Abstract
Monoclonal antibodies have provided many validated and potential new therapeutic candidates for various diseases encompassing the realms of neurology, ophthalmology, immunology, and especially oncology. The mechanism of action for these biological molecules typically involves specific binding to a soluble ligand or cell surface protein in order to block or alter a molecular pathway, induce a desired cellular response, or deplete a target cell. Many antigens reside within the interstitial space, the fluid-filled compartment that lies between the outer endothelial vessel wall and the plasma membranes of cells. This mini-review examines the concepts relevant to the kinetics and behavior of antibodies within the interstitium with a special emphasis on radiometric measurement of quantitative pharmacology. Molecular probes are discussed to outline chemical techniques, selection criteria, data interpretation, and relevance to the study of antibody pharmacokinetics. The importance of studying the tissue uptake of antibodies at a compartmental level is highlighted, including a brief overview of receptor occupancy and its interpretation in radiotracer studies. Experimental methods for measuring the spatial composition of tissues are examined in terms of relative vascular, interstitial, and cellular volumes using solid tumors as a representative example. Experimental methods and physiologically based pharmacokinetic modeling are introduced as distinct approaches to distinguish between free and bound fractions of interstitial antibody. Overall, the review outlines the available methods for pharmacokinetic measurements of antibodies and physiological measurements of the compartments that they occupy, while emphasizing that such approaches may not fully capture the complexities of dynamic, heterogeneous tumors and other tissues.
KEY WORDS: antibody, biodistribution, compartmental analysis, pharmacokinetics, physiology
INTRODUCTION
Monoclonal antibodies have emerged as an important class of therapeutic drugs in a variety of disease areas (1). The majority of therapeutic antibodies are designed to target either cell surface antigens or soluble ligands such as cytokines. Most antibodies evaluated for oncology have targeted various glycoproteins, glycolipids, and carbohydrates that populate the surface of cancerous cells, although a few have targeted soluble proteins (2). Consequently, the most relevant biological compartment for oncologic antibody therapy is often the interstitial fluid that lies between the outer endothelial vessel wall and the plasma membranes of cells (3). The transport of antibodies into and within the interstitium depends on the biological and physicochemical properties of the antibody itself and how it interacts within the interstitial compartment (4–6). For example, electrostatic interactions greatly affect the absorption, distribution, metabolism, and excretion (ADME) properties of antibodies due to the presence of negatively charged groups in the interstitial space including glycan chains on cell surfaces and heparin sulfate proteoglycans within the extracellular matrix (7).
Increased attention has been focused towards the pharmacokinetics of small molecule drugs within the interstitial space (8), with similar efforts for antibodies and other large molecule drugs (9,10). Unfortunately, such approaches have not become widely adopted as most quantitative measurements of concentration are still measured and interpreted merely in terms of plasma concentrations and total tissue uptake (11,12). This mini-review summarizes a presentation given at an AAPS Focus Group Workshop (ADME of Protein Therapeutics Introductory Workshop: Scientific, Technical Concepts and Case Studies; August 15, 2011; Buffalo, NY) and will highlight the available methodologies and significant advantages to drug development in dissecting total tissue uptake of antibody into the constituent plasma, interstitial, and cellular compartments, with further distinction between free and bound fractions.
RADIONUCLIDES FOR QUANTITATIVE PHARMACOLOGY OF ANTIBODIES: CHEMISTRY AND SELECTION CRITERIA
Radiohalogens Versus Radiometals
Although nonradioactive, bioanalytical methods are still regarded as the industry standard (13), the use of radionuclides in the quantitative pharmacology of antibodies boasts extremely high sensitivity and well-established methods for incorporation and detection (14). But perhaps the most important advantage lies in the facile detection of radionuclides in tissues for biodistribution studies (15,16). In fact, this process requires no special tissue handling, homogenization, bleaching, or quenching correction in the case of gamma-emitting radionuclides such as iodine-125 or indium-111.
Ideally, a radionuclide should be covalently linked to an antibody to create a stable linkage without impairing binding affinity to antigen or other receptors [e.g., Fc receptors (17–19)]. Radiolabeling procedures should also be simple, efficient, reproducible, affordable, and occurring under practical conditions. Chemical linkage methodologies comprising carbon–halogen, thiourea, thioether, amide, ester, and disulfide bonds are available. One of the most common methods for radiolabeling antibodies involves radioiodination of tyrosine residues, which should be performed using the Chizzonite or “Indirect Iodogen” method to minimize losses in immunoreactivity (20).
