was recovered in thoracic lymph in rabbits when IFN-α was administered at one SC site in the hind leg. Lymphatic transport of IFN-α, low in rabbits and high in sheep, may reflect species differences, including those in skin morphology, although experimental factors, including anesthesia, injection site, and methods of lymph collection in these studies, may also contribute to the observed differences. Low lymphatic uptake has been reported in rats (40), where three proteins, insulin, rHuEPO, and BSA, were administered SC in the lateral side of the thigh in rats. The authors reported that there was no significant contribution of the lymphatic system (3% or less) in absorption of these proteins, although they demonstrated a MW dependence. The extent of absorption was much less compared with that reported in studies with sheep (28,37). Kojima et al. (50) reported similar findings for another protein, tumor necrosis factor (TNF-α) in rats. After SC administration in rats, the amount of TNF-α present in the thoracic lymph was very limited, 0.02–0.03% of injected dose. Few studies have examined lymphatic uptake in mice. Based on uptake into lymph nodes draining the the front paw, SC injection site in mice, serum bovine albumin, and the monoclonal antibody bevacizumab also demonstrated limited uptake into the lymphatics (51).
Charge of the Proteins
The influence of charge on lymphatic uptake is likely dependent on the slightly negative charge present in the interstitial space (52). As previously described, the extracellular matrix is composed of collagen fibers that provide mechanical structure and glycosaminoglycans, which maintain fluid balance with their negative charge density (16). Electrostatic properties of glycosaminoglycans play an important role in interstitial transport of molecules. Therefore, the interstitial space poses one of the greatest barriers for the transport of macromolecules from the site of extravascular injection. In another study, five proteins in the MW range of 20–78 kDa were studied. It was reported that positively charged proteins appeared at delayed times in lymph compared to negatively charged proteins of same MW range (29,53).
These findings are supported by studies examining the lymphatic uptake of variously charged liposomes after SC administration, with the highest uptake with negatively charged liposomes, followed by positively charged, and then neutral liposomes (54). Similar observations were reported for surface-engineered liposomes of zidovudine in a recent publication (55). Kaledin et al. (56) reported increased lymphatic uptake in mice using negatively charged liposomes (size, 43 nm). Radiolabeled liposomes were injected SC in sheep, and the highest activity was observed in popliteal lymph, 5–50-fold higher than any other organ (56). Another study indicated that the localization of IgG-coated liposomes was influenced by charge where charged liposomes exhibited higher uptake than neutral liposomes (57).
Physiological or Other Factors Influencing the Lymphatic Uptake
In addition to the above-discussed factors, there are other determinants that affect the lymphatic uptake. These include respiration rate, blood pressure, anesthesia, volume of injection, massage, heat, and movement (16). A published abstract reported ∼100-fold lower lymphatic transport in anesthetized rats compared to normal rats, although further details were not provided (47). Massage of the injection site increased the rate and extent of uptake from the SC site for liposomes into the blood via lymphatics (58). Flow of lymph in a normal human leg was increased by 83% during 2 h of cycling exercise and by 117% during a 2-h warm water bath, suggesting exercise and heat may have significant impact on lymph flow and hence lymphatic transport of proteins (59). Elevation in temperature of the SC site resulted in increased absorption, presumably owing to increased SC blood flow (60). Overall heat or massage leads to increased blood and/or lymph flow that results in higher uptake of SC or intramuscularly administered drugs. In addition, changes in temperature affected transport of proteins in dog lymphatics (61). The transport rate of proteins was reduced by up to 49% when temperature was reduced to 4°C from 37°C, and the transport rate was increased by 48% when temperature was raised to 40°C. In another study, bradykinin or histamine was used to stimulate interstitial edema, which increased lymphatic transport of IFN-α2 (4). Similarly, lymphatic transport of interleukin was increased with co-administration of albumin or hyaluronidase (49). Hyaluronidase can increase bioavailability through the degradation of its hyaluronan that results in increased transport in the interstitium. Additionally, drug formulation factors that may affect absorption include ionic strength, concentration, osmolarity, viscosity, pH, and lipophilicity (4,47); however, the influence of these factors remains to be evaluated.
BIOAVAILABILITY FOLLOWING SC INJECTION
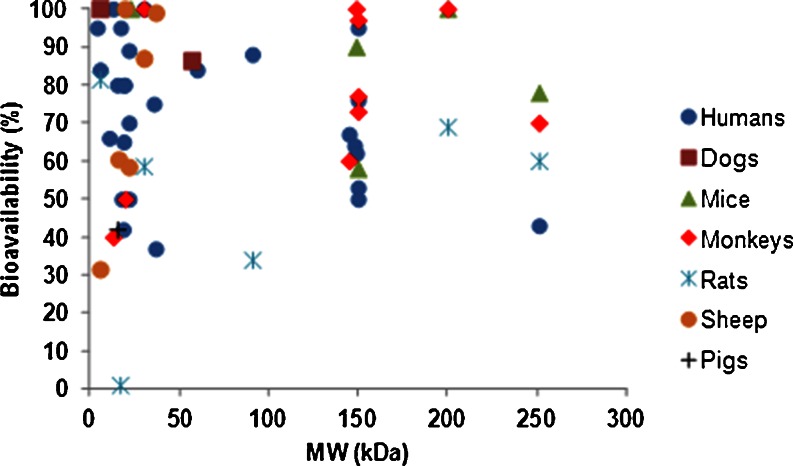
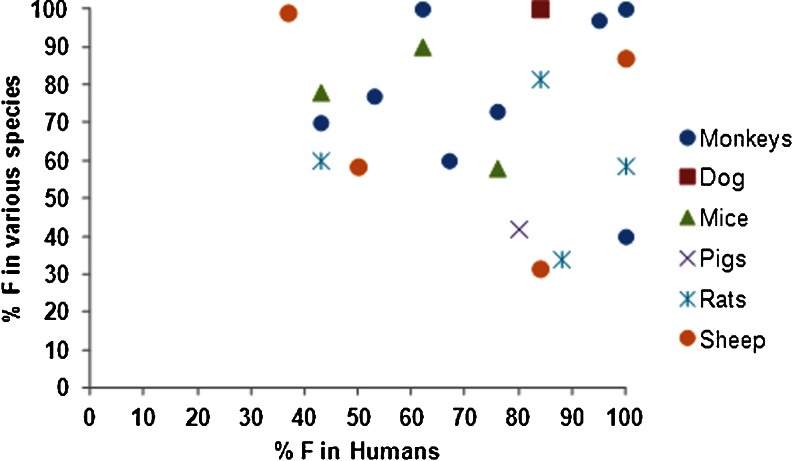
Bioavailability of therapeutic proteins demonstrates variability and species differences. Bioavailability of VEGF-C156S (MW 23 kDa) after SC administration to mice was observed to be complete (62), while bioavailability of interleukin-2 (IL-2) (MW 16 kDa) after SC administration was only 21–41% in mice, 4–24% in sheep, 42% in pigs, and ranged from 30 to 80% in humans (4,46) (Table I). There is no correlation between MW of a biotherapeutic and its bioavailability in various species (Fig. 3). These observations suggest the possibility of species differences in the absorption of macromolecules, although the results may be influenced by the site of SC administration, differences in catabolism at the site of injection, as well as other experimental details including the use of anesthesia in animal studies and protein formulation differences. Similarly, the influence of species was evident in the absorption of rHuEPO, ranging from 27% to 100% in humans, 59% in rats, 87% in sheep, and 65% in monkeys. For insulin, the bioavailability was 31% in sheep, 84% in humans, and estimated as 103% in dogs (Table I).
