Introduction
Over the last two decades, numerous advancements in medical therapies have improved patient outcomes in heart failure (HF). However, a significant number of patients still progress to end-stage HF, where treatment options are largely limited to cardiac transplantation. As patient demands for transplant continue to exceed the supply of available organs, mechanical assist devices – specifically, the left ventricular assist device (LVAD) – were initially introduced as a bridge to cardiac transplantation.
LVADs have two important beneficial effects. First, LVADs are placed in parallel to the native left ventricle (LV), causing pressure and volume unloading of the LV. Second, LVADs restore cardiac output and subsequent perfusion to the organs. As a result of these two effects, it became evident that some patients had actual improvement in left ventricular function after LVAD placement. The term reverse remodeling was used to describe the improvement in myocardial function that was observed in patients with a seemingly end-stage disease. With reverse remodeling, a new hope for the treatment of heart failure was born – using LVADs as a bridge to recovery, however to date this promise has largely been unrealized. This is likely reflective of the fact that the sequela of mechanical ventricular unloading are quite complex and appear to involve the engagement of competing biologic pathways including regression of cardiomyocyte hypertrophy as well as progressive cell atrophy. Thus while the promise of ventricular recovery still persists, its actualization will await a more comprehensive dissection of these competing biologic processes. This review will discuss the beneficial clinical effects of LVAD support as well as review what is known about the cellular and molecular response to mechanical unloading and mechanisms of reverse remodeling. Key research findings have been summarized in Table 1.
Table 1.
Summary of Research of Left Ventricular Assist Device (LVAD) Support on Clinical Effects and the Cellular and Molecular Changes that may Contribute to Reverse Remodeling
| Summary of Findings | References | |
|---|---|---|
| Clinical Effects | ||
| Mortality | Majority survive to transplant | 1 |
| Improved survival compared to medical therapy in non-transplant candidates | 2 | |
| Functional Status | Lower NYHA heart failure class | 3 |
| Improved 6-Minute walk test | 1,3 | |
| Quality of Life | Minnesota Living with heart failure questionnaire score improved | 1,3 |
| Kansas City Cardiomyopathy questionnaire score improved | 1,3 | |
| SF-36 score improved | 2 | |
| Beck Depression Score with less depression | 2 | |
| Organ Perfusion | Serum creatine lower | 1 |
| Blood urea nitrogen lower | 1 | |
| Serum liver function tests improved | 1 | |
| Chamber Size | Left atrial size decreased | 7 |
| Left ventricular size decreased | 6,7 | |
| Left ventricular mass decreased | 6 | |
| Contractility | Ejection fraction higher | 5–7 |
| LVAD explantation in majority | 5 | |
| LVAD explantation in minority | 6 | |
| Hemodynamics | Cardiac output/cardiac index increase | 1,3 |
| Mean pulmonary pressure lower | 1 | |
| Pulmonary capillary wedge pressure lower | 1,3 | |
| Neurohormones | Epinephrine and norepinephrine decreased | 18 |
| Renin decreased | 18 | |
| Angiotensin II decreased | 18 | |
| Arginine vasopressin decreased | 18 | |
| Natriuretic Peptides | Atrial natriuretic peptide decreased | 19 |
| Brain natriuretic peptide decreased (serum and tissue levels) | 19 | |
| Cytokines | TNF-α decreased (serum and tissue levels) | 6,20 |
| Electrical | QRS duration decreased | 21 |
| QT interval increased | 21 | |
| QTc interval increased in initial period after LVAD | 21 | |
| QTc interval shortened with sustained LVAD | 21 | |
| Action potential duration shortened | 21,22 | |
| Cellular and Molecular Changes | ||
| Myocyte Morphology | Left ventricular myocyte hypertrophy regresses | 23–30 |
| Right ventricular myocyte size unchanged | 25 | |
| Left ventricular myocyte atrophy in animals | 35–37 | |
| Myocyte Histology | Overall histology only partially improved | 7,39 |
| Distortion of sarcomeric proteins only partially improved | 26 | |
| Cardiomyocyte DNA content decreased | 40 | |
| Polyploid myocytes decreased and diploid myocytes increased | 40 | |
| Contractility | Basal myocyte contractility increased | 41,42 |
| Improved contractile response to β-adrenergic stimuli | 41,43,44 | |
| Abnormal force-frequency relationship either reversed or attenuated | 25,27,41,45 | |
| β-Adrenergic System | β-receptor density increased | 43,44,46–49 |
| Normalization in location of β-receptors | 29,47 | |
| Adenyl cyclase activity higher | 49 | |
| Calcium Handling | Sarcolemmal Ca2+ entry faster | 22,45,50 |
| Sarcoplasmic reticulum Ca2+ content higher | 22,45,50 | |
| SERCA abundance may increase to nonfailing levels | 25,27,45,52 | |
| Na+/Ca2+ exchanger abundance decreases to nonfailing levels | 27 | |
| Phospholamban abundance unchanged (ratio of SERCA/phospholamban may improve) | 27,53 | |
| Sarcomere Proteins | Abundance of some sarcomeric proteins may increase | 57 |
| Post-translational modifications of sarcomeric proteins may change | 48 | |
| Extracellular Matrix and Cytoskeleton | Collagen content may increase or decrease | 6,59–61 |
| Myocardial stiffness may decrease with angiotensin converting enzyme inhibitors | 64 | |
| Dystrophin improved | 67,68 | |
| Metabolism | Improved mitochondrial function | 69,70 |
| Cellular Response to Stress | Metallothionein reduced | 24 |
| Heme oxygenase-1 decreased | 71 | |
| Apoptosis and cell breakdown reduced | 72,73 | |
| Gene expression | Gene expression patterns may differentiate pre from post LVAD state | 74 |
| Many genes altered in heart failure, very few have complete reversal with LVAD | 84 | |
| Micro RNAs | Many up or downregulated in heart failure with normalization after LVAD | 85,86 |
| May be more sensitive than gene expression for reverse remodeling | 86 | |
Clinical Effects of LVADs
Clinical Efficacy as Bridge to Transplantation and Destination Therapy
LVADs were first introduced to support and stabilize patients with end-stage HF until a donor heart was available for cardiac transplantation. A multicenter prospective study has shown that these devices restore cardiac output, improve hemodynamics, and are successful in bridging the majority of LVAD supported patients to cardiac transplantation.1 The landmark REMATCH study further expanded the use of LVADs to patients with end-stage HF who were not transplant candidates, termed destination therapy.2 As confirmed in these trials as well as other trials and case series, patients who received LVADs demonstrated improved hemodynamics, functional status, and quality of life.
Despite the significantly improved survival among LVAD patients in the REMATCH study compared to medical therapy,2 widespread use of LVADs for destination therapy was somewhat limited, even in transplant centers, as the two year survival was still quite low. This mortality was largely related to problems with bleeding, infection, and pump failure that were likely compounded by the first generation pulsatile flow LVADs. The second generation continuous axial flow LVADs are advantageous in that they are smaller and are less susceptible to mechanical wear-and-tear. In a randomized trial comparing these two types of LVADs, patients supported by the continuous flow devices had superior survival with less adverse events related to LVAD device failure compared with the pulsatile flow devices.3 As a result, the continuous flow LVAD is now the mechanical therapy of choice for both bridge to transplant and destination therapy.