Alternatively, the stable attachment of radiometals to antibodies using chelates has been pursued to circumvent the susceptibility of radioiodine to dehalogenation (21). Some metal complexation reactions can be quite slow, requiring elevated temperature and/or high pH values over extended time periods and therefore compromising the use of such radiolabeling methodologies. However, metal complexes that form too easily tend to exhibit lower in vivo stability (21). Generally, DTPA derivatives and other acyclic chelates exhibit faster complex association and dissociation rates than 1,4,7,20-tetraazacyclododecane N,N′,N″,N″′-tetraacetic acid (DOTA) derivatives and other macrocyclic chelates. As for the extent of conjugation, an optimal chelate-to-protein ratio must be established wherein the degree of protein modification is optimized without reducing binding activity. This key variable is generally controlled by adjusting reaction stoichiometry and conditions (e.g., pH, temperature, and time).
Selection Criteria
The most obvious consideration in selecting an appropriate radiolabeling method is the physical half-life of the radionuclide (21). For instance, the half-life of iodine-125 is roughly 2 months, while that of indium-111 is less than 3 days. Longer lived metallic radionuclides of interest include gadolinium-153 and lutetium-177 and are governed by similar complexation chemistry as radioindium.
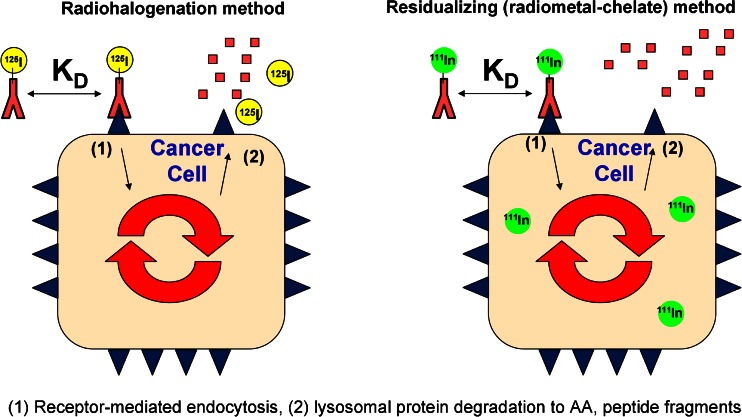
In addition to half-life, the distinction between residualizing (metallic) and non-residualizing (halogen) radionuclides must be considered. Radiocatabolites of antibodies labeled with metal radionuclides via DOTA or other polyaminopolycarboxylate chelators tend to become trapped inside cells and accumulate in antigen-expressing tissues following receptor-mediated endocytosis (22) due to the residualizing properties of this charged, highly polar probe (Fig. 1) (23,24). Importantly, while similar pharmacokinetic data in blood and antigen-negative tissues are typically obtained using either radioiodine or radiometal probes, a much different scenario exists in tissues that overexpress the antigen, especially if internalization occurs (25). Specifically, the true amount of antibody present in tissues that express internalizing antigen is often overestimated due to residualization or trapping of radiocatabolites derived from the cellular metabolism of antibodies labeled with radiometal–chelate complexes. As such, for internalizing antigens, radiometal probes give cumulative uptake in target tissues, whereas radiohalogen probes more closely approximate the “real-time” concentration of antibodies within tissues (i.e., kinetics in the tissue). Given the known normal metabolism of immunoglobulins into their constituent amino acids (26,27), the primary radiolabeled catabolite of indium–DOTA-labeled antibodies following lysosomal proteolytic degradation is indium-111–DOTA–lysine (28).
Fig. 1.
Figure adapted with permission from “Impact of Antibody Modifications on Tumor Targeting” by Stephen I. Rudnick, Ph.D. (AAPS-NBC 2009 Meeting). Differential cellular catabolite retention of non-residualizing (radiohalogen) or residualizing (radiometal–chelate) probes. For antibodies labeled with radiohalogens, following receptor-mediated internalization and lysosomal degradation, the resultant catabolic products are cleared from the cell. For antibodies labeled with radiometal–chelates, the resultant catabolized radiometal–chelate (usually present as an amino acid adduct) is trapped in the cell due to the highly polar nature of the chelate. The different cellular retention of their respective catabolic products can be used to understand antibody tissue kinetics (radiohalogen) or cumulative tissue exposure (radiometal–chelate)
A very nice agreement in blood pharmacokinetics (PK) between 125I and 111In-labeled antibodies has been demonstrated in two separate studies through 7 days (15,25). Similarly, close agreement between radiometric (111In) and ELISA-derived blood PK data has also been reported through 7 days (23). However, certain limitations exist regarding the extent to which radiohalogens and radiometals may be assumed to accurately represent real-time and cumulative tissue uptake of antibodies, respectively. These limitations vary with both time and tissues. Residualization of radiometals is never perfect and should instead be regarded as enhanced tissue retention. Furthermore, data in organs involved in clearance should be cautiously interpreted because transporters and other metabolic processes may impact the level and duration of residualization. Tumors have little to no lymphatic drainage and generally possess a high degree of residualization of radiometals; however, one must bear in mind that tumors are dynamic pseudo-organs that undergo continual growth, cell division, and cell death. Moreover, radioiodinated antibodies may somewhat underestimate the real-time concentration in tissues, particularly at late time points, due to dehalogenation; this is caused by a gradual removal of radioiodine from intact antibody by dehalogenase enzymes in vivo. Unfortunately, it is difficult to distinguish whether renal excretion of free 125I− is caused by complete proteolytic degradation of the radiolabeled antibody or by dehalogenation of the radionuclide from the intact antibody.