Table I.
Systemic Bioavailability of Proteins Shown as Percentage of Injected Dose
| Proteins | MW (kDa) | Biovailability (%) | Ref |
|---|---|---|---|
| Teriparatide | 4.2 | Humans: 95 | (1) |
| Insulin | 5.8 | Rats: 81.5 | (40) |
| Sheep: 31.5 | (28) | ||
| Dogs: 103a | (67) | ||
| Humans: 84 | (80) | ||
| IGF-1 | 7.6 | Humans: 100 | (81) |
| Follitropin | 10.2–12.5 | Humans: 66 | (1) |
| IL-3 | 13.2 | Monkeys: 40 | (82) |
| Humans: 100 | (83) | ||
| IL-2 | 15.5 | Humans: 30–80 | (84) |
| Pigs: 42b | (46) | ||
| GM-CSF | 15.5–19.5 | Humans: 50 | (4) |
| r-metHu-Leptin | 16.2 | Sheep: 60.4 | (39) |
| rHu TNF | 17–45c | Rats: 1.1 | (50) |
| Anakinra | 17.3 | Humans: 95 | (1) |
| G-CSF | 18–22 | Monkeys: 40–50 | (85) |
| IL-10 | 18.7 | Humans: 42 | (86) |
| IL-11 | 19 | Humans: 65 | (1,87) |
| IFNα | 19.5 | Humans: >80 | (88) |
| rhLIF | 19.7 | Sheep: 100 | (89) |
| IFNγ 1b | 20–25 | Humans: >89 | (1) |
| IFNγ | 20–25 | Humans: 30–70 | (4) |
| hGH | 22 | Humans: 50 | (90) |
| Sheep: 58.4 | (38) | ||
| IFNβ | 23 | Rabbits: 12 | (91) |
| VEGF-C156S | 23 | Mice: 100 | (62) |
| rHuEPO | 30.4 | Humans: 36–100d | (92,93) |
| Rats: 58.6 | (94) | ||
| Sheep: 87 | (37) | ||
| Monkeys: 27–100d | (95) | ||
| FSH | 36 | Humans: 66–75 | (96) |
| Darbepoetinα | 37 | Humans: 36.9 | (97) |
| Sheep: 83–99 | (41,45) | ||
| Factor IXe | 57 | Dogs: 63.5–86.4 | (98,99) |
| Peginterferon alfa-2a | 60 | Humans: 84 | (1) |
Proteins are arranged in ascending order based on their MW
aBioavailability estimated from infusion dose response curve
bFor PEG-IL2, bioavailability was 100% in pigs
cMW determined by two different methods
dBioavailability after SC administration was dose dependent
eThe SC injection of factor IX (MW 56 kDa) in dogs resulted in 63.5% BA whereas IM injection resulted in 83% BA (98)
Fig. 3.
Relationship between systemic availability of biotherapeutics and their molecular weight in various species. The data are provided in Tables I and II
Monoclonal antibodies have a MW of ∼150 kDa and show variable bioavailability, 50–100% (44). Following SC administration, the reported bioavailability of mAbs and Fc fusion proteins in humans are: adalimumab, 64%; canakinumab, 63–67%; certolizumab pegol, 76–88%; efalizumab, 50%; etanercept, 76%; golimumab, 53%; omalizumab, 53–71%; rilonacept, 43%; ustekinumab, 57% (2,63), and CAT-354 an anti-IL-13 human monoclonal IgG4 antibody 60–62% (64). Systemic bioavailability data of mAbs in various species are listed in Table II.
Table II.
Systemic Bioavailability of Monoclonal Antibodies and Fc-Fusion Proteins Shown as Percentage of Injected Dose
| Antibodies | MW (kDa) | Biovailability (%) | Ref |
|---|---|---|---|
| Certolizumab pegola | 91 | Rats: 24–34 | (2) |
| Humans: 76–88 | |||
| Canakinumab | 145 | Monkeys: 60 | (2) |
| Humans: 63–67 | |||
| Adalimumab | 148 | Monkeys: 96 | (1,2) |
| Humans: 64 | |||
| Omalizumab | 149 | Mice: 90 | (1,2) |
| Monkeys: 64–104 | |||
| Humans: 62 | |||
| Etanercept | 150 | Mice: 58 | (2) |
| Monkeys: 73 | |||
| Humans: 76 | |||
| Golimumab | 150 | Monkeys: 77 | (2) |
| Humans: 53 | |||
| Ustekinumab | 150 | Monkeys: 97 | (2) |
| Humans: 24–95 | |||
| Efalizumab | 150 | Humans: 50 | (100) |
| rhuMAb VEGF | 150 | Rats: 69 | (2) |
| Monkeys: 98 | |||
| Mice: >100 | |||
| Rilonacept | 251 | Rats: 60 | (2) |
| Mice: 78 | |||
| Monkeys: 70 | |||
| Humans: 43 |
mAbs are arranged in ascending order based on their molecular weight (MW)
aPEGylated Fab fragment of a humanized TNF inhibitor monoclonal antibody
The reasons for low bioavailability of therapeutic proteins in humans may be attributed, in part, to degradation at the injection site, but the quantitative significance is unknown. Catabolism at the injection site may be dependent on rates of extracellular degradation via proteolysis. Interestingly, proteases can be affected by disease states and are reported to be upregulated with disease progression (65). Cancer, thrombosis, hypertension, diabetes, and infectious diseases, among others, are associated with abnormal proteolysis (65). Berger and co-workers (66) reported that a significant (up to 21% of injected dose) amount of SC-administered insulin was degraded at the injection site in pigs, whereas other studies have shown minimal or no degradation of insulin administered by the same route (67). Another study involved the use of hGH in growth hormone-deficient humans and indicated the possibility of local degradation of protein at the injection site, based on a comparison of results between IV vs. SC infusions of protein (4). Despite this report, a later study (30) suggested SC administration as the most effective route of administration for hGH in growth hormone-deficient children for most efficient promotion of growth.