Clinical Efficacy as Bridge to Recovery
Early in the use of LVADs as a bridge to transplant, isolated reports and case series noted some patients having sufficient recovery of native LV function to allow for LVAD explantation – leading to the idea of using LVADs as a bridge to recovery. The largest of these early reports was from Germany where investigators noted successful weaning and LVAD explantation in 32 of 131 patients with chronic idiopathic dilated cardiomyopathy (mean duration of HF prior to LVAD implantation 4.3 years).4 All of these patients received medical therapy with β-blockers, angiotensin converting enzyme inhibitors, and aldosterone antagonists while being supported with a LVAD for a mean duration of 4.6 months. The 5 year survival among patients who had LVAD explantation was 78.3% with a HF recurrence rate of 31.3% in the first 3 years after LVAD removal.4
Further enthusiasm for LVAD-induced reverse remodeling and use of LVADs as a bridge and facilitator for LV recovery was bolstered by a report from the United Kingdom of successful LVAD explantation in 11 of 15 patients with nonischemic cardiomyopathy.5 While receiving LVAD support for a mean duration of 320 days, patients in this series were initially treated with a combination of traditional HF medications including angiotensin enzyme inhibitors, angiotensin receptor blockers, β-blockers, and aldosterone antagonists. With echocardiographic evidence of regression of LV enlargement, the β2-agonist clenbuterol was added to the pharmacologic regimen to counter-act atrophy. The authors reported that of the 11 patients who underwent successful LVAD explantation, only 2 patients died in the 4 year follow-up period (one of arrhythmias 24 hours after explantation and one of lung carcinoma 27 months after explantation). Survival free of HF in this subpopulation was 100% at 1 year and 88.9% at 4 years.5
Unfortunately, these initial optimistic single-center reports have yet to be replicated in larger multicenter studies. In the Multi-Institutional LVAD Working Group Study from the United States, only 6 of 67 patients (9%) underwent successful LVAD explantation for recovery.6 Thus, investigations into the mechanical, biochemical, cellular, and molecular effects of LVADs have become increasingly important in advancing our ability to optimize this therapy.
Chamber Size
It is clear that mechanical unloading with a LVAD successfully unloads the LV and results in reduction in the LV dilation seen in end-stage HF. Imaging evidence of decreased LV size is evident immediately post-LVAD implantation on transesophageal echocardiography in the operating room7 and reductions in LV dilation and LV mass have been noted to persist for extended periods of time after LVAD placement6 as illustrated in Figure 1. In addition, LV pressure-volume recordings confirm lower LV volumes for equivalent pressure loads with LVAD support; however, these physiologic changes may not be evident until greater than 40 days after LVAD placement.8
Figure 1.
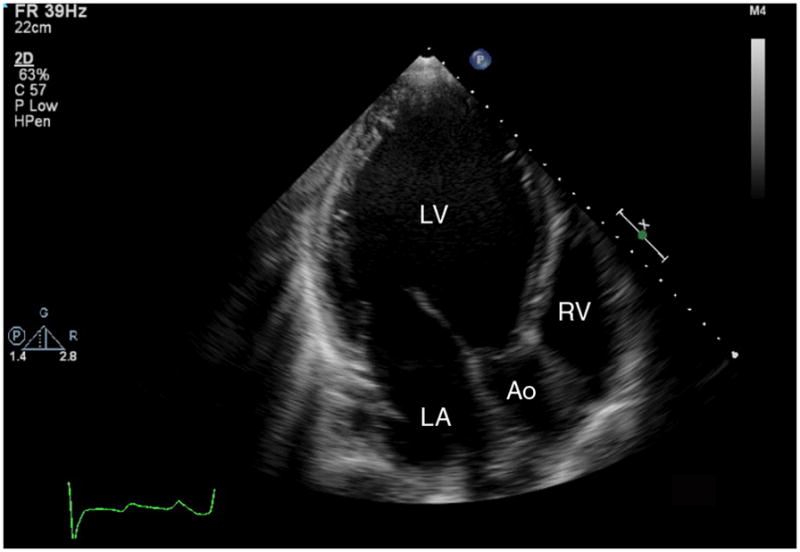
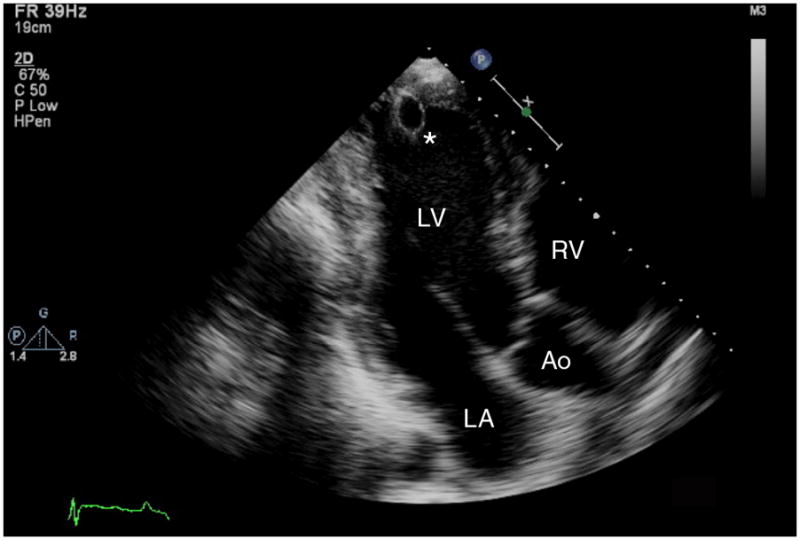
(a) Transthoracic apical echocardiographic image obtained in end-diastole from a patient with idiopathic dilated cardiomyopathy that demonstrates severe left ventricular dilation. (b) Follow up image obtained in end-diastole from the same patient after placement of a continuous axial flow left ventricular assist device (LVAD) demonstrates mechanical unloading with normalization of left ventricular dimensions. The LVAD inflow cannula (*) is visible in the left ventricular apex. LV=left ventricle, LA=left atrium, RV=right ventricle, Ao=aorta.
The degree of unloading may vary based on the type of LVAD used. Some have suggested that using pulsatile flow support may unload the heart to a greater degree than the newer continuous flow LVADs. In one study, echocardiographic LV dimensions as well as LV mass were more improved in patients supported with pulsatile LVADs compared to continuous flow LVADs.9 However, their larger size, poorer durability, and greater complication rate have limited the use of pulsatile flow LVADs. Whether there is an optimal ventricular geometry that might result in sustained ventricular recovery is an open question.
