Abstract
Pediatric drug development is a required consideration for all drug development programs. Age-appropriate formulations such as suspensions, chewable tablets, oral disintegrating tablets, etc., are typically developed and used in the pediatric clinical studies. However, it is not uncommon to use enabling formulations in the pivotal pediatric clinical study followed by bridging bioavailability and/or bioequivalence studies. Development of age-appropriate formulations is an essential part of pediatric drug development and adds additional biopharmaceutical considerations to an already complex problem. Careful planning of biopharmaceutic data collection during the adult and pediatric development program can contribute significantly to the biopharmaceutic risk assessment and planning of appropriate clinical studies leading to successful development of pediatric formulations.
Key words: bioavailability, bioequivalence, biopharmaceutics, formulations, pediatric
INTRODUCTION
Pediatric research to determine the safe and effective usage of drugs in children is essential for making life-saving medications available to children and is an important component of today’s drug development programs. Legislations in the USA (BPCA1 and PREA2),3(1,2) and the European Union (EU; 3) make it mandatory for sponsors to develop drugs for pediatric populations while providing exclusivity incentives. In the USA, a PREA assessment is required at the time of filing the NDA4; while in the EU, an agreed-upon-pediatric investigation plan (PIP) is required prior to filing the MAA.5 There is a strong focus on developing age-appropriate formulations by both regulatory agencies with the objective of maintaining the safety and efficacy while improving compliance in the pediatric populations.
Pediatric drug development presents a unique set of challenges due to the dynamic physiological changes that the population undergoes as they progress through infancy, childhood, and adolescence. Developmental differences in the barriers between children and adults can significantly affect rate of absorption and bioavailability (4). Several excellent reviews that address this subject are available (4–6). Pediatric development plans are designed to understand efficacy, safety, and pharmacokinetic (PK) differences between adults and children and to identify the safe and efficacious exposures. Age-appropriate formulations play a significant role in delivering the medications at the targeted exposures while ensuring compliance in children. Age-appropriate formulations are likely to be a requirement if the pediatric development will include neonates (birth to 1 month), infants (1 month to 2 years), and children (2–12 years), as these age groups are unlikely to swallow solid oral dosage forms. It is imperative that sponsors begin planning for pediatric development early, with an eye on collection of important biopharmaceutic data, during the adult development which will aid the future biopharmaceutic risk assessment, planning for age-appropriate formulations, and execution of the pediatric drug development program.
Collection of detailed biopharmaceutic data in children is difficult due to ethical issues. Additionally, volunteers in biopharmaceutic clinical studies are usually healthy adult individuals who do not receive any medical benefit, making it difficult to justify similar studies in a vulnerable pediatric population. Hence, we have to rely on data generated from adults in bioavailability and bioequivalence studies. Although collection of elaborate biopharmaceutic data in children is unlikely, carefully planned pharmacokinetic sampling in combination with quantitative methods can generate pediatric biopharmaceutics data where there is none. This paper will highlight the various stages of adult and pediatric drug development where one can plan to generate biopharmaceutic data to aid and de-risk pediatric drug development.
PEDIATRIC DEVELOPMENT PLANNING AND BIOPHARMACEUTIC RISK ASSESSMENT
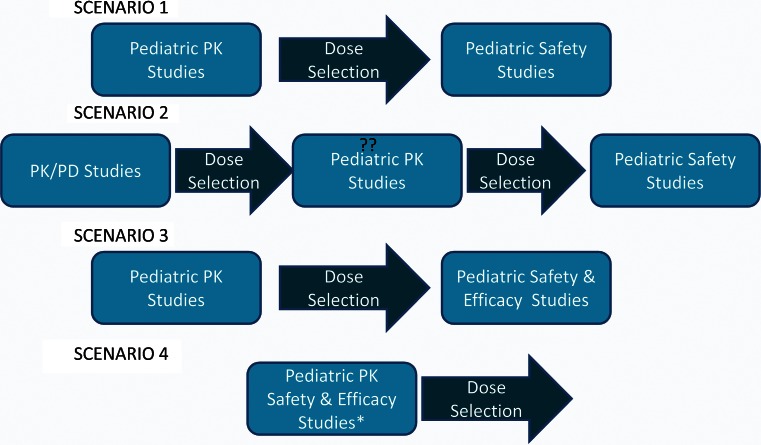
The earliest one starts planning for a pediatric development program is after completion of the phase 1 studies in adults as suggested by EU regulations for submission of a PIP or as late as after completion of the positive proof of concept (POC) trial. Considering the high failure rates of POC trials, it may be advantageous to wait until POC is achieved to prevent the effort of developing a pediatric program to be at risk. Broadly, the pediatric development strategy can be envisioned to consist of a pediatric pharmacokinetic study followed by efficacy and/or safety studies (7). Depending on the disease area and the drug, various pediatric development strategies may be adopted (Fig. 1.). An abridged development strategy comprised of pediatric PK studies followed by safety study at the selected dose is usually adopted, for disease areas where disease progression, response and concentration-response relationship can be assumed to be comparable to adults (scenario 1). A full development plan is required where any of the above assumptions do not hold or for a disease area specific for pediatric population (scenario 3).