For monoclonal antibodies, rates of endocytosis and recycling through the interactions with the Brambell receptor (FcRn) may also be important. FcRn is widely present in endothelial and epithelial cells including in skin, muscle, kidney, liver, and placenta. The binding of IgG with FcRn is highly pH dependent. IgG enters endothelial cells by endocytosis and binds to FcRn in acidic environment (pH 6.0). The unbound IgG in the endosome is subjected to proteolysis in lysosomes, whereas FcRn-bound IgG is recycled to the cell surface where it is released at the physiological pH of 7.4 (44). This recycling by FcRn protects IgG from lysosomal degradation (68) and is believed to contribute towards high bioavailability of mAbs (44). Systemic bioavailability of the IgG antibody, 7E3, was 3-fold higher in wild-type mice (82.5%) than in FcRn knockout mice (28.3%) after SC administration (44). Recently, it was reported that it was necessary to incorporate FcRn binding as a part of the absorption mechanism to appropriately capture the PK of the antibody rituximab in rats (42). Studies in mice with mAbs of differing FcRn-binding affinities demonstrated a small change in bioavailability (12%) after subcutaneous administration (69). The variant N434H had the highest binding affinity to murine FcRn at pH 7.4 and the lowest bioavailability (61.3%) compared with the N434A variant (72.4%) and the wild type (73.2%) (69). An inverse relationship between bioavailability and SC dose has been reported for efalizumab in patients (70) and rituximab in rats (42). Such a relationship may suggest saturable endocytosis, saturable FcRn binding, and/or saturable catabolism at the SC site.
Other factors that may affect the bioavailability after SC administration include blood and lymph flow at the site of SC injection, uptake of macromolecules from the site, and skin morphology, among others.
ANIMAL MODELS—SCALE-UP TO HUMANS
During the preclinical development phase of protein therapeutics, there is great interest in studying the SC pharmacokinetics in animal models that translate to humans. Data from such translatable animal models may be used to predict key PK parameters in humans, including absorption rate and SC bioavailability. In addition, animal models may be used for SC formulation testing. To date, our understanding is limited on the translation of animal SC PK data to humans. The topic was subject of a recent review (2).
For the translatability of SC animal models, one needs to consider all relevant steps in the absorption and disposition of the SC-administered biotherapeutics, i.e., (1) absorption from the SC tissue; (2) first-pass catabolism, endocytosis, and FcRn recycling before reaching the systemic circulation, and (3) disposition after having reached systemic circulation. The predictability of animal models for the disposition of biotherapeutics (both proteins and mAbs) has been reviewed elsewhere (68,71).
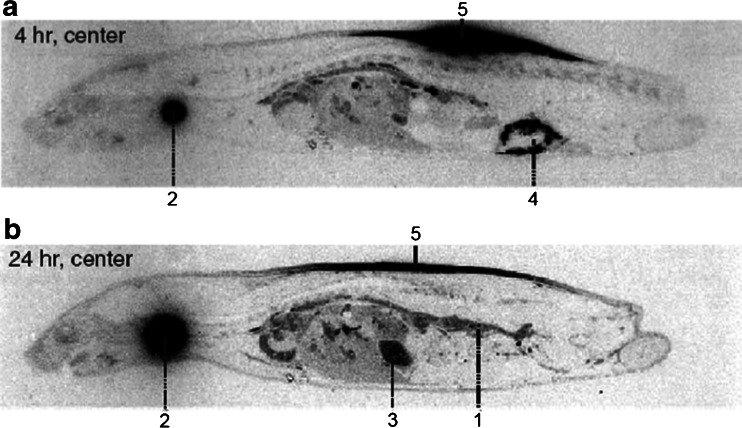
The processes involved in the first-pass catabolism after SC dosing are poorly understood. Therefore, considerations on the predictability of animal models will remain empirical for the time being (see also below). For mAbs, however, species differences in the FcRn binding need to be considered. FcRn has been demonstrated to protect IgG from first-pass catabolism after SC administration to mice (44). Assuming a similar FcRn role across species, the binding affinity of the tested antibody to the animal FcRn should preferably be similar to the binding affinity to human FcRn. First-pass catabolism may occur in the hypodermis or, in case of lymphatic absorption, in the draining lymphatics. The degree of first-pass catabolism in the hypodermis may depend on the residence time of the biotherapeutic in the SC tissue. The latter reflects the transport rate from the administration site to the absorbing lymph or blood capillary. The transport rate may depend on SC tissue structure, which influences the spreading behavior of a SC-administered biotherapeutic. The structure of the hypodermis differs across species. By contrast to humans, furred animals tend to have a loose connection between skin and muscle (15). In rodents such as rats, the SC connective tissue consists of multiple layers of connective tissue (Fig. 2), which are only loosely connected with each other due to the scarcity of fibers interconnecting adjacent collagen sheets (72). This loosely connected tissue allows SC administration of a relatively large fluid volume (15). As expected from such tissue structure, SC-administered biotherapeutics show a wide lateral spreading within the SC tissue. Following SC administration of 125I-darbepoetin α, at a dose volume of 1 mL/kg, whole body autoradiograms showed a wide spreading of radioactivity in the hypodermis of rats at 4 and 24 h post-dose (Fig. 4) (73). Martin and co-workers demonstrated the wide lateral distribution of an antibody after SC administration to mice by positron-emission tomography (74). The structure of the hypodermis in pigs, however, differs from that in furred animals and resembles that in humans. Similar to humans, the skin is connected to the deep fascia via a fibrous network in the hypodermis (75). Thus, in pigs or minipigs, the spreading behavior of SC-administered biotherapeutics should be more similar to humans in contrast to that in rodents. Experimental studies on this, however, are still lacking.
Fig. 4.
Wide lateral spreading of SC administered biotherapeutics in rodents: whole-body autoradiograms 4 and 24 h after a single SC administration of 125I-darbepoetin α to rats (1, blood; 2, thyroid; 3, gastric content; 4, caecal content; 5, injection site). Reproduced with permission from Informa Healthcare (73)
Another relevant difference between human and animal hypodermis is the presence of the panniculus carnosus, a striated muscle, in the hypodermis of animals. By contrast, the panniculus carnosus is reduced in non-human primates and nearly missing in humans (2). In rats, the panniculus carnosus is located close to the dermis (76), while in the pig, the panniculus carnosus divides the fatty tissue of the hypodermis (75). It is of note that in some parts of the pig, the panniculus carnosus is missing (e.g., forelimb, thigh, groin, and back). The role of the panniculus carnosus in the absorption of SC-administered biotherapeutics is not understood. Thus, transport of SC-administered biotherapeutics across the panniculus carnosus needs to be considered including the potential presence and abundance of lymph capillaries to facilitate absorption of larger biotherapeutics.