Furthermore, most studies examining the effects of LVAD on chamber size have been done with full unloading of the LV. Some studies have examined the role of echocardiographic assessment of LV size after temporarily decreasing or turning off LVAD flow support as a predictor of recovery and successful LVAD explantation – where persistently small LV dimensions or a limited increase in LV dimensions both correlate with better outcomes.6,10–13 Similarly, small LV dimensions with administration of dobutamine or during exercise may also be predictors for recovery during LVAD support.10,13
Contractility and Hemodynamics
In addition to improving LV dimensions, LVAD support can greatly improve blood flow, thus increasing cardiac output and lowering filling pressure.1,3 Moreover, patients also demonstrate recovery of LV contractile function as evidenced by improved ejection fraction (EF). In a large recent cohort study, nearly a third of patients showed an EF of greater than 40% with partial LVAD support.6 Interestingly, though the EF appeared to improve by 30 days, further support up to 120 days resulted in declining EF which approached pre-LVAD values.6 It has been postulated that LV atrophy may occur with prolonged LVAD support. Animal studies in which normal hearts were subjected to sustained mechanical unloading have shown cardiac atrophy, a decline in contractile function,14 an increase in activation of the fetal gene program,15 and activation of protein degradation pathways.16 However, there may be differential effects on unloading normal versus failing hearts. One study reported improvements in calcium handling properties and myocardial contractility from unloaded failing hearts only – unloading of normal hearts did not demonstrate such effects.17 Understanding clinical predictors of improved EF after LVAD placement and how to clinically balance the presumed beneficial impact of reverse remodeling with the potentially deleterious impact of muscle atrophy is an important area of clinical investigation.
In addition, it is unclear how to predict whether the improvements in contractility and hemodynamics during LVAD support will persist after LVAD explantation. Higher EF values as assessed by echocardiography during full and partial LVAD support may be predictive of LV recovery and successful LVAD explant.6,11–13 Furthermore, inotropic reserve as assessed by echocardiographic EF and dP/dt during administration of dobutamine may also have a role.6,10 The role of exercise echocardiographic and hemodynamic testing with partial and full LVAD support has also been studied in small numbers of patients – with lower filling pressure and greater exercise tolerance being more predictive than oxygen consumption or cardiac output.11,13 The optimal modalities and protocols that should be used to predict LV recovery during LVAD support and successful LVAD explant remains largely unknown and is an area of active clinical investigation.
Neurohormone, Natriuretic Peptide, and Cytokine Levels
The initial compensatory neurohormonal activation of the failing heart chronically creates a vicious cycle that can exacerbate the failing phenotype. Many current evidence-based HF therapies such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers, aldosterone antagonists, and β-blockers are meant to counteract this chronically maladaptive neurohormonal activation. There is clear evidence that by normalizing hemodynamics, LVADs result in great improvements in the neurohormonal milieu that is deranged in HF. Plasma epinephrine, norepinephrine, renin, angiotensin II, and arginine vasopressin levels all significantly decrease after LVAD implantation. 18 Circulating as well as LV tissue levels of natriuretic peptides decrease after LVAD placement.19 Systemic cytokines like TNF-α also decrease in the periphery as well as the LV tissue.6,20 These improvements in systemic circulating and paracrine factors suggest that the failing heart may have a more favorable environment to recover in after LVAD placement.
Electrical Remodeling
In addition to the structural and contractile effects of LVAD support, LVAD placement appears to have an effect on the heart’s electrical system. In ECG analysis of patients before and after LVAD placement, the QRS duration decreased, the QT interval increased, and the QTc increased.21 With sustained unloading however, the QTc decreased. This finding was confirmed in isolated myocytes as demonstrated by a shortened duration of the action potential,21,22 suggesting an electrical contribution to the improved contractile performance seen after LVAD therapy.
Cellular and Molecular Changes
Myocyte Morphology
Pathologic hypertrophy is a hallmark feature of the failing myocyte. Numerous studies have suggested that LVAD support helps myocytes regress toward the dimensions of nonfailing myocytes.23–30 While there does appear to be improvement in length, width, and thickness (thus decreasing myocyte volume and cross-sectional area), cell length appears to decrease the most with LVAD support.23 The relation of myocyte hypertrophy with LV recovery during LVAD support remains unclear. One study examining myocytes from LVAD supported hearts with improved EF compared to those without improved EF suggested that patients with smaller myocytes and less fibrosis at baseline (the time of LVAD implantation) would be more likely to recover contractile function as assessed by EF with LVAD support.28 Another study examining myocytes from LVAD patients with clinical recovery compared with LVAD patients without clinical recovery found that myocytes from both groups showed similar reductions in cell size, suggesting that reduction in myocyte hypertrophy is not associated with LV recovery.22
The stimulus for the regression of hypertrophy appears to be related to mechanical unloading rather than changes in circulating systemic factors. It has been suggested that hypertrophy regression is more prominent with longer duration of support, and that the greatest regression of hypertrophy occurs in the sub-endocardium.24 This theory is further supported by the fact that regression of hypertrophy appears to be more prominent in myocytes from the LV as opposed to the right ventricle (RV).25 The biochemical mechanisms of myocyte hypertrophy regression with LVAD therapy are not well understood. Alterations in a number of signaling pathways have been examined. Specifically, a number of studies have noted that regulation of mitogen-activated protein kinases may lead to reduced myocyte hypertrophy and less myocyte apoptosis.31,32 In addition, paradoxical enhancement of transcription regulation of ribosomal biogenesis33 and activation of insulin-like growth factor-I34 – both of which may serve to limit and/or counterbalance the parallel engagement of protein degradation pathways – have been described both in human and animal models. Interestingly, the elevated insulin-like growth factor-I expression found in patients with clinical recovery during LVAD placement correlated with stem cell recruitment factor SDF-1 expression.34 Insulin-like growth-factor-I and SDF-1 have been shown to be involved in stem cell recruitment and in limiting apoptosis, both of which may have beneficial roles in the myocardial response to damage and the process of reverse remodeling with mechanical unloading.
While there is reduction in myocyte hypertrophy with LVAD support, it is not clear to what extent atrophy occurs in human myocyte. Several animal models of unloading do suggest that myocyte atrophy can occur with prolonged mechanical unloading.35–38 One these studies utilized a rat heterotopic transplant model and found that LV unloading initially led to normalization of myocyte size with improvement in papillary muscle contractile function, but prolonged LV unloading resulted in myocyte atrophy, fibrosis, and worsening of papillary muscle function.38 If atrophy does in fact occur in human myocytes supported with LVADs, consideration may need to be given to partial LVAD unloading or pharmacologic therapy with β2-adrenergic agonists such as clenbuterol to avoid atrophy and to enhance cellular mechanisms that might better allow for LV recovery.
Myocyte Histology
Though the literature suggests there is a clear reduction in myocyte size with LVAD support, whether there are improvements in other histological features is unclear. An autopsy study of myocardial tissue from patients before and after LVAD found that LVAD support reduced the amount of coagulative necrosis, myocytolysis, and myocyte waviness, but did not change fibrosis, eosinophilia, or contraction bands.39 Another study showed reductions in wavy fibers and contraction band necrosis, but myocardial fibrosis increased.7
Further evidence that myocyte hypertrophy reduction may not correlate to biochemical improvement came from histological staining and immunohistochemistry from a number of contractile proteins within the myocyte. Despite improvement in myocyte size, the widespread evidence of sarcomeric disarray including distortion of actin, tropomyosin, troponin C, troponin T, and titan before LVAD only partially recovered after LVAD.26 One protein, myosin, actually had a worse histological appearance after LVAD.26
Cellular level changes appear to be accompanied by changes within the myocyte nucleus as well including possible reactivation of the cell cycle. The median cardiomyocyte DNA content and number of polyploid myocytes declined after LVAD, while diploid cardiomyocytes and binucleated cardiomyocytes increased after LVAD.40 This evidence suggests that an increase in diploid myocytes either through cell cycle progression with completion of mitosis or by an increase in differentiation of pluripotent stem cells may allow for cardiac regeneration.40
Contractility
In vitro assessments of contractility have suggested that there is an improvement in myocyte force with LVAD support. In a study of myocytes obtained from patients with HF at transplant compared to patients with HF supported with a LVAD at the time of transplant, the myocytes from LVAD supported patients had greater magnitude of shortening, faster time to peak contraction, and faster time to shortening.41 In paired samples from the same patient before and after LVAD, in vitro motility and force assays demonstrated a near normalization of maximal calcium activated force with LVAD42 and our lab has demonstrated similar improvements in isometric force generation in skinned cell fragments after LVAD support.