Interpretation of Tissue Distribution Data
Proper interpretation of tissue distribution data is contingent upon the biological question to be addressed. Expressing tissue uptake in virtually any unit can reveal the degree to which the antibody distributes to various tissues. Units of micrograms per milliliter or nanomolar are appropriate to demonstrate the antibody concentration in tissues. Importantly, demonstrating the specificity of distribution—whether the uptake is dose-dependent and/or saturable—is best addressed by use of the dose-normalized unit of concentration, percentage of injected dose per gram of tissue (%ID/g). Conversion between non-dose normalized (e.g., micrograms per milliliter) and dose normalized (e.g., percentage of injected dose per gram of tissue) units of concentration may be easily achieved based on the concept of specific activity (see below). Finally, expressing data as percentage of injected dose (%ID) can convey the fraction of the antibody (or catabolite thereof) that is present within a given tissue or excreta; this is often appropriate for mass balance studies. In this context, distinction between intact antibodies and catabolites is often pursued using size exclusion radio-HPLC or trichloroacetic acid (TCA) precipitation of plasma, excreta, and/or tissue homogenates. However, tissues containing antibodies labeled with radiometal–chelate complexes should not be analyzed by TCA precipitation due to radiometal decomplexation in acidic conditions.
The specific activity of the radiotracer (i.e., the antibody labeled with the radionuclide) is defined as the ratio of radioactivity in microcuries or counts per minute (CPM) to the amount of protein in micromoles or micrograms. Using this quantity, the amounts of radioactivity (decay-corrected) in blood and tissues may be converted into absolute amounts of protein. Furthermore, a convenient formula describing the relationship between percentage of injected dose per gram of tissue (%ID/g) and the antibody concentration in micrograms per milliliter is as follows:
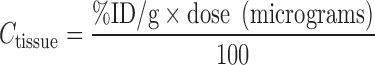 |
1 |
where “dose” represents the total administered dose of antibody in units of micrograms. Conceptually, the key to understanding this formula is that the injected dose “ID” refers to the dose in terms of both radioactivity and protein since the specific activity must be regarded as a constant based on the stable attachment of the radionuclide to the antibody.
INTERPRETATION OF RECEPTOR OCCUPANCY IN RADIOTRACER STUDIES
Receptor Theory
When evaluated as a function of both dose and time, receptor occupancy can be used to evaluate optimal doses and dosing regimens for antibody therapeutics and other drugs. Receptor occupancy (RO) of an antibody is defined as the proportion of receptors (R) occupied by an antibody (A) at a given time (29):
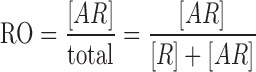 |
2 |
The basic underlying concept is that administration of a given dose of antibody will yield a certain plasma level as a function of time. Consequently, this plasma level will be associated with a dynamic receptor occupancy level that elicits an associated drug response (Fig. 2). In some circumstances, it is reasonable to assume that the maximum drug effect will be achieved at or near 100% receptor occupancy.
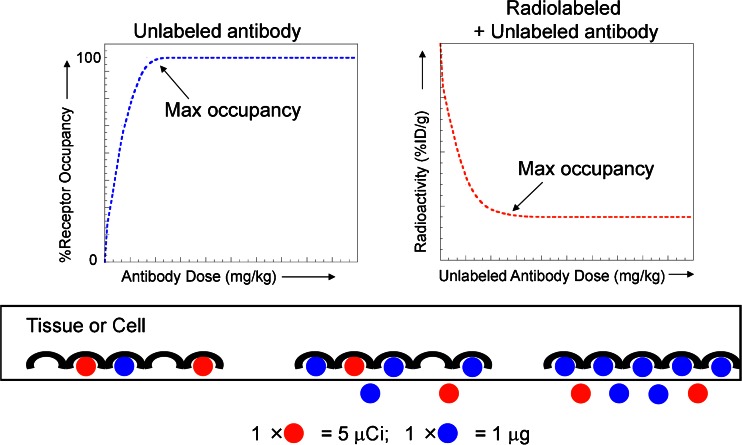
Fig. 2.