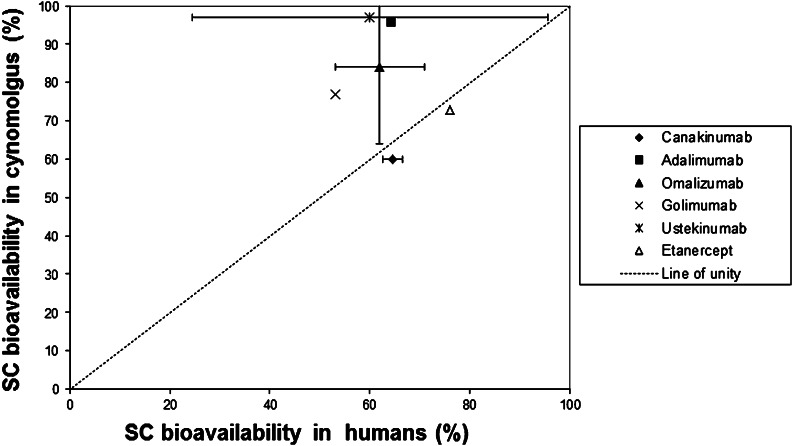
Due to the unknowns in the translation of absorption and first-pass catabolic processes from animals to humans, our knowledge on predictability of animal data for humans is largely empirical. McDonald et al. compiled an overview on comparative bioavailability data in animals and humans (2). Non-human primates tend to overestimate the SC bioavailability of mAbs (Fig. 5). For mAbs in rodents, as well as for other biotherapeutics in all species, no clear pattern was observed when correlating SC bioavailability in humans and in the various animal species. As pointed out above, the hypodermis of humans resembles that in pigs/minipigs more than that in other species, so that pigs or minipigs appear to be a promising animal species for SC testing of biotherapeutics. Only a few studies, however, have been published in pigs, but these studies have shown promising results with regard to extrapolation to humans. The SC bioavailability of IL-2 and PEG-IL-2 (IL-2 derivatized with three to four polyethylene glycol molecules of approximately 7 kDa each) in pigs was found to be 42.2% and “essentially complete,” respectively (46). These data compare well with data in patients (35–47% and 83%, respectively). For both compounds, furred animals underestimated the SC bioavailability. For etanercept (molecular weight of about 150 kDa), Harvey et al. found an SC bioavailability of 50% in minipigs (77), which is slightly lower than the human SC bioavailability of 76% (2). We compared bioavailability of 13 biotherapeutics in humans with at least one of the following species: monkeys, dogs, rats, mice, pigs, or sheep. None of the species exhibited strong correlation (Fig. 6).
Fig. 5.
Comparison of SC bioavailabilities of monoclonal antibodies and IgG Fc fusion proteins in humans and cynomolgus monkeys (mean values and data range) (data from (2))
Fig. 6.
Systemic availability of biotherapeutics in various species with respect to their availability in humans after SC administration (n = 13). Data are provided in Tables I and II
The comparison of SC absorption rates across species has received little attention in the literature. SC absorption rates in animals appear to be more rapid than in humans. The SC absorption rate of erythropoietin in rat, cynomolgus monkeys, and humans is inversely related to body weight (78). The logarithm of the absorption rate scales with the logarithm of the body weight with an allometric exponent of −0.349.
KNOWLEDGE GAPS
Several aspects of SC absorption of biotherapeutics are not well understood despite the common use of this administration route for biotherapeutics. These include, for instance, the reason(s) for the incomplete bioavailability after SC dosing and the translation of SC animal data to humans. Catabolic first-pass clearance after SC administration is likely to contribute to the incomplete SC bioavailability of many SC-administered biotherapeutics. The catabolic processes in the SC tissue or in the draining lymph pathways are poorly understood for biotherapeutics. Further research will be required in this area.
As described in the previous section, the translation of SC animal data to humans also remains uncertain, both with regard to rate and extent of absorption. A few empirical approaches for comparison of absorption rates in animals and man have been published (78,79). The reasons for the reported species differences are not known. Scaling of the extent of absorption/bioavailability has met even less success. For biotherapeutics with molecular weights <40 kDa, no pattern in the bioavailability values was found across species (2), so that there is no sound basis for an empirical scaling of bioavailabilities across species. For mAbs, the cynomolgus monkey tends to overestimate the extent of absorption/bioavailability (2) (Fig. 5). Recently the minipig was suggested as an alternative model to the monkey (79).
Based on current knowledge, it will be difficult to improve or replace empirical approaches for translation of SC animal data to humans by mechanistic approaches. The knowledge about the processes determining both rate and extent of absorption is limited. More information about transport processes both in the ECM and the lymphatic system, as well as about catabolic processes during SC absorption, will be needed to develop suitable mechanistic models for translation from animals to humans. Thus, for instance, more information will be needed on metabolic/catabolic processes as potential reasons for the marked SC bioavailability differences across compounds and across species. Furthermore, the anatomy and physiology differ across species (e.g., presence of panniculus carnosus in many laboratory animals or different thickness/structure of the hypodermis). Also, lymphatic absorption may differ across species (36). The consequences of those differences on SC absorption are not understood. In the absence of such knowledge, it will be important to control and standardize experimental approaches in, e.g., animal models as much as possible, in order to reduce sources of variability (2).
The absorption of a biotherapeutic after SC administration may be also influenced by factors related to the formulation and its fate in the ECM. After injection, the composition of the formulation may change, e.g., with regard to pH or ionic composition. Such changes in turn may influence absorption behavior. Alternatively, the formulation may cause reactions such as water inflow at the administration site, which may change the transport of the administered biotherapeutics. Sustained release formulations for SC administration will require additional considerations. The formulation-related aspects of SC absorption are beyond the scope of this review.
OUTLOOK
The SC route is of growing importance in the parenteral administration of biotherapeutics. Nevertheless, the mechanisms involved in the SC absorption of biotherapeutics have received relatively little attention in the literature. This review tries to summarize some aspects of the mechanistic determinants of SC absorption. The physiology of the hypodermis and its difference across species need to be considered when dealing with the SC absorption of biotherapeutics, since transport and catabolism in the hypodermis are key events in the SC absorption process. For larger biotherapeutics including mAbs, transport in the ECM is usually followed by lymphatic absorption, which will eventually lead to availability of the biotherapeutic in the systemic circulation. The mechanistic knowledge about these events is still relatively scarce. More research will be needed to improve our understanding of the events during SC absorption. More mechanistic insights into SC absorption will facilitate the development of improved SC formulations and dosing regimens. In addition, more mechanistic insights may allow a more rational translation of SC animal data to humans, as compared to the current situation, including the use of physiologically based pharmacokinetic models.