There also appears to be improvement in the myocardial contractile response to β-adrenergic stimulation with LVAD support in patients with HF. Force measured from LV trabeculae from HF patients showed a blunted response to isoproterenol. By contrast, the response to isoproterenol in LV trabeculae from HF patients supported with a LVAD was similar to that of nonfailing myocardial tissue.43,44 Similar improvements were noted in myocyte sample preparations from HF patients with and without LVAD support at the time of transplant.41
Finally, the failing heart is characterized by a negative force frequency relationship, and it is unclear whether LVAD support can reverse this abnormal finding. Two in vitro studies using LV trabeculae from nonfailing hearts, failing hearts, and failing hearts supported with a LVAD suggest that there is reversal of the force frequency relationship.25,45 Two other similar studies using trabeculae and myocyte preparations found an attenuated decline in the force-frequency relationship in samples from failing hearts supported with a LVAD, but not restoration to the normal positive force-frequency relationship.27,41
β-Adrenergic System
β-adrenergic receptors are downregulated in patients with chronic heart failure. The improvement in contractile response to β-adrenergic stimulation likely involves reversal of this β-receptor downregulation and restoration of normal receptor density. The downregulation of β-receptors in HF requires targeting of phosphoinositide 3-kinase (PI3K)-gamma and redistribution of β-receptors into endosomal compartments. One study of myocardial tissue from patients prior to LVAD implantation compared to tissue from nonfailing hearts found that increased β-receptor associated PI3K activity was associated with downregulation of β-receptors from the plasma membrane and sequestration into endosomes compared with tissue obtained following LVAD support.46 In short, LVAD support reversed PI3Kgamma activation, normalized levels of β-receptors in the plasma membranes, and depleted β-receptors from endosomes. Multiple other studies have also noted increased β-adrenergic receptor density after LVAD placement.43,44,47–49 In addition to greater β-receptor density, two studies have suggested that β-receptors relocalize from an abnormal punctate/clumped pattern to the normal homogenous pattern after LVAD support.29,47
Improvements in the adrenergic response after LVAD may also be related to changes in adenyl cyclase. Basal adenyl cyclase activity is lower in myocardium from failing hearts compared to nonfailing hearts and isoproterenol stimulated adenyl cyclase activity is blunted in failing hearts. LVAD support has been shown to increase basal and isoproterenol stimulated adenyl cyclase activity to levels closer to that seen in nonfailing hearts.49
Calcium Handling
Calcium is required for muscle contraction and alterations in calcium handling of the cell may contribute to the decreased contractility and negative force-frequency relationship seen in heart failure. LVAD support does seem to be associated with changes in the calcium handling properties of the myocyte. Two studies have assessed calcium transients of myocytes from patients with HF and from those with HF supported with a LVAD. The LVAD supported myocytes had calcium transients more similar to nonfailing myocytes, with a greater peak systolic ratio and a faster rate of decay than myocytes from failing hearts.27,41 Such changes in calcium transients are associated with faster calcium entry via sarcolemmal calcium channels, higher sarcoplasmic reticulum calcium content, and shorter action potential durations.22,45,50
Changes in calcium cycling related to changes in the balance of the calcium regulatory proteins, sarcoplasmic reticulum calcium adenosine triphosphatase (SERCA), the sarcolemmal Na/Ca exchanger, and phospholamban, have also been seen. LVAD support appears to increase Na/Ca exchanger and SERCA gene expression,8,51 but the effect of LVAD support on SERCA protein abundance is unclear. Some studies suggest no change in SERCA content with LVAD support and others show an increase in SERCA content closer to nonfailing levels.25,27,45,52 Na/Ca exchanger abundance and activity decreased with LVAD support to levels closer to nonfailing hearts.27 There appears to be no change in phospholamban abundance, but the ratio of SERCA to phospholamban, an indicator of calcium uptake by the sarcoplasmic reticulum and of diastolic relaxation, appears to normalize early after LVAD.27,53 Further alterations in calcium cycling may be the result of changes in L-type calcium channel function and ryanodine receptor function, as LVADs have been shown to affect the function of these channels via post-translational modifications.44,54–56
While the impact of LVAD support on these calcium handling proteins seems evident, the effects of LVADs on calcium handling are incompletely understood. Likewise, the stimulus that drives these changes in calcium regulatory proteins is unclear. Systemic neurohormones seem unlikely to significantly contribute to such changes as variation in SERCA content appears to be localized to the LV and not the RV.25 These changes may also vary with the duration of LVAD support, as one study described normalization of SERCA and SERCA/phospholamban ratios early after LVAD implantation with reversion back to failing levels with prolonged LVAD support53 – again raising the question of optimal timing.
Sarcomeric Proteins
The sarcomeric contractile proteins of actin, myosin, tropomyosin, and the troponin complex are responsible for cross-bridge cycling of actin thin filaments and myosin thick filaments – ultimately generating the force in striated muscle. As noted above, immunohistochemical analysis confirms wide spread distortion of several of these sarcomeric proteins in failing hearts, and these are only partially improved with LVAD placement.26 Furthermore, alterations in the abundance of these sarcomeric proteins with LVAD support may contribute to the reverse remodeling process. In patients with evidence of LV recovery with LVAD placement, there were statistically significant increases in the sarcomeric proteins myosin heavy chain, troponin C, troponin T, and actin,57 although whether this translates into an increase in functional contractile elements is not clear.
In addition, there may be changes in some of the post-translational modifications of sarcomeric proteins after LVAD placement. For example, protein kinase A dependent phosphorylation of troponin I in LVAD supported hearts was lower compared to measurements before LVAD implantation.48 Animal models of unloading with heterotropic heart transplantation suggest that there are alterations in the protein kinase-phosphatase balance. These alterations may affect the phosphorylation state of sarcomeric proteins – which play a key role in the regulation of contractility and relaxation of the heart.58 There is still a gap in the knowledge of the effects of these proteins after LVAD support and this remains an area for potential research and therapeutics.