Receptor occupancy as it relates to dose in terms of absolute versus radiotracer uptake. In a direct binding model (left), as the dose of total antibody increases, the percent receptor occupancy also increases, approaching 100% or maximum occupancy. In contrast, in a competitive binding model (right), the radiotracer is used as a marker to follow the antibody levels in the tissue. At a fixed dose of radiotracer, radioactivity levels in the tissue decrease with increasing dose of unlabeled antibody due to competitive binding, reaching a bottom plateau at maximum occupancy. The cartoon below the graphs illustrates these concepts at the receptor level, with the unlabeled antibody (measured in micrograms) represented in blue and the radiolabeled antibody (measured in microcuries) represented in red
For an antibody binding to its receptor in a steady-state equilibrium, the equilibrium dissociation constant (KD) is defined as an inverse measure of binding affinity and also represents the concentration of antibody that produces 50% receptor occupancy (30):
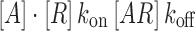 |
3 |
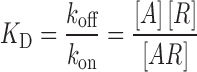 |
4 |
Substituting the above definition for receptor occupancy and solving for the concentration of free receptors ([R]) yield the Hill–Langmuir equation:
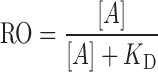 |
5 |
where [A] is the free concentration of interstitial antibody. At high doses, it is a common practice to make the assumption that the free interstitial concentration approximates the total interstitial concentration; however, caution must be exercised in tissues having extremely high receptor expression levels. Calculations of receptor occupancy are based on an assumption that the target receptor is freely accessible to the antibody within the interstitial fluid space. This assumption may not be valid in some situations, particularly for tumors with areas of necrosis. Further complications arise when one considers that dose-dependent spatial heterogeneity in receptor occupancy may exist within a given solid tumor (31,32).
Competitive Binding Inhibition
Despite the simplicity of the Hill–Langmuir equation and knowledge of KD values for many antibodies, determination of specific values for receptor occupancy, especially across a dynamic dose range, is often impossible due to the aforementioned technical challenges in measuring free interstitial concentrations at steady state. As such, it is often more straightforward to employ radiotracer studies designed to reveal the dose at which maximum receptor occupancy is attained at a given time. It is extremely critical to bear in mind that, as the dose of unlabeled antibody is increased, increasing receptor occupancy levels means that the radioactivity levels in tissues actually decrease based on the concept of competitive binding inhibition (Fig. 2) (33). In this situation, the radiotracer is used as a marker to follow the antibody levels in the tissue. At a fixed dose of radiotracer, radioactivity levels in the tissue decrease with increasing dose of unlabeled antibody due to competitive binding, reaching a bottom plateau at maximum occupancy.
COMPARTMENTAL PHYSIOLOGICAL MEASUREMENTS
Measurement of Vascular and Interstitial Volumes
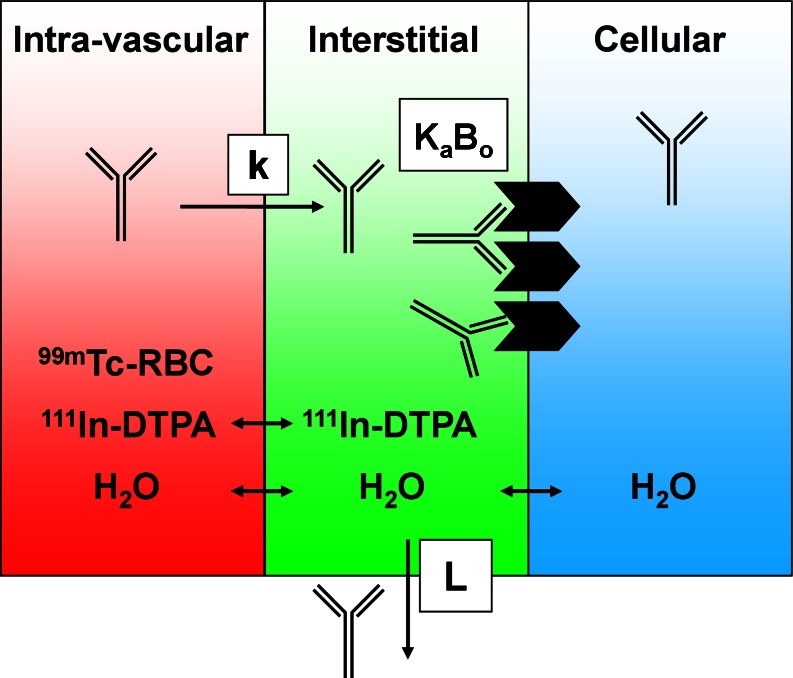
If antibody concentrations are measured in terms of total, whole-tissue uptake, then a physiologically based correction is necessary to derive compartmental concentrations. Such corrections require knowledge of the relative tissue spaces that are occupied by blood and interstitial fluid. A considerable amount of physiological data for laboratory animals and humans is reported in the literature (34); however, the manner in which it is utilized should be approached with an understanding of several limitations since measurement techniques vary widely and the use of assumed nominal values is common (35). Furthermore, the physiology of disease tissues such as xenograft models is highly variable and largely unknown. As such, preclinical methods for measurement of vascular volume (Table I) have been developed to allow subtractive correction for the amount of antibody within the blood of tissues (35). This measurement is based on a clinically utilized blood pool nuclear imaging protocol and relies on radiolabeling of red blood cells (RBCs) with technetium-99m and measuring the amount of radioactivity in CPM in tissues and blood using a gamma counter (Fig. 3), yielding vascular volume (Vv) in units of microliters per gram of tissue:
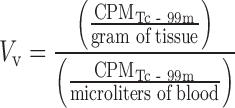 |
6 |
Table I.