ACKNOWLEDGMENTS
This study was supported in part by the Center for Protein Therapeutics, University at Buffalo, State University of New York (for MEM). The authors thank Elke Atzpodien, F. Hoffmann-La Roche, Basel, for providing the section of rat skin.
REFERENCES
- 1.Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93(9):2184–2204. doi: 10.1002/jps.20125. [DOI] [PubMed] [Google Scholar]
- 2.McDonald TA, Zepeda ML, Tomlinson MJ, Bee WH, Ivens IA. Subcutaneous administration of biotherapeutics: current experience in animal models. Curr Opin Mol Ther. 2010;12(4):461–470. [PubMed] [Google Scholar]
- 3.Hale G, Rebello P, Brettman LR, Fegan C, Kennedy B, Kimby E, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104(4):948–955. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- 4.Porter CJ, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89(3):297–310. doi: 10.1002/(SICI)1520-6017(200003)89:3<297::AID-JPS2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Buttel IC, Chamberlain P, Chowers Y, Ehmann F, Greinacher A, Jefferis R, et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals. 2011;39(2):100–109. doi: 10.1016/j.biologicals.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–169. doi: 10.1023/A:1015880819328. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JH. The route and rate of absorption of subcutaneously injected serum in relation to the occurrence of sudden death after injection of antitoxic horse serum. JAMA. 1921;76:1342–1345. doi: 10.1001/jama.1921.02630200014009. [DOI] [Google Scholar]
- 8.Field ME, Drinker CK. The permeability of the capillaries of the dog to protein. Am J Physiol. 1931;97:40–51. [Google Scholar]
- 9.Weinstein JN, Steller MA, Covell DG, Holton OD, Keenan AM, Sieber SM, et al. Monoclonal antitumor antibodies in the lymphatics. Cancer Treat Rep. 1984;68(1):257–264. [PubMed] [Google Scholar]
- 10.Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519–1530. doi: 10.1185/03007995.2010.481203. [DOI] [PubMed] [Google Scholar]
- 11.Mirrashed F, Sharp JC, Krause V, Morgan J, Tomanek B. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10(3):161–168. doi: 10.1111/j.1600-0846.2004.00072.x. [DOI] [PubMed] [Google Scholar]
- 12.Lancerotto L, Stecco C, Macchi V, Porzionato A, Stecco A, De Caro R. Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat. 2011;33(10):835–842. doi: 10.1007/s00276-010-0772-8. [DOI] [PubMed] [Google Scholar]
- 13.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73(1):1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31(12):446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4(4):427–440. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- 16.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50(1–2):3–20. doi: 10.1016/S0169-409X(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 17.Kretsos K, Kasting GB. Dermal capillary clearance: physiology and modeling. Skin Pharmacol Physiol. 2005;18(2):55–74. doi: 10.1159/000083706. [DOI] [PubMed] [Google Scholar]
- 18.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–256. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 19.Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114(2):230–241. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Reed RK, Lepsoe S, Wiig H. Interstitial exclusion of albumin in rat dermis and subcutis in over- and dehydration. Am J Physiol. 1989;257(6 Pt 2):H1819–H1827. doi: 10.1152/ajpheart.1989.257.6.H1819. [DOI] [PubMed] [Google Scholar]
- 21.Parker JC, Gilchrist S, Cartledge JT. Plasma-lymph exchange and interstitial distribution volumes of charged macromolecules in the lung. J Appl Physiol. 1985;59(4):1128–1136. doi: 10.1152/jappl.1985.59.4.1128. [DOI] [PubMed] [Google Scholar]
- 22.Mrsny RJ. Metabolic processes at injection sites affecting pharmacokinetics, pharmacodynamics, and metabolism of protein and peptide therapeutics. In: Mrsny RJ, Daugherty A, editors. Proteins and peptides—pharmacokinetics, harmacodynamic, and metabolic outcomes. New York: Informa healthcare; 2009. [Google Scholar]
- 23.Braverman IM, Keh-Yen A. Ultrastructure of the human dermal microcirculation. III. The vessels in the mid- and lower dermis and subcutaneous fat. J Investig Dermatol. 1981;77(3):297–304. doi: 10.1111/1523-1747.ep12482470. [DOI] [PubMed] [Google Scholar]
- 24.Ryan TJ, Mortimer PS, Jones RL. Lymphatics of the skin. Neglected but important. Int J Dermatol. 1986;25(7):411–419. doi: 10.1111/j.1365-4362.1986.tb03443.x. [DOI] [PubMed] [Google Scholar]
- 25.Schacht V, Luedemann W, Abels C, Berens von Rautenfeld D. Anatomy of the subcutaneous lymph vascular network of the human leg in relation to the great saphenous vein. Anat Rec (Hoboken) 2009;292(1):87–93. doi: 10.1002/ar.20765. [DOI] [PubMed] [Google Scholar]
- 26.Leak LV. Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue-lymph interface. Microvasc Res. 1970;2(4):361–391. doi: 10.1016/0026-2862(70)90031-2. [DOI] [PubMed] [Google Scholar]
- 27.Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5(1):14–19. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 28.Charman SA, McLennan DN, Edwards GA, Porter CJ. Lymphatic absorption is a significant contributor to the subcutaneous bioavailability of insulin in a sheep model. Pharm Res. 2001;18(11):1620–1626. doi: 10.1023/A:1013046918190. [DOI] [PubMed] [Google Scholar]
- 29.Reddy ST, Berk DA, Jain RK, Swartz MA. A sensitive in vivo model for quantifying interstitial convective transport of injected macromolecules and nanoparticles. J Appl Physiol. 2006;101(4):1162–1169. doi: 10.1152/japplphysiol.00389.2006. [DOI] [PubMed] [Google Scholar]
- 30.Beshyah SA, Anyaoku V, Niththyananthan R, Sharp P, Johnston DG. The effect of subcutaneous injection site on absorption of human growth hormone: abdomen versus thigh. Clin Endocrinol (Oxf) 1991;35(5):409–412. doi: 10.1111/j.1365-2265.1991.tb03557.x. [DOI] [PubMed] [Google Scholar]