Extracellular Matrix and Cytoskeleton
Numerous studies have assessed changes in the extracellular matrix with LVAD support. Results have been inconsistent with regard to LVAD effects on the degree of collagen formation and fibrosis. Some studies have suggested decreased collagen deposition and collagen content after LVAD6,59,60 whereas others describe increased collagen cross-linking with subsequent increased myocardial stiffness.61 There is some indication that the balance of tissue inhibitors of metalloproteinases (TIMPs) and matrix metalloproteinases (MMPs) does change with LVAD support, thus potentially altering the balance of collagen breakdown and production.61–63
However, such changes in collagen do not appear to affect the RV—suggesting the driving force is mechanical unloading as opposed to changes in systemic factors.61 Reverse remodeling cardiac medications may have a role in combating the increased myocardial stiffness observed related to increased collagen cross-linking. In patients who received an angiotensin converting enzyme inhibitor in addition to a LVAD, myocardial collagen content and myocardial stiffness decreased compared to patients with LVADs alone.64 There may be a time course of support causing changes as well, as collagen turnover was noted to increase in the first 200 days of LVAD support, followed by decreased turnover and restoration of the collagen network.65 These data suggest that there is value in designing adjunctive pharmacologic therapies in patients undergoing LVAD support.
Changes in the cytoskeleton have been described with LVAD support,57,66 but the role of the cytoskeleton in reverse remodeling is unclear. One cytoskeletal protein, dystrophin, has been shown to be preferentially disrupted in failing hearts. This finding was reversed in hearts from LVAD support.67,68 Further work in this area is needed.
Metabolism
Mitochondrial structure and function and myocardial oxygen consumption and metabolism may be abnormal in HF, yet very little is known about the effects of LVAD support on these parameters. One report found that mitochondria isolated from hearts of LVAD supported patients had improved respiratory capacity suggesting improvement in mitochondrial function.69 Some of these changes may be related to potentiation of endogenous nitric oxide mediated regulation of mitochondrial respiration with chronic LVAD support.70 Much remains for future study.
Cellular Response to Stress
Some data now suggest that LVAD support may alter the myocardial susceptibility to oxidative stress. For example, there is regression of the stress inducible protein, metallothionein, with LVAD support of more than 88 days, with the greatest reductions in sub-endocardium.24 Another example is heme oxygenase-1, a stress protein induced by hypoxia. LVAD reduced the heme oxygenase-1 content in the failing heart, supporting the concept that normoxia by mechanical unloading may contribute to reverse remodeling.71 Again improvement with LVAD was greater in the more vulnerable sub-endocardium compared to the epicardium.71
Two studies suggest there may be less cell breakdown and apoptosis with LVAD placement. Markers for autophagy, the molecular process that breaks down damaged cellular organelles and yields amino acids for de novo protein synthesis or energy provision, appear to decrease after LVAD.72 The anti-apoptotic protein, Bcl-2, and the repair/proliferation marker, proliferating cell nuclear antigen, were over-expressed before LVAD and tended to normalize with LVAD – suggesting that these indicators of cellular stress and DNA degradation reversed with unloading.73
Gene Expression
Microarray technology allows for rapid analysis of patterns of gene expression before and after LVAD implantation. The first study in this area, based on data from six patients, suggested that gene expression patterns could be used to differentiate patients before and after LVAD placement.74 Subsequent studies have suggested that up or downregulation of a variety of genes may be involved in the pathogenesis of heart failure and the reverse remodeling process, but as with most microarray studies, the volume of data available has not yet provided mechanistic clarity.34,75–83
A recent report of a large gene microarray from before and after LVAD samples reported 3088 transcripts significantly altered in HF.84 Only 238 demonstrated a consistent response to LVAD support. Of this small fraction, 75% exhibited persistence or exacerbation of HF abnormalities, 11% had partial recovery, 5% showed normalization, and 2% were over-corrected.84 This report suggests that the reverse remodeling process may occur without normalization of abnormal gene expression and that other mechanisms may be more important in regulating myocardial structure and function. Indeed, if the transcriptional stimulus to abnormal gene expression persists despite mechanical support, it is not surprising that sustained mechanical recovery is illusive.
MicroRNAs
MicroRNAs are small noncoding RNAs that can regulate cellular function by binding to multiple mRNAs and stimulating mRNA degradation or inhibiting protein translation – thus inducing a pattern of mRNA expression. Many microRNAs can be up or downregulated in response to cellular stimuli, and they can subsequently modify cellular functions like proliferation, differentiation, and apoptosis. Two studies to date have addressed changes in microRNAs with LVAD support.85,86 In the most comprehensive of these, investigators performed parallel microarray profiling of microRNAs and mRNAs from myocardial tissue from nonfailing hearts, failing hearts, and failing hearts supported with a LVAD. They found that 28 microRNAs more than doubled in failing hearts, and there was near complete normalization of the failing microRNA signature in the LVAD supported hearts. By contrast, 444 mRNAs were altered by 1.3 fold in failing hearts, and only 29 of these normalized by 25% in the LVAD supported hearts.86 This suggests that microRNAs may be more sensitive than mRNAs to functional changes related to end-stage HF or those pressures on mRNA expression include factors other than microRNA regulation.
Conclusions
Though heart failure has been conceptualized as an end-stage disease, many physiologic parameters can improve and even normalize with LVAD therapy. Such changes can be observed clinically, but also extend to the most basic aspects of genetic regulation and involve a complex, interconnected cascade of changes. LVAD implantation allows for both mechanical unloading as well as positively impacting systemic and biochemical responses that might ultimately recover contractile performance. However, many questions remain and it is still a mystery why some patients recover and others do not. The current literature suggests that there are a number of biologically relevant parameters beyond mechanical unloading that might well influence functional recovery. Going forward, studies are needed to best understand optimal duration of LVAD therapy, what adjunctive mechanical and pharmacologic treatments are most likely to foster positive outcomes, and how to successfully wean and explant the device. Ultimately, understanding which mechanisms are the most essential for sustained reverse remodeling of the left ventricle is paramount in fostering improved recovery.