Mean Physiological Values with Standard Deviations in Tumor and Various Tissues
| V v (n = 5) | V i (n = 5) | |
|---|---|---|
| Tumor | 8.2 ± 1.3 | 170 ± 25 |
| Liver | 55 ± 11 | 72 ± 8.5 |
| Spleen | 117 ± 36 | n.r.a |
| Kidney | 108 ± 28 | 1,200 ± 300b |
| Lung | 214 ± 78 | 270 ± 120 |
| Heart | 49 ± 9 | 190 ± 92 |
| Muscle | 5.4 ± 2.0 | 48 ± 8.0 |
| Fat | 9.3 ± 4.0 | 49 ± 12 |
Vascular volumes (V v) and interstitial volumes (V i) are in microliters per gram. Adapted from Pastuskovas et al. (15)
aVi values are not reported (n.r.) for spleen due to dependence of V i on V v and the spleen’s role in RBC sequestration, which results in calculation of negative values
bRenal V i values are not physiologically accurate due to the kidney’s role in radioactive tracer clearance
Fig. 3.
Adapted with permission from Boswell et al. (36). Tissue compartments and the radioactive tracers used to assess their physiological parameters. Each tissue can be separated into three compartments: intravascular, interstitial, and cellular. Technetium-99m-labeled red blood cells (99m Tc-RBC) and 111In-DTPA allow measurement of vascular and extracellular volumes, respectively. The antibody’s receptor, if present, may be expressed on the cell surface, exposed to the interstitial fluid. An antibody in circulation may extravasate from blood into interstitial space at a rate (k), where it may encounter a number (B o) of receptors for which it has binding affinity (K a). The antibody may also return to circulation via lymphatic flow (L)
In addition, similar methods have been established for measurement of extracellular volume following intravenous infusion of the extracellular marker indium-111-pentetate (36). Subtracting the vascular volume (Tc-99m) from the extracellular volume (In-111) allows derivation of the pharmacologically relevant quantity, the interstitial volume in units of microliters per gram of tissue (Table I).
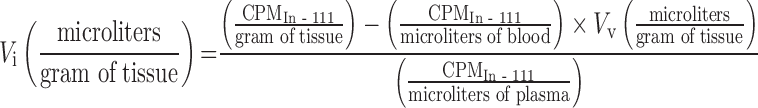 |
7 |
The interstitial volume is also referred to as the “biophase” due to its central role in the biological mechanism of action for many drugs.
Direct Measurement of Interstitial Antibody Concentration
Another approach to obtaining antibody concentrations within the interstitial space involves direct sampling of the interstitial fluid itself. Although several technical challenges exist, this method has the advantage of not relying on physiological parameters and is therefore a more direct approach. Specifically, Wiig and coworkers have developed a low-speed centrifugation technique as a method to isolate interstitial fluid from tumors (9,37). However, extreme care and appropriate controls are necessary to ensure that the measurements are not confounded by blood contamination, inaccurate volumes, and even evaporation due to the very low volumes of collected interstitial fluid.
Ex Vivo Strategies for Assessing Interstitial Antibody Concentration
As an alternative to direct sampling of interstitial fluid, others have chosen to pursue ex vivo methods for measuring receptor occupancy. For instance, Bumbaca et al. administered various levels of unlabeled antibody, followed by tumor harvest, homogenation, and incubation in the presence of the radiolabeled version of the same antibody (16). This approach allowed for estimation of the dose at which maximal receptor occupancy was reached; however, an actual value could not be determined. Furthermore, such ex vivo systems may underestimate the saturating dose because more receptors are potentially accessible for antibody binding in the processed tumor than in vivo.