Fig. 1.
The pediatric drug development strategies one may adopt for a specific disease area. Scenarios 1 and 3 are explained in the text. Scenario 2 is also an abbreviated strategy where concentration-response relationships are reconfirmed in a pediatric PK/PD study followed by pediatric safety study. Scenario 4 is a combination of the pediatric PK study and the safety/efficacy study. Double question marks The pediatric PK study can be eliminated with a well-designed pediatric PK/PD study. Asterisk this strategy requires the pivotal study to be a dose ranging study to allow for dose selection at the end of the study
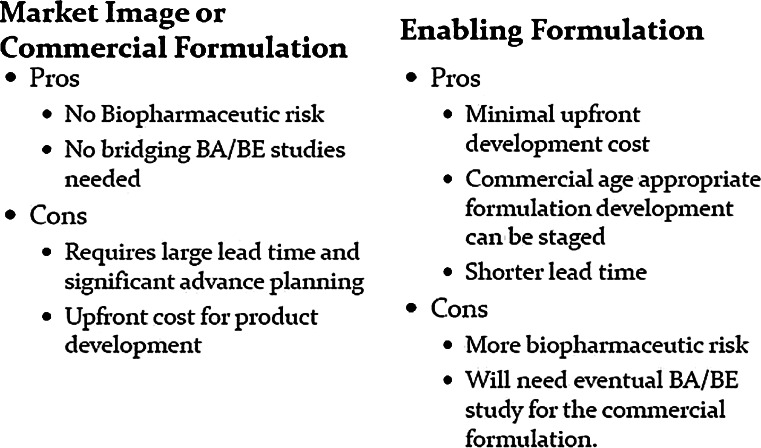
An important consideration for planning pediatric studies is the use of enabling formulations over market image or final commercial formulations. The use of enabling formulations becomes an important consideration when age-appropriate formulations are required for neonates, infants, and children. There are obvious pros and cons of selecting either strategy (Fig. 2.). The choice between the two options is mostly dominated by timeline and cost considerations but, should also include a thorough biopharmaceutic risk assessment. It is important to note that the choice of an enabling formulation is likely to require a bioavailability (BA) or bioequivalence (BE) study between the enabling formulation and the final commercial formulation unless a biowaiver can be requested based on Biopharmaceutics Classification System (BCS) class. The biopharmaceutic risk assessment begins with a thorough understanding of the biopharmaceutic properties of the drug. Important considerations are BCS class (permeability and solubility), taste characteristics, gastric stability, anticipated dosage forms for various age groups with dosing instructions, and variability of PK parameters. All the above constitute the overall biopharmaceutic risk one assumes if enabling formulations are chosen for the pivotal pediatric studies.
Fig. 2.