Acknowledgments
Funding/Support: Dr. Ambardekar was supported by a 2009 Research Fellowship Award from the Heart Failure Society of America (St. Paul, MN). Additional work on this review was supported by grants from the National Institutes of Health (HL077195 and HL101435) and the Temple Hoyne Buell endowment.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 4.Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB, Hetzer R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist device. Circulation. 2005;112(suppl I):I37–I45. doi: 10.1161/CIRCULATIONAHA.104.525352. [DOI] [PubMed] [Google Scholar]
- 5.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 6.Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, Miller LW, Margulies K, McRee S, Frazier OH, Torre-Amione G. Cardiac improvement during mechanical circulatory support: A prospective multicenter study of the LVAD working group. Circulation. 2007;115:2497–2505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy PM, Nakatani S, Vargo R, Kottke-Marchant K, Harasaki H, James KB, Savage RM, Thomas JD. Structural and left ventricular histologic changes after implantable LVAD insertion. Ann Thorac Surg. 1995;59:609–613. doi: 10.1016/0003-4975(94)00953-8. [DOI] [PubMed] [Google Scholar]
- 8.Madigan JD, Barbone A, Choudhri AF, Morales DLS, Cai B, Oz MC, Burkhoff D. Time course of reverse remodeling of the left ventricle during support with a left ventricular assist device. J Thorac Cardiovasc Surg. 2001;121:902–908. doi: 10.1067/mtc.2001.112632. [DOI] [PubMed] [Google Scholar]
- 9.Thohan V, Stetson SJ, Nagueh SF, Rivas-Gotz C, Koerner MM, Lafuente JA, Loebe M, Noon GP, Torre-Amione G. Cellular and hemodynamics responses of failing myocardium to continuous flow mechanical circulatory support using the DeBakey-Noon left ventricular assist device: A comparative analysis with pulsatile-type devices. J Heart Lung Transplant. 2005;24:566–575. doi: 10.1016/j.healun.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Khan T, Delgado RM, Radovancevic B, Torre-Amione G, Abrams J, Miller K, Myers T, Okerberg K, Stetson SJ, Gregoric I, Hernandez A, Frazier OH. Dobutamine stress echocardiography predicts myocardial improvement in patients supported by left ventricular assist devices (LVADs): Hemodynamic and histologic evidence of improvement before LVAD explantation. J Heart Lung Transplant. 2003;22:137–146. doi: 10.1016/s1053-2498(02)00485-0. [DOI] [PubMed] [Google Scholar]
- 11.George RS, Yacoub MH, Tasca G, Webb C, Bowles CT, Tansley P, Hardy JP, Dreyfus G, Khaghani A, Birks EJ. Hemodynamic and echocardiographic responses to acute interruption of left ventricular assist device support: Relevance to assessment of myocardial recovery. J Heart Lung Transplant. 2007;26:967–973. doi: 10.1016/j.healun.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Dandel M, Weng Y, Siniawski H, Potapov E, Drews T, Lehmkuhl HB, Knosalla C, Hetzer R. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118(suppl 1):S94–S105. doi: 10.1161/CIRCULATIONAHA.107.755983. [DOI] [PubMed] [Google Scholar]
- 13.Formica P, Murthy S, Edwards P, Goldstein D, Maybaum S. A structured 3-step approach to evaluate cardiac recovery with continuous flow circulatory support. J Heart Lung Transplant. 2010;29:1440–1442. doi: 10.1016/j.healun.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Kent RL, Uboh CE, Thompson EW, Gordon SS, Marino TA, Hoober JK, Cooper G. Biochemical and structural correlates in unloaded and reloaded cat myocardium. J Mol Cell Cardiol. 1985;17:153–165. doi: 10.1016/s0022-2828(85)80018-3. [DOI] [PubMed] [Google Scholar]
- 15.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 16.Razeghi P, Volpini KC, Wang M, Youker KA, Stepkowski S, Taegtmeyer H. Mechanical unloading of the heart activates the calpain system. J Mol Cell Cardiol. 2007;42:449–452. doi: 10.1016/j.yjmcc.2006.08.114. [DOI] [PubMed] [Google Scholar]
- 17.Takaseya T, Ishimatsu M, Tayama E, Nishi A, Akasu T, Aoyagi S. Mechanical unloading improves intracellular Ca2+ regulation in rats with doxorubicin-induced cardiomyopathy. J Am Coll Cardiol. 2004;44:2239–46. doi: 10.1016/j.jacc.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 18.James KB, McCarthy PM, Thomas JD, Vargo R, Hobbs RE, Sapp S, Bravo E. Effect of implantable left ventricular assist device on neuroendocrine activation in heart failure. Circulation. 1995;92(suppl II):II191–II195. doi: 10.1161/01.cir.92.9.191. [DOI] [PubMed] [Google Scholar]
- 19.Bruggink AH, de Jonge N, van Oosterhout MF, VanWichen DF, de Koning E, Lahpor JR, Kemperman H, Gmeligh-Meyling FH, de Weger RA. Brain natriuretic peptide is produced both by cardiomyocytes and cells infiltrating the heart in patients with severe heart failure supported by a left ventricular assist device. J Heart Lung Transplant. 2006;25:174–180. doi: 10.1016/j.healun.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Torre-Amione G, Stetson SJ, Youker KA, Durand JB, Radovancevic B, Delgado RM, Frazier OH, Entman ML, Noon GP. Decreased expression of tumor necrosis factor-α in failing human myocardium after mechanical circulatory support: A potential mechanism for cardiac recovery. Circulation. 1999;100:1189–1193. doi: 10.1161/01.cir.100.11.1189. [DOI] [PubMed] [Google Scholar]
- 21.Harding JD, Piacentino V, Gaughan JP, Houser SR, Margulies KB. Electrophysiological alterations after mechanical circulatory support in patients with advanced cardiac failure. Circulation. 2001;104:1241–1247. doi: 10.1161/hc3601.095718. [DOI] [PubMed] [Google Scholar]
- 22.Terracciano CMN, Hardy J, Birks EJ, Khaghani A, Banner NR, Yacoub MH. Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation. 2004;109:2263–2265. doi: 10.1161/01.CIR.0000129233.51320.92. [DOI] [PubMed] [Google Scholar]
- 23.Zafeiridis A, Jeevanandam V, Houser SR, Margulies KB. Regression of cellular hypertrophy after left ventricular assist device support. Circulation. 1998;98:656–662. doi: 10.1161/01.cir.98.7.656. [DOI] [PubMed] [Google Scholar]
- 24.Baba HA, Grabellus F, August C, Plenz G, Takeda A, Tijan TD, Schmid C, Deng MC. Reversal of metallothionein expression is different throughout the human myocardium after prolonged left-ventricular mechanical support. J Heart Lung Transplant. 2000;19:668–674. doi: 10.1016/s1053-2498(00)00074-7. [DOI] [PubMed] [Google Scholar]
- 25.Barbone A, Holmes JW, Heerdt PM, The AH, Naka Y, Joshi N, Daines M, Marks AR, Oz MC, Burkhoff D. Comparison of right and left ventricular responses to left ventricular assist device support in patients with severe heart failure: A primary role mechanical unloading underlying reverse remodeling. Circulation. 2001;104:670–675. doi: 10.1161/hc3101.093903. [DOI] [PubMed] [Google Scholar]