Blood and Interstitial Corrections
The vascular and interstitial volumes may be utilized to calculate the amounts of antibody in the appropriate compartments. First, the amount of antibody within the blood of tissues is computed:
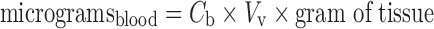 |
8 |
Secondly, the amount of antibody (in micrograms) within the blood of tissues is subtracted from the total tissue uptake:
 |
9 |
Finally, the interstitial concentration is calculated using the fractional interstitial volume (Φ):
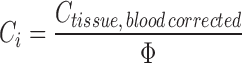 |
10 |
The fractional interstitial volume (Φ) may be easily derived from interstitial volume (Vi) data in traditional units of volume per gram of tissue. For example, a tissue having an interstitial volume of 100 μL of blood per gram of tissue has a fractional interstitial volume of 0.100 since each gram of tissue occupies 1,000 μL of space (assuming a tissue density of 1 g/mL). The same relationship exists between the γ and vascular volume (Vv). Once the interstitial concentration of antibody is calculated, its units may be converted from micrograms per milliliter to nanomolars using the molecular weight of approximately 150,000 g/mol for most antibodies.
Distinguishing Between Free and Bound Antibody
Interstitial concentrations measured directly by collection of interstitial fluid only include free, unbound antibody molecules (see “Direct Measurement of Interstitial Antibody Concentration” section). In contrast, interstitial concentrations derived from compartmental correction of total tissue uptake include both free and bound fractions of interstitial antibody since radiometric detection methods are unable to distinguish between the two populations. This problem is not specific to radiometric methods, however, as the existence of similar challenges for bioanalytical methods has been documented (38). One solution to this problem is to neglect the amount of bound antibody and assume that the free interstitial concentration of antibody approximates the total concentration; however, this is not a valid assumption at low doses or in tissues having high receptor expression levels.
Physiologically based pharmacokinetic modeling represents an alternative approach to distinguish between free and bound fractions of antibody in the interstitial space (10,39,40). This approach is based on the law of mass balance, with blood concentrations dominating (Cb) the interstitial concentration (Ci) of antibody in a given tissue and the rates of extravasation (k) and lymphatic fluid flow (L):
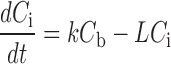 |
11 |
In tumors, where lymphatic fluid flow is virtually nonexistent, the parameter L may instead represent the leaving rate of antibody from the interstitial compartment, most likely via binding and/or internalization by tumor cells. The major advantage of the modeling approach is that it provides an estimate of free interstitial concentration based on inputting known physiological values (e.g., Φ) as fixed parameters and curve fitting a model-derived equation to mathematically solve for unknown, variable parameters, including B0, the total number of binding sites (Fig. 3) (10,40). The major disadvantages include a dependency on the accuracy of fixed parameters, validity of model assumptions, and a requirement for use of a non-binding control antibody.
CONCLUSION
In conclusion, radioactive probes are useful preclinical development tools that may be used to measure concentrations of antibody in blood and tissues and to further derive physiologically relevant concentrations within distinct biological compartments. The chemistry and selection criteria for radionuclides should depend on the specific biological questions to be addressed, and robust characterization of immunoconjugates is critical to ensure that labeling procedures do not affect immunoreactivity. Mechanistic studies support development of antibody therapeutics by identifying specific tissue uptake and by allowing estimation of receptor occupancy, which may be related to expected drug effect. Finally, both pharmacokinetic and physiological (compartmental) measurements are feasible, but may not fully reflect the complexities of dynamic, heterogeneous tumors and other disease tissues.
ACKNOWLEDGMENTS
The authors would like to thank Ruedi Port, Frank-Peter Theil, Gregory Ferl, and Martin Brechbiel for helpful discussions.
Disclosure Statement
All authors hold financial interest in Hoffmann-La Roche.
