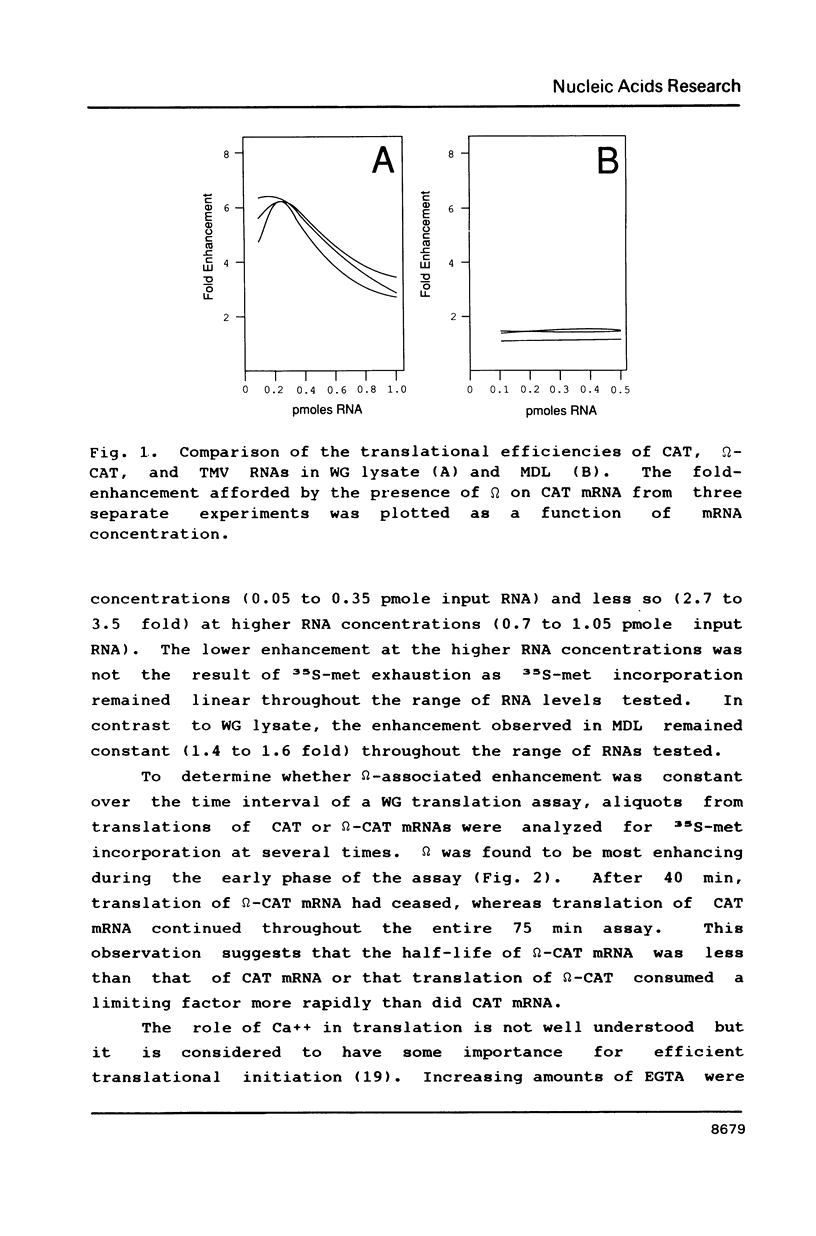
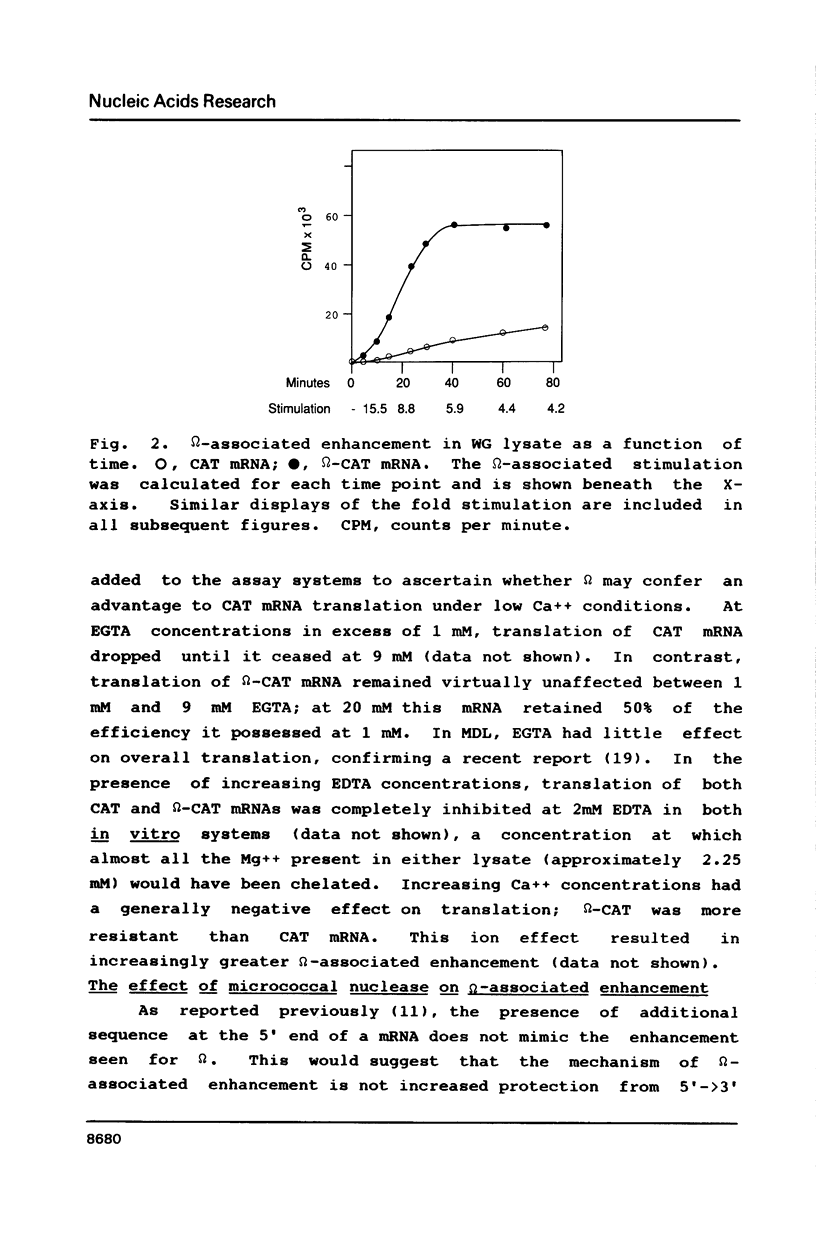
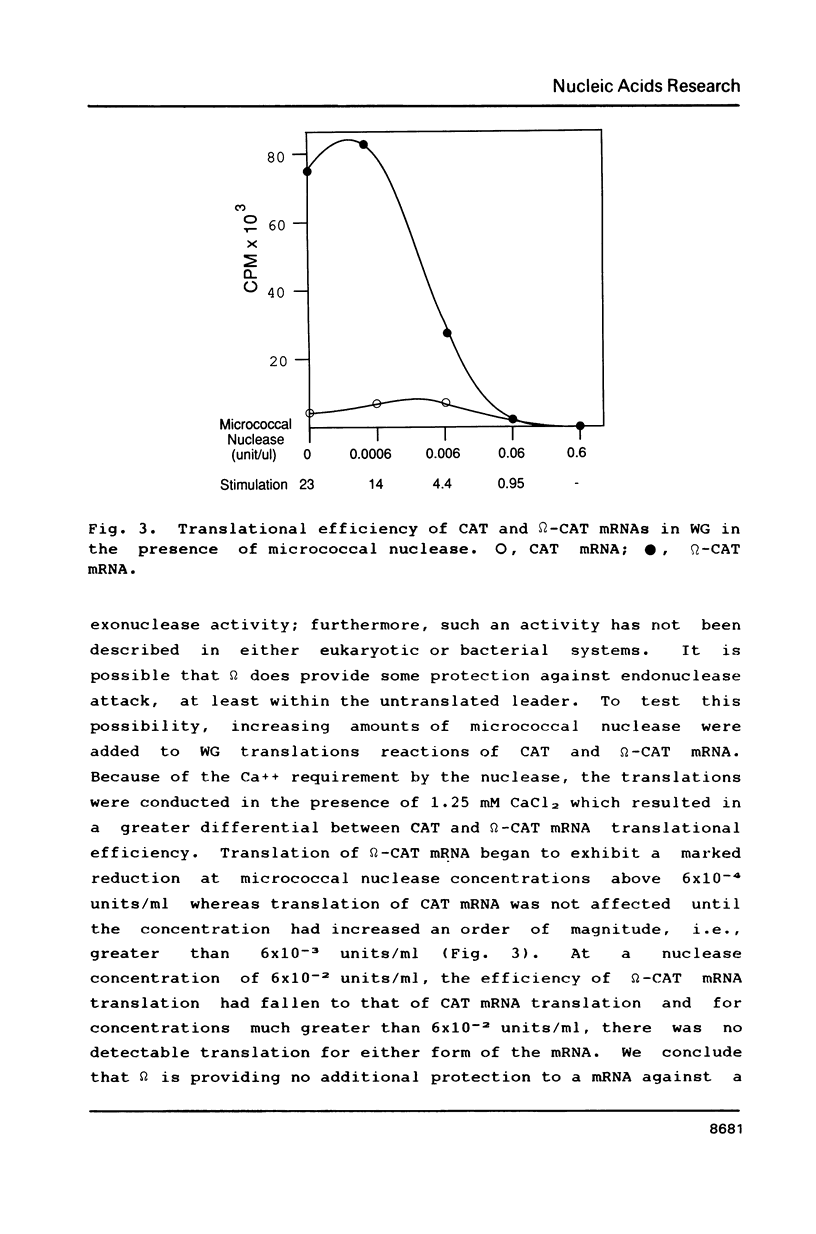
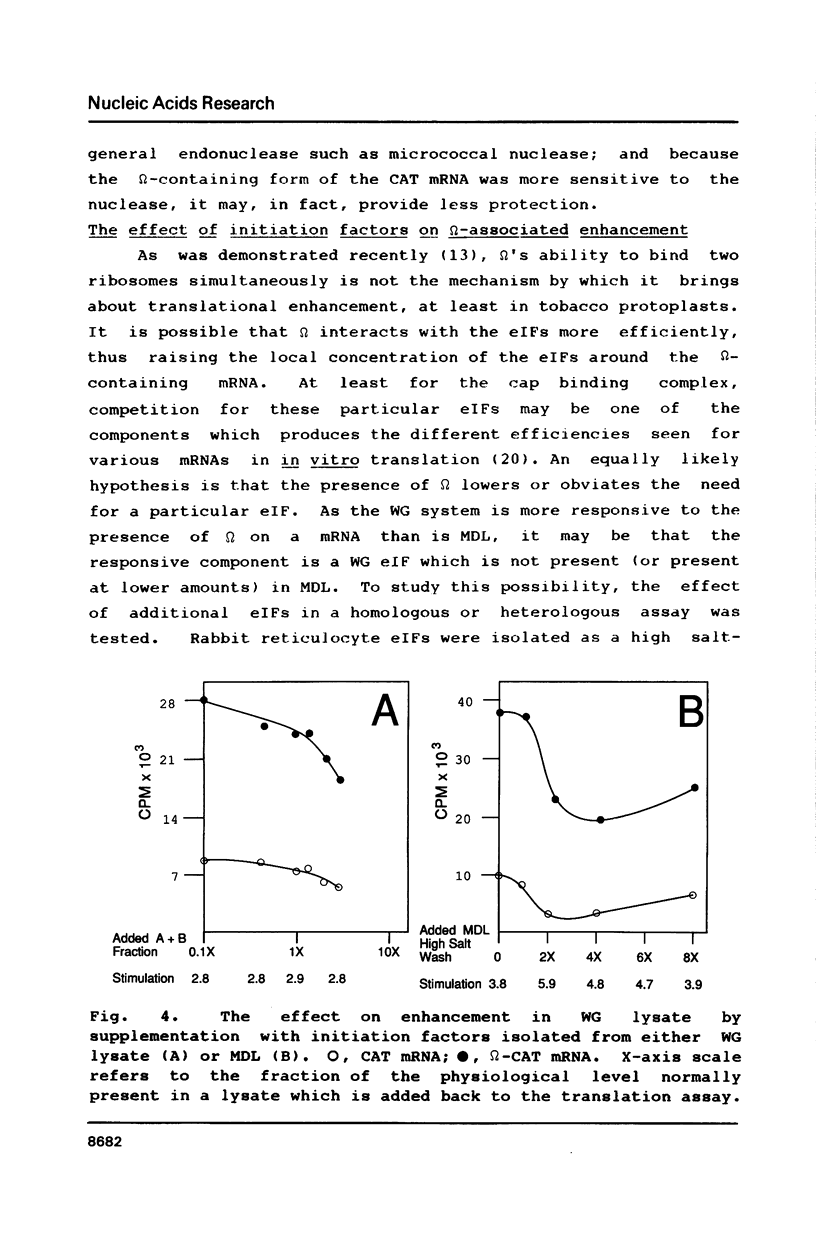
Abstract
The omega sequence at the 5'-terminus of tobacco mosaic virus (TMV) RNA acts as a translational enhancer. The differential in omega-associated translational enhancement between the in vitro translation system derived from wheat germ (WG) and that from rabbit reticulocytes (MDL) was exploited to identify that lysate component which was responsible for a lysate's characteristic response to omega. Using fractionated MDL and WG lysates, which were reconstituted in various combinations, the high salt-washed ribosomal fraction was determined to be the responsive element in a lysate. Analysis of omega's ability to enhance translation was greatest at low mRNA and high ribosomal concentrations and to occur in the early phase of an in vitro translation assay. Translation of omega-containing CAT mRNA was more sensitive to the presence of micrococcal nuclease than CAT mRNA without an omega. In substitution experiments, WG ribosomes functioned at much reduced efficiency in MDL as did MDL ribosomes in WG lysate. The