- 26.de Jonge N, van Wichen DF, Schipper ME, Lahpor JR, Gmelig-Meyling FH, Robles de Medina EO, de Weger RA. Left ventricular assist device in end-stage heart failure: Persistence of structural myocyte damage after unloading: An immunohistochemical analysis of the contractile myofilaments. J Am Coll Cardiol. 2002;39:963–969. doi: 10.1016/s0735-1097(02)01713-8. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary KW, Rossman EI, Piacentino V, Kenessey A, Weber C, Gaughan JP, Ojamaa K, Klein I, Bers DM, Houser SR, Margulies KB. Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J Am Coll Cardiol. 2004;44:837–845. doi: 10.1016/j.jacc.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 28.Bruckner BA, Razeghi P, Stetson S, Thompson L, Lafuente J, Entman M, Loebe M, Noon G, Taegtmeyer H, Frazier OH, Youker K. Degree of cardiac fibrosis and hypertrophy at time of implantation predicts myocardial improvement during left ventricular assist device support. J Heart Lung Transplant. 2004;23:36–42. doi: 10.1016/s1053-2498(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 29.Schnee PM, Shah N, Bergheim M, Poindexter BJ, Buja LM, Gemmato C, Radovancevic B, Letsou GV, Frazier OH, Bick RJ. Location and density of α- and β-adrenoreceptor sub-types in myocardium after mechanical left ventricular unloading. J Heart Lung Transplant. 2008;27:710–717. doi: 10.1016/j.healun.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Drakos SG, Kfoury AG, Hammond EH, Reid BB, Revelo MP, Rasmusson BY, Whitehead KJ, Salama ME, Selzman CH, Stehlik J, Clayson SE, Bristow MR, Renlund DG, Li DY. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol. 2010;56:382–391. doi: 10.1016/j.jacc.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flesch M, Margulies KB, Mochmann HC, Engel D, Sivasubramanian N, Mann DL. Differential regulation of mitogen-activated protein kinases in the failing human heart in response to mechanical unloading. Circulation. 2001;104:2273–2276. doi: 10.1161/hc4401.099449. [DOI] [PubMed] [Google Scholar]
- 32.Baba HA, Stypmann J, Grabellus F, Kirchhof P, Sokoll A, Schafers M, Takeda A, Wilhelm MJ, Scheld HH, Takeda N, Breithardt G, Levkau B. Dynamic regulation of MEK/Erks and Akt/GSK-3β in human end-stage heart failure after left ventricular mechanical support: Myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovascular Research. 2003;59:390–399. doi: 10.1016/s0008-6363(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 33.Razeghi P, Buksinska-Lisik M, Palanichamy N, Stepkowski S, Frazier OH, Taegtmeyer H. Transcriptional regulators of ribosomal biogenesis are increased in the unloaded heart. FASEB J. 2006;20:1090–1096. doi: 10.1096/fj.06-5718com. [DOI] [PubMed] [Google Scholar]
- 34.Barton PJR, Felkin LE, Birks EJ, Cullen ME, Banner NR, Grindle S, Hall JL, Miller LW, Yacoub MH. Myocardial insulin-like growth factor-I gene expression during recovery from heart failure after combined left ventricular assist device and clenbuterol therapy. Circulation. 2005;112(suppl I):I46–I50. doi: 10.1161/01.CIRCULATIONAHA.105.525873. [DOI] [PubMed] [Google Scholar]
- 35.Kinoshita M, Takano H, Takaichi S, Taenaka Y, Nakatani T. Influence of prolonged ventricular assistance on myocardial histopathology in intact heart. Ann Thorac Surg. 1996;61:640–645. doi: 10.1016/0003-4975(95)01087-4. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S, Ying J, Razeghi P, Stepkowski S, Taegtmeyer H. Atrophic remodeling of the transplanted rat heart. Cardiology. 2006;105:128–136. doi: 10.1159/000090550. [DOI] [PubMed] [Google Scholar]
- 37.Brinks H, Tevaearai H, Muhlfeld C, Bertschi D, Gahl B, Carrel T, Giraud MN. Contractile function is preserved in unloaded hearts despite atrophic remodeling. J Thorac Cardiovasc Surg. 2009;137:742–746. doi: 10.1016/j.jtcvs.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Oriyanhan W, Tsuneyoshi H, Nishina T, Matsuoka S, Ikeda T, Komeda M. Determination of optimal duration of mechanical unloading for failing hearts to achieve bridge to recovery in a rat heterotropic heart transplantation model. J Heart Lung Transplant. 2007;26:16–23. doi: 10.1016/j.healun.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Rose AG, Park SJ. Pathology in patients with ventricular assist devices: A study of 21 autopsies, 24 ventricular apical core biopsies and 24 explanted hearts. Cardiovascular Pathology. 2005;14:19–23. doi: 10.1016/j.carpath.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Wohlschlaeger J, Levkau B, Brockhoff G, Schmitz KJ, von Winterfeld M, Takeda A, Takeda N, Stypmann J, Vahlhaus C, Schmid C, Pomjanski N, Bocking A, Baba HA. Hemodynamic support by left ventricular assist device reduces cardiomyocyte DNA content in the failing human heart. Circulation. 2010;121:989–996. doi: 10.1161/CIRCULATIONAHA.108.808071. [DOI] [PubMed] [Google Scholar]
- 41.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 42.Noguchi T, Hunlich M, Camp PC, Begin KJ, El-Zaru M, Patten R, Leavitt BJ, Ittleman FP, Alpert NR, LeWinter MM, VanBuren P. Thin filament-based modulation of contractile performance in human heart failure. Circulation. 2004;110:982–987. doi: 10.1161/01.CIR.0000139334.43109.F9. [DOI] [PubMed] [Google Scholar]
- 43.Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation. 2001;104:881–886. doi: 10.1161/hc3301.094911. [DOI] [PubMed] [Google Scholar]
- 44.Klotz S, Barbone A, Reiken S, Holmes JW, Naka Y, Oz MC, Marks AR, Burkhoff D. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J Am Coll Cardiol. 2005;45:668–676. doi: 10.1016/j.jacc.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 45.Heerdt PM, Holmes JW, Cai B, Barbone A, Madigan JD, Reiken S, Lee DL, Oz MC, Marks AR, Burkhoff D. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation. 2000;102:2713–2719. doi: 10.1161/01.cir.102.22.2713. [DOI] [PubMed] [Google Scholar]
- 46.Perrino C, Schroder JN, Lima B, Villamizar N, Nienaber JJ, Milano CA, Naga Prasad SV. Dynamic regulation of phosphoinositide 3-kinase-gamma activity and beta-adrenergic receptor trafficking in end-stage human heart failure. Circulation. 2007;116:2571–2579. doi: 10.1161/CIRCULATIONAHA.107.706515. [DOI] [PubMed] [Google Scholar]
- 47.Bick RJ, Grigore AM, Poindexter BJ, Schnee PM, Nussmeier NA, Gregoric ID, Shah NA, Myers TJ, Buja LM, Frazier OH. Left ventricular unloading with an assist device results in receptor relocalization as well as increased beta-adrenergic receptor numbers: Are these changes indications for outcome? J Card Surg. 2005;20:332–336. doi: 10.1111/j.1540-8191.2005.2004105.x. [DOI] [PubMed] [Google Scholar]
- 48.Milting H, Scholz C, Arusoglu L, Freitag M, Cebullar R, Jaquet K, Korfer R, Lewinski DV, Kassner A, Brodde OE, Kogler H, El Banayosy A, Pieske B. Selective upregulation of β1-adrenergic receptors and dephosphorylation of troponin I in end-stage heart failure patients supported by ventricular assist devices. J Mol Cell Cardiol. 2006;41:441–450. doi: 10.1016/j.yjmcc.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Pandalai PK, Bulcao CF, Merrill WH, Akhter SA. Restoration of myocardial β-adrenergic receptor signaling after left ventricular assist device support. J Thorac Cardiovasc Surg. 2006;131:975–980. doi: 10.1016/j.jtcvs.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 50.Terracciano CMN, Harding SE, Adamson D, Koban M, Tansley P, Birks EJ, Barton PJ, Yacoub MH. Changes in sarcolemmal Ca entry and sarcoplasmic reticulum Ca content in ventricular myocytes from patients with end-stage heart failure following myocardial recovery after combined pharmacological and ventricular assist device therapy. Eur Heart J. 2003;24:1329–1339. doi: 10.1016/s0195-668x(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 51.Terracciano CM, Koban MU, Soppa GK, Siedlecka U, Lee J, Stagg MA, Yacoub MH. The role of the cardiac Na+/Ca2+ exchanger in reverse remodeling: Relevance for LVAD-recovery. Ann N Y Acad Sci. 2007;1099:349–360. doi: 10.1196/annals.1387.061. [DOI] [PubMed] [Google Scholar]