- 31.Patel TV, Robinson K, Singh AK. Is it time to reconsider subcutaneous administration of epoetin? Nephrol News Issues. 2007;21(11):57. [PubMed] [Google Scholar]
- 32.ter Braak EW, Woodworth JR, Bianchi R, Cerimele B, Erkelens DW, Thijssen JH, et al. Injection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulin. Diabetes Care. 1996;19(12):1437–1440. doi: 10.2337/diacare.19.12.1437. [DOI] [PubMed] [Google Scholar]
- 33.Jensen JD, Jensen LW, Madsen JK. The pharmacokinetics of recombinant human erythropoietin after subcutaneous injection at different sites. Eur J Clin Pharmacol. 1994;46(4):333–337. doi: 10.1007/BF00194401. [DOI] [PubMed] [Google Scholar]
- 34.Yoshikawa H, Satoh Y, Naruse N, Takada K, Muranishi S. Comparison of disappearance from blood and lymphatic delivery of human fibroblast interferon in rat by different administration routes. J Pharmacobiodyn. 1985;8(3):206–210. doi: 10.1248/bpb1978.8.206. [DOI] [PubMed] [Google Scholar]
- 35.Binder C. Absorption of injected insulin. A clinical–pharmacological study. Acta Pharmacol Toxicol (Copenh) 1969;27(Suppl 2):1–84. doi: 10.1111/j.1600-0773.1969.tb03069.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z, Wang Q, Zhuang Y, Frederick B, Yan H, Bouman-Thio E, et al. Subcutaneous bioavailability of golimumab at 3 different injection sites in healthy subjects. J Clin Pharmacol. 2010;50(3):276–284. doi: 10.1177/0091270009340782. [DOI] [PubMed] [Google Scholar]
- 37.McLennan DN, Porter CJ, Edwards GA, Martin SW, Heatherington AC, Charman SA. Lymphatic absorption is the primary contributor to the systemic availability of epoetin alfa following subcutaneous administration to sheep. J Pharmacol Exp Ther. 2005;313(1):345–351. doi: 10.1124/jpet.104.078790. [DOI] [PubMed] [Google Scholar]
- 38.Charman SA, Segrave AM, Edwards GA, Porter CJ. Systemic availability and lymphatic transport of human growth hormone administered by subcutaneous injection. J Pharm Sci. 2000;89(2):168–177. doi: 10.1002/(SICI)1520-6017(200002)89:2<168::AID-JPS4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 39.McLennan DN, Porter CJ, Edwards GA, Brumm M, Martin SW, Charman SA. Pharmacokinetic model to describe the lymphatic absorption of r-metHu-leptin after subcutaneous injection to sheep. Pharm Res. 2003;20(8):1156–1162. doi: 10.1023/A:1025036611949. [DOI] [PubMed] [Google Scholar]
- 40.Kagan L, Gershkovich P, Mendelman A, Amsili S, Ezov N, Hoffman A. The role of the lymphatic system in subcutaneous absorption of macromolecules in the rat model. Eur J Pharm Biopharm. 2007;67(3):759–765. doi: 10.1016/j.ejpb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Kota J, Machavaram KK, McLennan DN, Edwards GA, Porter CJ, Charman SA. Lymphatic absorption of subcutaneously administered proteins: influence of different injection sites on the absorption of darbepoetin alfa using a sheep model. Drug Metab Dispos. 2007;35(12):2211–2217. doi: 10.1124/dmd.107.015669. [DOI] [PubMed] [Google Scholar]
- 42.Kagan L, Turner MR, Balu-Iyer SV, Mager DE. Subcutaneous absorption of monoclonal antibodies: role of dose, site of injection, and injection volume on rituximab pharmacokinetics in rats. Pharm Res. 2012;29(2):490–499. doi: 10.1007/s11095-011-0578-3. [DOI] [PubMed] [Google Scholar]
- 43.Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50(1–2):143–156. doi: 10.1016/S0169-409X(01)00154-5. [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 45.McLennan DN, Porter CJ, Edwards GA, Heatherington AC, Martin SW, Charman SA. The absorption of darbepoetin alfa occurs predominantly via the lymphatics following subcutaneous administration to sheep. Pharm Res. 2006;23(9):2060–2066. doi: 10.1007/s11095-006-9064-8. [DOI] [PubMed] [Google Scholar]
- 46.Chen SA, Sawchuk RJ, Brundage RC, Horvath C, Mendenhall HV, Gunther RA, et al. Plasma and lymph pharmacokinetics of recombinant human interleukin-2 and polyethylene glycol-modified interleukin-2 in pigs. J Pharmacol Exp Ther. 2000;293(1):248–259. [PubMed] [Google Scholar]
- 47.Porter CJ, Edwards GA, Charman SA. Lymphatic transport of proteins after s.c. injection: implications of animal model selection. Adv Drug Deliv Rev. 2001;50(1–2):157–171. doi: 10.1016/S0169-409X(01)00153-3. [DOI] [PubMed] [Google Scholar]
- 48.Supersaxo A, Hein W, Gallati H, Steffen H. Recombinant human interferon alpha-2a: delivery to lymphoid tissue by selected modes of application. Pharm Res. 1988;5(8):472–476. doi: 10.1023/A:1015957022073. [DOI] [PubMed] [Google Scholar]
- 49.Bocci V, Muscettola M, Grasso G, Magyar Z, Naldini A, Szabo G. The lymphatic route. 1) Albumin and hyaluronidase modify the normal distribution of interferon in lymph and plasma. Experientia. 1986;42(4):432–433. doi: 10.1007/BF02118644. [DOI] [PubMed] [Google Scholar]
- 50.Kojima K, Takahashi T, Nakanishi Y. Lymphatic transport of recombinant human tumor necrosis factor in rats. J Pharmacobiodyn. 1988;11(10):700–706. doi: 10.1248/bpb1978.11.700. [DOI] [PubMed] [Google Scholar]
- 51.Wu F, Bhansali SG, Tamhane M, Kumar R, Vathy LA, Ding H, et al. Noninvasive real-time fluorescence imaging of the lymphatic uptake of BSA-IRDye 680 conjugate administered subcutaneously in mice. J Pharm Sci. 2012;101:1744–54. doi: 10.1002/jps.23058. [DOI] [PubMed] [Google Scholar]
- 52.Hawley AE, Davis SS, Illum L. Targeting of colloids to lymph nodes: influence of lymphatic physiology and colloidal characteristics. Adv Drug Deliv Rev. 1995;17:129–148. doi: 10.1016/0169-409X(95)00045-9. [DOI] [Google Scholar]
- 53.Xie DD, Hale V. Factors affecting the lymphatic absorption of macromolecules following extravascular administration. Pharm Res. 1996;13:S396. [Google Scholar]