initiation factor-containing fraction of one system could not, as a whole, functionally replace that of the other and actually acted to inhibit translation in the heterologous system.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J., Fromm M., Walbot V. Expression of mRNA electroporated into plant and animal cells. Nucleic Acids Res. 1987 Jul 24;15(14):5823–5831. doi: 10.1093/nar/15.14.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K. V., Cade C., Brostrom C. O., Galuska E. M., Brostrom M. A. Calcium-dependent regulation of protein synthesis at translational initiation in eukaryotic cells. J Biol Chem. 1987 Dec 5;262(34):16509–16514. [PubMed] [Google Scholar]
- Davies J. W., Aalbers A. M., Stuik E. J., Van Kammen A. Translation of cowpea mosaic virus RNA in a cell-free extract from wheat germ. FEBS Lett. 1977 May 15;77(2):265–269. doi: 10.1016/0014-5793(77)80248-2. [DOI] [PubMed] [Google Scholar]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. In vivo uncoating and efficient expression of foreign mRNAs packaged in TMV-like particles. Science. 1987 May 29;236(4805):1122–1124. doi: 10.1126/science.3472350. [DOI] [PubMed] [Google Scholar]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. Mutational analysis of the tobacco mosaic virus 5'-leader for altered ability to enhance translation. Nucleic Acids Res. 1988 Feb 11;16(3):883–893. doi: 10.1093/nar/16.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. The 5'-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 1987 Apr 24;15(8):3257–3273. doi: 10.1093/nar/15.8.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geballe A. P., Spaete R. R., Mocarski E. S. A cis-acting element within the 5' leader of a cytomegalovirus beta transcript determines kinetic class. Cell. 1986 Sep 12;46(6):865–872. doi: 10.1016/0092-8674(86)90068-1. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Jobling S. A., Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987 Feb 12;325(6105):622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Binding of ribosomes to linear and circular forms of the 5'-terminal leader fragment of tobacco-mosaic-virus RNA. Eur J Biochem. 1981 Feb;114(2):221–227. doi: 10.1111/j.1432-1033.1981.tb05139.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Inability of circular mRNA to attach to eukaryotic ribosomes. Nature. 1979 Jul 5;280(5717):82–85. doi: 10.1038/280082a0. [DOI] [PubMed] [Google Scholar]
- Lax S. R., Lauer S. J., Browning K. S., Ravel J. M. Purification and properties of protein synthesis initiation and elongation factors from wheat germ. Methods Enzymol. 1986;118:109–128. doi: 10.1016/0076-6879(86)18068-2. [DOI] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985 Oct;42(3):903–911. doi: 10.1016/0092-8674(85)90286-7. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ray B. K., Brendler T. G., Adya S., Daniels-McQueen S., Miller J. K., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc Natl Acad Sci U S A. 1983 Feb;80(3):663–667. doi: 10.1073/pnas.80.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walthall B. J., Spremulli L. L., Lax S. R., Ravel J. M. Isolation and purification of protein synthesis initiation factors from wheat germ. Methods Enzymol. 1979;60:193–204. doi: 10.1016/s0076-6879(79)60016-2. [DOI] [PubMed] [Google Scholar]
- Werner M., Feller A., Messenguy F., Piérard A. The leader peptide of yeast gene CPA1 is essential for the translational repression of its expression. Cell. 1987 Jun 19;49(6):805–813. doi: 10.1016/0092-8674(87)90618-0. [DOI] [PubMed] [Google Scholar]