The pros and cons of selecting market image/commercial or enabling formulation for pivotal pediatric clinical study
As an illustrative example, let us assume a very bitter, BCS class 1 drug which will eventually be formulated into a taste masked powder for oral suspension (POS) formulation for pediatric use. A very rapidly dissolving, enabling formulation will be used for the pediatric clinical studies. The biopharmaceutic risk for this scenario is minimal assuming that the final taste masked POS formulation is also very rapidly dissolving. A formulation risk assessment should be conducted to evaluate if a very rapidly dissolving taste masked formulation is feasible. A similar scenario with a BCS class 2 or 4 drug; however, will have additional biopharmaceutic risk considering inherent low solubility of the drug and the additional taste-masking technology. Depending on the taste-masking technology employed, additional dissolution rate-limiting effects may be introduced, that may further retard dissolution, hence increasing the risk of differences in bioavailability of the enabling and final commercial formulation. This scenario requires sufficient upfront investigation and de-risking. Additional in vitro and/or clinical data may have to be generated in such scenarios where a significant biopharmaceutic risk is evident. Collection of data for biopharmaceutic risk assessment should be planned for and integrated into the adult development program. An additional period in an already planned BA study for the adult program can provide invaluable data to de-risk the pediatric program at minimal cost. Relative bioavailability data of solution or suspension versus tablets/capsules, crushed tablets on soft foods versus intact tablets can be very useful for the biopharmaceutic risk assessment for pediatric programs. Comparable bioavailability seen in the above comparisons will aid in the informed selection of an enabling formulation. Conversely, if significant differences in bioavailability are observed, then it should prompt a more conservative approach to selection of enabling formulation and may require additional clinical BA studies with candidate formulations. A scenario similar to the above mentioned illustrative example can also be envisioned for drugs having compromised gastric stability e.g., acid labile drugs. Enabling formulations for such drugs should provide the same degree of protection from gastric contents as would be anticipated for the final commercial formulation to ensure similar bioavailability.
An additional factor that requires attention is the intrasubject PK variability of the compound in question. Highly variable drugs (HVDs) are defined as drugs with intra-subject variability in Cmax or AUC of 30% or more (8). HVDs constitute a substantial number of drugs across all BCS classes (9). These drugs usually have high failure rate of BE studies with one estimate suggesting failure rates of 85% with intrasubject CV >35% (10). The choice of enabling formulation or the final commercial formulation for the pivotal pediatric clinical studies has to be carefully considered for HVDs. The consequences of not being able to bridge the exposures between enabling formulations used in pivotal pediatric clinical studies and the final commercial formulation can be significant, requiring additional safety/efficacy studies, and may delay pediatric filing.
IS PEDIATRIC RELATIVE BIOAVAILABILITY DATA POSSIBLE?
Ethical considerations prevent us from generating detailed biopharmaceutic data in pediatric population and hence the reliance on adult data with the assumption that the adult observations can be extrapolated to children. However the question remains if BA or BE observed in adults is applicable for children. A carefully planned pediatric program can however avail of opportunities during the pediatric clinical studies to generate pediatric PK data from different formulations (enabling and final commercial formulation) which can then be used to determine relative bioavailability using population pharmacokinetic approaches. However, the above is only possible when the bridging BA/BE study between the enabling and commercial pediatric formulation have been completed while the pediatric clinical studies are still ongoing or are yet to be conducted. Having completed the bridging studies and provided the formulations can be considered equivalent, a planned switch of the formulations during the pediatric study combined with sparse PK sampling for the respective formulations, can enable a relative bioavailability assessment. Population PK methods combined with a parametric bootstrap can estimate the relative bioavailability as well as evaluate the sensitivity or power of the method to detect actual differences, thereby validating the results (11). Although the above data is unlikely to obviate the need of formal BA/BE studies in adults, it does generate relative BA data in the target population and thereby help identify possible risk to pediatric patients. The above strategy can be implemented fairly easily by development teams in a well planned development program and will generate data for direct comparison of formulations in pediatric populations where no data is usually forthcoming.
DISCUSSION AND CONCLUSIONS
Failure to bridge the clinical formulation used in the pivotal pediatric trial to the final commercial formulation can have significant consequences where additional clinical efficacy or safety studies may be required. The above situation, given the target population would not only be difficult to accomplish but would be costly and irresponsible, delaying availability of important medications to pediatric populations. Biopharmaceutic risk assessment is a critical component of the pediatric development plan and should be undertaken at the earliest conceptualization of the pediatric development strategy. Need for additional data to inform the biopharmaceutic risk assessment should be identified early so its collection can be synchronized with the adult development program for efficiency. The biopharmaceutic risk assessment should inform the eventual formulation strategy in support of the pediatric development plan.