- 54.Patel HM, Boodle KM, Vaughan-Jones R. Assessment of the potential uses of liposomes for lymphoscintigraphy and lymphatic drug delivery. Failure of 99 m-technetium marker to represent intact liposomes in lymph nodes. Biochim Biophys Acta. 1984;801(1):76–86. doi: 10.1016/0304-4165(84)90214-9. [DOI] [PubMed] [Google Scholar]
- 55.Kaur CD, Nahar M, Jain NK. Lymphatic targeting of zidovudine using surface-engineered liposomes. J Drug Target. 2008;16(10):798–805. doi: 10.1080/10611860802475688. [DOI] [PubMed] [Google Scholar]
- 56.Kaledin VI, Matienko NA, Nikolin VP, Gruntenko YV, Budker VG, Vakhrusheva TE. Subcutaneously injected radiolabeled liposomes: transport to the lymph nodes in mice. J Nat Cancer Inst. 1982;69(1):67–71. [PubMed] [Google Scholar]
- 57.Mangat S, Patel HM. Lymph node localization of non-specific antibody-coated liposomes. Life Sci. 1985;36(20):1917–1925. doi: 10.1016/0024-3205(85)90440-0. [DOI] [PubMed] [Google Scholar]
- 58.Trubetskoy VS, Whiteman KR, Torchilin VP, Wolf GL. Massage-induced release of subcutaneously injected liposome-encapsulated drugs to the blood. J Control Release. 1998;50(1–3):13–19. doi: 10.1016/S0168-3659(97)00104-1. [DOI] [PubMed] [Google Scholar]
- 59.Olszewski W, Engeset A, Jaeger PM, Sokolowski J, Theodorsen L. Flow and composition of leg lymph in normal men during venous stasis, muscular activity and local hyperthermia. Acta Physiol Scand. 1977;99(2):149–155. doi: 10.1111/j.1748-1716.1977.tb10365.x. [DOI] [PubMed] [Google Scholar]
- 60.Astrup A, Bulow J, Madsen J. Skin temperature and subcutaneous adipose blood flow in man. Scand J Clin Lab Invest. 1980;40(2):135–138. doi: 10.3109/00365518009093015. [DOI] [PubMed] [Google Scholar]
- 61.O'Morchoe CC, Jones WR, 3rd, Jarosz HM, O'Morchoe PJ, Fox LM. Temperature dependence of protein transport across lymphatic endothelium in vitro. J Cell Biol. 1984;98(2):629–640. doi: 10.1083/jcb.98.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhansali SG, Balu-Iyer SV, Morris ME. Influence of route of administration and liposomal encapsulation on blood and lymph node exposure to the protein VEGF-C156S. J Pharm Sci. 2012;101(2):852–859. doi: 10.1002/jps.22795. [DOI] [PubMed] [Google Scholar]
- 63.Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Oh CK, Faggioni R, Jin F, Roskos LK, Wang B, Birrell C, et al. An open-label, single-dose bioavailability study of the pharmacokinetics of CAT-354 after subcutaneous and intravenous administration in healthy males. Br J Clin Pharmacol. 2010;69(6):645–655. doi: 10.1111/j.1365-2125.2010.03647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Law B, Tung CH. Proteolysis: a biological process adapted in drug delivery, therapy, and imaging. Bioconjug Chem. 2009;20(9):1683–1695. doi: 10.1021/bc800500a. [DOI] [PubMed] [Google Scholar]
- 66.Berger M, Halban PA, Girardier L, Seydoux J, Offord RE, Renold AE. Absorption kinetics of subcutaneously injected insulin. Evidence for degradation at the injection site. Diabetologia. 1979;17(2):97–99. doi: 10.1007/BF01222209. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe RM, Volund A, Bergman RN. Intravenous insulin infusion to simulate subcutaneous absorption. Bioavailability and metabolic sequelae. Diabetes Care. 1991;14(11):1021–1030. doi: 10.2337/diacare.14.11.1021. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Prueksaritanont T. Prediction of human clearance of therapeutic proteins: simple allometric scaling method revisited. Biopharm Drug Dispos. 2010;31(4):253–263. doi: 10.1002/bdd.708. [DOI] [PubMed] [Google Scholar]
- 69.Deng R, Loyet KM, Lien S, Iyer S, DeForge LE, Theil FP, et al. Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{alpha} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab Dispos. 2010;38(4):600–605. doi: 10.1124/dmd.109.031310. [DOI] [PubMed] [Google Scholar]
- 70.Mortensen DL, Walicke PA, Wang X, Kwon P, Kuebler P, Gottlieb AB, et al. Pharmacokinetics and pharmacodynamics of multiple weekly subcutaneous efalizumab doses in patients with plaque psoriasis. J Clin Pharmacol. 2005;45(3):286–298. doi: 10.1177/0091270004270260. [DOI] [PubMed] [Google Scholar]
- 71.Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3(1):61–66. doi: 10.4161/mabs.3.1.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawamata S, Ozawa J, Hashimoto M, Kurose T, Shinohara H. Structure of the rat subcutaneous connective tissue in relation to its sliding mechanism. Arch Histol Cytol. 2003;66(3):273–279. doi: 10.1679/aohc.66.273. [DOI] [PubMed] [Google Scholar]
- 73.Yoshioka E, Kato K, Shindo H, Mitsuoka C, Kitajima SI, Ogata H, et al. Pharmacokinetic study of darbepoetin alfa: absorption, distribution, and excretion after a single intravenous and subcutaneous administration to rats. Xenobiotica. 2007;37(1):74–90. doi: 10.1080/00498250600987929. [DOI] [PubMed] [Google Scholar]
- 74.Martin SM, O'Donnell RT, Kukis DL, Abbey CK, McKnight H, Sutcliffe JL, et al. Imaging and pharmacokinetics of (64)Cu-DOTA-HB22.7 administered by intravenous, intraperitoneal, or subcutaneous injection to mice bearing non-Hodgkin's lymphoma xenografts. Mol Imaging Biol. 2009;11(2):79–87. doi: 10.1007/s11307-008-0148-1. [DOI] [PubMed] [Google Scholar]
- 75.Rose EH, Vistnes LM, Ksander GA. The panniculus carnosus in the domestic pig. Plast Reconstr Surg. 1977;59(1):94–97. doi: 10.1097/00006534-197701000-00017. [DOI] [PubMed] [Google Scholar]
- 76.Wells MY, Voute H, Bellingard V, Fisch C, Boulifard V, George C, et al. Histomorphology and vascular lesions in dorsal rat skin used as injection sites for a subcutaneous toxicity study. Toxicol Pathol. 2010;38(2):258–266. doi: 10.1177/0192623309357953. [DOI] [PubMed] [Google Scholar]