ABBREVIATIONS
- ADME
Adsorption distribution, metabolism, and excretion
- Fc
Crystallizable fragment
- DTPA
Diethylenetriaminepentaacetic acid
- DOTA
1,4,7,20-Tetraazacyclododecane N,N′,N″,N″′-tetraacetic acid
- %ID/g
Percentage of injected dose per gram of tissue
- Ctissue
Whole-tissue antibody concentration (μg/mL)
- RO
Receptor occupancy
- AR
Antibody–receptor complex
- R
Free receptor
- A
Free antibody
- kon/koff
Rates of association/dissociation
- KD
Dissociation constant
- CPM
Counts per minute
- Vv
Vascular volume
- Vi
Interstitial volume
- Cb
Concentration of antibody in whole blood
- Ctissue, blood-corrected
Blood-corrected tissue concentration
- Ci
Concentration of antibody in interstitial fluid
- ϕ
Fractional interstitial volume
- γ
Fractional vascular volume
- k
Rate of extravasation from blood to interstitial space
- L
Rate of lymphatic fluid flow
- Ka
Association constant
- B0
Total number of binding sites
- RBC
Red blood cell
REFERENCES
- 1.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 2.Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6(5):349–356. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 3.Wiig H, Tenstad O, Iversen PO, Kalluri R, Bjerkvig R. Interstitial fluid: the overlooked component of the tumor microenvironment? Fibrogenesis Tissue Repair. 2010;3:12. doi: 10.1186/1755-1536-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039–3051. [PubMed] [Google Scholar]
- 5.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 6.Thurber GM, Zajic SC, Wittrup KD. Theoretic criteria for antibody penetration into solid tumors and micrometastases. J Nucl Med. 2007;48(6):995–999. doi: 10.2967/jnumed.106.037069. [DOI] [PubMed] [Google Scholar]
- 7.Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem. 2010;21(12):2153–2163. doi: 10.1021/bc100261d. [DOI] [PubMed] [Google Scholar]
- 8.Levitt DG. The pharmacokinetics of the interstitial space in humans. BMC Clin Pharmacol. 2003;3:3. doi: 10.1186/1472-6904-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiig H, Gyenge CC, Tenstad O. The interstitial distribution of macromolecules in rat tumours is influenced by the negatively charged matrix components. J Physiol. 2005;567(Pt 2):557–567. doi: 10.1113/jphysiol.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung C, Youle RJ, Dedrick RL. Pharmacokinetic analysis of immunotoxin uptake in solid tumors: role of plasma kinetics, capillary permeability, and binding. Cancer Res. 1990;50(22):7382–7392. [PubMed] [Google Scholar]
- 11.Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab’)2, and Fab’ in mice. Cancer Res. 1986;46(8):3969–3978. [PubMed] [Google Scholar]
- 12.Weinstein JN, Eger RR, Covell DG, Black CD, Mulshine J, Carrasquillo JA, et al. The pharmacology of monoclonal antibodies. Ann N Y Acad Sci. 1987;507:199–210. doi: 10.1111/j.1749-6632.1987.tb45802.x. [DOI] [PubMed] [Google Scholar]
- 13.Tabrizi M, Funelas C, Suria H. Application of quantitative pharmacology in development of therapeutic monoclonal antibodies. AAPS J. 2010;12(4):592–601. doi: 10.1208/s12248-010-9220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holton OD, Black CD, Parker RJ, Covell DG, Barbet J, Sieber SM, et al. Biodistribution of monoclonal IgG1, F(ab’)2, and Fab’ in mice after intravenous injection. Comparison between anti-B cell (anti-Lyb8.2) and irrelevant (MOPC-21) antibodies. J Immunol. 1987;139(9):3041–3049. [PubMed] [Google Scholar]
- 15.Pastuskovas CV, Mundo EE, Williams SP, Nayak TK, Ho J, Ulufatu S, et al. Effects of anti-VEGF on pharmacokinetics, biodistribution and tumor penetration of trastuzumab in a preclinical breast cancer model. Mol Cancer Ther. 2012;11(3):752–762. doi: 10.1158/1535-7163.MCT-11-0742-T. [DOI] [PubMed] [Google Scholar]
- 16.Bumbaca D, Xiang H, Boswell CA, Port RE, Stainton SL, Mundo EE, et al. Maximizing anti-neuropilin-1 tumour exposure requires saturation of non-tumour tissue antigenic sinks in mice. Br J Pharmacol. 2012;166(1):368–377. doi: 10.1111/j.1476-5381.2011.01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010;24(1):23–39. doi: 10.2165/11530560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11(1–2):81–88. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- 19.Prabhu S, Boswell CA, Leipold D, Khawli LA, Li D, Lu D, et al. Antibody delivery of drugs and radionuclides: factors influencing clinical pharmacology. Ther Deliv. 2011;2(6):769–791. doi: 10.4155/tde.11.41. [DOI] [PubMed] [Google Scholar]
- 20.Chizzonite R, Truitt T, Podlaski FJ, Wolitzky AG, Quinn PM, Nunes P, et al. IL-12: monoclonal antibodies specific for the 40-kDa subunit block receptor binding and biologic activity on activated human lymphoblasts. J Immunol. 1991;147(5):1548–1556. [PubMed] [Google Scholar]