Pediatric clinical studies are difficult to plan and conduct. However, careful planning may allow us to gather considerably more information than traditional approaches allow. Use of more advanced quantitative methods along with intelligent sparse PK sampling may allow us to generate pediatric biopharmaceutic data. This can answer important questions regarding the applicability of adult bioavailability data to children. The mentioned quantitative approach maximizes the minimal data collection opportunities that are available in pediatric trials and should be considered by development teams. An important consideration for constituting the pediatric development team is the inclusion of a clinical pharmacologist with extensive knowledge of pediatric pharmacology as a team member or a consultant for development planning and biopharmaceutic risk assessment. This ensures that the team has the essential expertise in identifying the data gaps and can plan appropriately.
In summary, the planning for pediatric development plans begins long before it is actually executed and when informed by a thorough biopharmaceutic risk assessment can make pediatric clinical studies more efficient and may help accelerate the development without taking unnecessary risks.
Acknowledgments
The author would like to thank Jack Cook and Xiang Gao for valuable discussions and suggestions.
Footnotes
BPCA = Best Pharmaceuticals for Children Act
PREA = Pediatric Research Equity Act
Only BPCA provides exclusivity incentives
NDA = New Drug Application
MAA = Marketing Authorization Application
References
- 1.Pediatric Research Equity Act of 2007, Title IV. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM049870.pdf. Accessed on: 17 Jan 2012.
- 2.Best Pharmaceuticals for Children Act of 2007, Title V. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM049870.pdf. Accessed on 17 Jan 2012.
- 3.Regulation (EC) No. 1901/2006 of the European Parliament and of the Council of December 12, 2006, on medicinal products for paediatric use and amending Regulation (EEC) No. 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No. 726/2004. Available from: http://ec.europa.eu/health/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf. Accessed on 17 Jan 2012.
- 4.Benedetti MS, Whomsley R, Baltes EL. Differences in absorption, distribution, metabolism and excretion of xenobiotics between the pediatric and adult populations. Expert Opin Drug Metab Toxicol. 2005;1(3):447–471. doi: 10.1517/17425255.1.3.447. [DOI] [PubMed] [Google Scholar]
- 5.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;359:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 6.Johnson TN, Thomson M. Intestinal metabolism and transport of drugs in children: the effects of age and disease. J Pediatr Gastroenterol Nutr. 2008;47:3–10. doi: 10.1097/MPG.0b013e31816a8cca. [DOI] [PubMed] [Google Scholar]
- 7.Roth-Cline M, Gerson J, Bright P, Lee CS, Nelson RM. Ethical considerations in conducting pediatric research. pediatric clinical pharmacology. Handb Exp Pharmacol. 2011;205(Part 2):219–244. doi: 10.1007/978-3-642-20195-0_11. [DOI] [PubMed] [Google Scholar]
- 8.Haidar SH, Davit B, Chen ML, Conner D, Lee L, Li QH, et al. Bioequivalence approaches for highly variable drugs and drug products. Pharm Res. 2008;25:237–241. doi: 10.1007/s11095-007-9434-x. [DOI] [PubMed] [Google Scholar]
- 9.Cook JA, Davit BM, Polli JE. Impact of biopharmaceutics classification system-based biowaivers. Mol Pharm. 2010;7:1539–1544. doi: 10.1021/mp1001747. [DOI] [PubMed] [Google Scholar]
- 10.Tanguay M, Potvin D, Haddad J, Lavigne J, Marier JF, DiMarco M, et al. When will a drug formulation pass or fail bioequivalence criteria? Experience from 1,200 studies. AAPS Pharm Sci. 2002;4(S1). Abstract R6193.
- 11.Purohit VS, Harnisch L, Gao X. Relative bioavailability of different sildenafil formulations: a population pharmacokinetic based approach. Abstract R6349. AAPS Annual Meeting, 2011, Washington DC. Available from: www.aapsj.org/abstracts/AM_2011/R6349.pdf.