- 77.Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28(1):107–116. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 78.Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos. 2007;35(9):1672–1678. doi: 10.1124/dmd.107.015248. [DOI] [PubMed] [Google Scholar]
- 79.Zheng Y, Tesar DB, Benincosa L, Birnbock H, Boswell CA, Bumbaca D, et al. Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. MAbs. 2012;4(2):243–255. doi: 10.4161/mabs.4.2.19387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffman A, Ziv E. Pharmacokinetic considerations of new insulin formulations and routes of administration. Clin Pharmacokinet. 1997;33(4):285–301. doi: 10.2165/00003088-199733040-00004. [DOI] [PubMed] [Google Scholar]
- 81.Grahnen A, Kastrup K, Heinrich U, Gourmelen M, Preece MA, Vaccarello MA, et al. Pharmacokinetics of recombinant human insulin-like growth factor I given subcutaneously to healthy volunteers and to patients with growth hormone receptor deficiency. Acta Paediatr Suppl. 1993;82(Suppl 391):9–13. doi: 10.1111/j.1651-2227.1993.tb12918.x. [DOI] [PubMed] [Google Scholar]
- 82.van Gils FC, Westerman Y, van den Bos C, Burger H, van Leen RW, Wagemaker G. Pharmacokinetic basis for optimal hemopoietic effectiveness of homologous IL-3 administered to rhesus monkeys. Leukemia. 1993;7(10):1602–1607. [PubMed] [Google Scholar]
- 83.Biesma B, Pokorny R, Kovarik JM, Duffy FA, Willemse PH, Mulder NH, et al. Pharmacokinetics of recombinant human interleukin 3 administered subcutaneously and by continuous intravenous infusion in patients after chemotherapy for ovarian cancer. Cancer Res. 1993;53(24):5915–5919. [PubMed] [Google Scholar]
- 84.Konrad MW, Hemstreet G, Hersh EM, Mansell PW, Mertelsmann R, Kolitz JE, et al. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 1990;50(7):2009–2017. [PubMed] [Google Scholar]
- 85.Tanaka H, Tanaka Y, Shinagawa K, Yamagishi Y, Ohtaki K, Asano K. Three types of recombinant human granulocyte colony-stimulating factor have equivalent biological activities in monkeys. Cytokine. 1997;9(5):360–369. doi: 10.1006/cyto.1996.0177. [DOI] [PubMed] [Google Scholar]
- 86.Radwanski E, Chakraborty A, Van Wart S, Huhn RD, Cutler DL, Affrime MB, et al. Pharmacokinetics and leukocyte responses of recombinant human interleukin-10. Pharm Res. 1998;15(12):1895–1901. doi: 10.1023/A:1011918425629. [DOI] [PubMed] [Google Scholar]
- 87.Aoyama K, Uchida T, Takanuki F, Usui T, Watanabe T, Higuchi S, et al. Pharmacokinetics of recombinant human interleukin-11 (rhIL-11) in healthy male subjects. Br J Clin Pharmacol. 1997;43(6):571–578. doi: 10.1046/j.1365-2125.1997.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wills RJ, Dennis S, Spiegel HE, Gibson DM, Nadler PI. Interferon kinetics and adverse reactions after intravenous, intramuscular, and subcutaneous injection. Clin Pharmacol Ther. 1984;35(5):722–727. doi: 10.1038/clpt.1984.101. [DOI] [PubMed] [Google Scholar]
- 89.Segrave AM, Mager DE, Charman SA, Edwards GA, Porter CJ. Pharmacokinetics of recombinant human leukemia inhibitory factor in sheep. J Pharmacol Exp Ther. 2004;309(3):1085–1092. doi: 10.1124/jpet.103.063289. [DOI] [PubMed] [Google Scholar]
- 90.Laursen T, Grandjean B, Jorgensen JO, Christiansen JS. Bioavailability and bioactivity of three different doses of nasal growth hormone (GH) administered to GH-deficient patients: comparison with intravenous and subcutaneous administration. Eur J Endocrinol. 1996;135(3):309–315. doi: 10.1530/eje.0.1350309. [DOI] [PubMed] [Google Scholar]
- 91.Bocci V, Muscettola M, Naldini A. The lymphatic route—III. Pharmacokinetics of human natural interferon-beta injected with albumin as a retarder in rabbits. Gen Pharmacol. 1986;17(4):445–448. doi: 10.1016/0306-3623(86)90189-8. [DOI] [PubMed] [Google Scholar]
- 92.Salmonson T, Danielson BG, Wikstrom B. The pharmacokinetics of recombinant human erythropoietin after intravenous and subcutaneous administration to healthy subjects. Br J Clin Pharmacol. 1990;29(6):709–713. doi: 10.1111/j.1365-2125.1990.tb03692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramakrishnan R, Cheung WK, Wacholtz MC, Minton N, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2004;44(9):991–1002. doi: 10.1177/0091270004268411. [DOI] [PubMed] [Google Scholar]
- 94.Woo S, Krzyzanski W, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharmacol Exp Ther. 2006;319(3):1297–1306. doi: 10.1124/jpet.106.111377. [DOI] [PubMed] [Google Scholar]
- 95.Ramakrishnan R, Cheung WK, Farrell F, Joffee L, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous dose administration in cynomolgus monkeys. J Pharmacol Exp Ther. 2003;306(1):324–331. doi: 10.1124/jpet.102.047191. [DOI] [PubMed] [Google Scholar]
- 96.Karlsson MO, Wade JR, Loumaye E, Munafo A. The population pharmacokinetics of recombinant—and urinary—human follicle stimulating hormone in women. Br J Clin Pharmacol. 1998;45(1):13–20. doi: 10.1046/j.1365-2125.1998.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10(11):2392–2395. doi: 10.1681/ASN.V10112392. [DOI] [PubMed] [Google Scholar]
- 98.Liles D, Landen CN, Monroe DM, Lindley CM, Read MS, Roberts HR, et al. Extravascular administration of factor IX: potential for replacement therapy of canine and human hemophilia B. Thromb Haemost. 1997;77(5):944–948. [PubMed] [Google Scholar]
- 99.McCarthy K, Stewart P, Sigman J, Read M, Keith JC, Jr, Brinkhous KM, et al. Pharmacokinetics of recombinant factor IX after intravenous and subcutaneous administration in dogs and cynomolgus monkeys. Thromb Haemost. 2002;87(5):824–830. [PubMed] [Google Scholar]
- 100.Joshi A, Bauer R, Kuebler P, White M, Leddy C, Compton P, et al. An overview of the pharmacokinetics and pharmacodynamics of efalizumab: a monoclonal antibody approved for use in psoriasis. J Clin Pharmacol. 2006;46(1):10–20. doi: 10.1177/0091270005283282. [DOI] [PubMed] [Google Scholar]