- 21.Boswell CA, Brechbiel MW. Development of radioimmunotherapeutic and diagnostic antibodies: an inside-out view. Nucl Med Biol. 2007;34(7):757–778. doi: 10.1016/j.nucmedbio.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wall DA, Maack T. Endocytic uptake, transport, and catabolism of proteins by epithelial cells. Am J Physiol. 1985;248(1 Pt 1):C12–C20. doi: 10.1152/ajpcell.1985.248.1.C12. [DOI] [PubMed] [Google Scholar]
- 23.Boswell CA, Mundo EE, Zhang C, Bumbaca D, Valle NR, Kozak KR, et al. Impact of drug conjugation on pharmacokinetics and tissue distribution of anti-STEAP1 antibody-drug conjugates in rats. Bioconjug Chem. 2011;22(10):1994–2004. doi: 10.1021/bc200212a. [DOI] [PubMed] [Google Scholar]
- 24.Shih LB, Thorpe SR, Griffiths GL, Diril H, Ong GL, Hansen HJ, et al. The processing and fate of antibodies and their radiolabels bound to the surface of tumor cells in vitro: a comparison of nine radiolabels. J Nucl Med. 1994;35(5):899–908. [PubMed] [Google Scholar]
- 25.Perera RM, Zoncu R, Johns TG, Pypaert M, Lee FT, Mellman I, et al. Internalization, intracellular trafficking, and biodistribution of monoclonal antibody 806: a novel anti-epidermal growth factor receptor antibody. Neoplasia. 2007;9(12):1099–1110. doi: 10.1593/neo.07721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49(4):673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegelberg HL, Fishkin BG, Grey HM. Catabolism of human gammaG-immunoglobulins of different heavy chain subclasses. I. Catabolism of gammaG-myeloma proteins in man. J Clin Invest. 1968;47(10):2323–2330. doi: 10.1172/JCI105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers BE, Franano FN, Duncan JR, Edwards WB, Anderson CJ, Connett JM, et al. Identification of metabolites of 111In-diethylenetriaminepentaacetic acid-monoclonal antibodies and antibody fragments in vivo. Cancer Res. 1995;55(23 Suppl):5714s–5720s. [PubMed] [Google Scholar]
- 29.Ploeger BA, van der Graaf PH, Danhof M. Incorporating receptor theory in mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling. Drug Metab Pharmacokinet. 2009;24(1):3–15. doi: 10.2133/dmpk.24.3. [DOI] [PubMed] [Google Scholar]
- 30.Kenakin T. Receptor theory. Current Protocols in Pharmacology. 2008; Unit 1.2. doi:10.1002/0471141755.ph0102s41. [DOI] [PubMed]
- 31.Yang FE, Brown RS, Koral KF, Clavo AC, Jackson GA, Wahl RL. Quantitative autoradiographic evaluation of the influence of protein dose on monoclonal antibody distribution in human ovarian adenocarcinoma xenografts. Cancer Immunol Immunother. 1992;35(6):365–372. doi: 10.1007/BF01789014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurber GM, Schmidt MM, Wittrup KD. Factors determining antibody distribution in tumors. Trends Pharmacol Sci. 2008;29(2):57–61. doi: 10.1016/j.tips.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckman RA, von Roemeling R, Scott AM. Monoclonal antibody dose determination and biodistribution into solid tumors. Ther Deliv. 2011;2(3):333–344. doi: 10.4155/tde.10.91. [DOI] [PubMed] [Google Scholar]
- 34.Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55(20):4611–4622. [PubMed] [Google Scholar]
- 35.Boswell CA, Ferl GZ, Mundo EE, Schweiger MG, Marik J, Reich MP, et al. Development and evaluation of a novel method for preclinical measurement of tissue vascular volume. Mol Pharm. 2010;12:12. doi: 10.1021/mp100183k. [DOI] [PubMed] [Google Scholar]
- 36.Boswell CA, Ferl GZ, Mundo EE, Bumbaca D, Schweiger MG, Theil FP, et al. Effects of anti-VEGF on predicted antibody biodistribution: roles of vascular volume, interstitial volume, and blood flow. PLoS One. 2011;6(3):e17874. doi: 10.1371/journal.pone.0017874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiig H, Aukland K, Tenstad O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. Am J Physiol Heart Circ Physiol. 2003;284(1):H416–H424. doi: 10.1152/ajpheart.00327.2002. [DOI] [PubMed] [Google Scholar]
- 38.Lee JW, Kelley M, King LE, Yang J, Salimi-Moosavi H, Tang MT, et al. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J. 2011;13(1):99–110. doi: 10.1208/s12248-011-9251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danhof M, de Jongh J, De Lange EC, Della Pasqua O, Ploeger BA, Voskuyl RA. Mechanism-based pharmacokinetic-pharmacodynamic modeling: biophase distribution, receptor theory, and dynamical systems analysis. Annu Rev Pharmacol Toxicol. 2007;47:357–400. doi: 10.1146/annurev.pharmtox.47.120505.105154. [DOI] [PubMed] [Google Scholar]
- 40.Shockley TR, Lin K, Sung C, Nagy JA, Tompkins RG, Dedrick RL, et al. A quantitative analysis of tumor specific monoclonal antibody uptake by human melanoma xenografts: effects of antibody immunological properties and tumor antigen expression levels. Cancer Res. 1992;52(2):357–366. [PubMed] [Google Scholar]