- 52.Takeishi Y, Jalili T, Hoit BD, Kirkpatrick DL, Wagoner LE, Abraham WT, Walsh RA. Alterations in Ca2+ cycling proteins and Gαq signaling after left ventricular assist device support in failing human hearts. Cardiovascular Research. 2000;45:883–888. doi: 10.1016/s0008-6363(99)00415-0. [DOI] [PubMed] [Google Scholar]
- 53.Ogletree ML, Sweet WE, Talerico C, Klecka ME, Young JB, Smedira NG, Starling RC, Moravec CS. Duration of left ventricular assist device support: Effects on abnormal calcium cycling and functional recovery in the failing heart. J Heart Lung Transplant. 2010;29:554–561. doi: 10.1016/j.healun.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, Piacentino V, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanic assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Zhang X, Harris DM, Piacentino V, Berretta RM, Margulies KB, Houser SR. Reduced effects of BAY K 8644 on L-type Ca2+ current in failing human cardiac myocytes are related to abnormal adrenergic regulation. Am J Physiol Heart Circ Physiol. 2008;294:H2257–H2267. doi: 10.1152/ajpheart.01335.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latif N, Yacoub MH, George R, Barton PJR, Birks EJ. Changes in sarcomeric and non-sarcomeric cyctoskeletal proteins and focal adhesion molecules during clinical myocardial recovery after left ventricular assist device support. J Heart Lung Transplant. 2007;26:230–235. doi: 10.1016/j.healun.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Schwoerer AP, Neuber C, Schmechel A, MeInychenko I, Mearini G, Boknik P, Kirchhefer U, Schmitz W, Ehmke H, Eschenhagen T, El-Armouche A. Mechanical unloading of the rat heart involves marked changes in the protein kinase-phosphatase balance. J Mol Cell Cardiol. 2008;45:846–852. doi: 10.1016/j.yjmcc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Bruckner BA, Stetson SJ, Farmer JA, Radovancevic B, Frazier OH, Noon GP, Entman ML, Torre-Amione G, Youker KA. The implications for cardiac recovery of left ventricular assist device support on myocardial collagen content. Am J Surg. 2000;180:498–501. doi: 10.1016/s0002-9610(00)00553-5. [DOI] [PubMed] [Google Scholar]
- 60.Bruckner BA, Stetson SJ, Perez-Verdia A, Youker KA, Radovancevic B, Connelly JH, Koerner MM, Entman ME, Frazier OH, Noon GP, Torre-Amione G. Regression of fibrosis and hypertrophy in failing myocardium following mechanical circulatory support. J Heart Lung Transplant. 2001;20:457–64. doi: 10.1016/s1053-2498(00)00321-1. [DOI] [PubMed] [Google Scholar]
- 61.Klotz S, Foronjy RF, Dickstein ML, Gu A, Garrelds IM, Danser AH, Oz MC, D’Armiento J, Burkhoff D. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation. 2005;112:364–374. doi: 10.1161/CIRCULATIONAHA.104.515106. [DOI] [PubMed] [Google Scholar]
- 62.Li YY, Feng Y, McTiernan CF, Pei W, Moravec CS, Wang P, Rosenblum W, Kormos RL, Feldman AM. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation. 2001;104:1147–1152. doi: 10.1161/hc3501.095215. [DOI] [PubMed] [Google Scholar]
- 63.Bruggink AH, van Oosterhout MF, de Jonge N, Cleutjens JP, van Wichen DF, van Kuik J, Tilanus MG, Gmelig-Meyling FH, van den Tweel JG, de Weger RA. Type IV collagen degradation in the myocardial basement membrane after unloading of the failing heart by a left ventricular assist device. Laboratory Invest. 2007;87:1125–1137. doi: 10.1038/labinvest.3700670. [DOI] [PubMed] [Google Scholar]
- 64.Klotz S, Danser AHJ, Foronjy RF, Oz MC, Wang J, Mancini D, D’Armiento J, Burkhoff D. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–74. doi: 10.1016/j.jacc.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 65.Bruggink AH, van Oosterhout MF, de Jonge N, Ivangh B, van Kuik J, Voorbij RH, Cleutjens JP, Gmelig-Meyling FH, de Weger RA. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. J Heart Lung Transplant. 2006;25:1091–1098. doi: 10.1016/j.healun.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human myocytes. J Heart Lung Transplant. 2004;23:954–963. doi: 10.1016/j.healun.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 67.Vatta M, Stetson SJ, Perez-Verdia A, Entman ML, Noon GP, Torre-Amione G, Bowles NE, Towbin JA. Molecular remodeling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936–941. doi: 10.1016/S0140-6736(02)08026-1. [DOI] [PubMed] [Google Scholar]
- 68.Vatta M, Stetson SJ, Jimenez S, Entman ML, Noon GP, Bowles NE, Towbin JA, Torre-Amione G. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J Am Coll Cardiol. 2004;43:811–817. doi: 10.1016/j.jacc.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 69.Lee SH, Doliba N, Osbakken M, Oz M, Mancini D. Improvement of myocardial mitochondrial function after hemodynamic support with left ventricular assist devices in patients with heart failure. J Thorac Cardiovasc Surg. 1998;116:344–349. doi: 10.1016/s0022-5223(98)70136-9. [DOI] [PubMed] [Google Scholar]
- 70.Mital S, Loke KE, Addonizio LJ, Oz MC, Hintze TH. Left ventricular assist device implantation augments nitric oxide dependent control of mitochondrial respiration in failing human hearts. J Am Coll Cardiol. 2000;36:1897–1902. doi: 10.1016/s0735-1097(00)00948-7. [DOI] [PubMed] [Google Scholar]
- 71.Grabellus F, Schmid C, Levkau B, Breukelmann D, Halloran PF, August C, Takeda N, Takeda A, Wilhelm M, Deng MC, Baba HA. Reduction of hypoxia-inducible heme oxygenase-1 in the myocardium after left ventricular mechanical support. J Pathol. 2002;197:230–237. doi: 10.1002/path.1106. [DOI] [PubMed] [Google Scholar]
- 72.Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, Frazier OH, Taegtmeyer H. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation. 2009;120(suppl):S191–S197. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Francis GS, Anwar F, Bank AJ, Kubo SH, Jessurun J. Apoptosis, Bcl-2, and proliferating cell nuclear antigen in the failing human heart: Observations made after implantation of left ventricular assist device. J Cardiac Failure. 1999;5:308–315. doi: 10.1016/s1071-9164(99)91335-0. [DOI] [PubMed] [Google Scholar]
- 74.Blaxall BC, Tschannen-Moran BM, Milano CA, Koch WJ. Differential gene expression and genomic patient stratification following left ventricular assist device support. J Am Coll Cardiol. 2003;41:1096–1106. doi: 10.1016/s0735-1097(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 75.Bartling B, Milting H, Schumann H, Darmer D, Arusoglu L, Koerner MM, El-Banayosy A, Koerfer R, Holtz J, Zerkowski HR. Myocardial gene expression of regulators of myocyte apoptosis and myocyte calcium homeostasis during hemodynamic unloading by ventricular assist devices in patients with end-stage heart failure. Circulation. 1999;100(suppl II):II216–II223. doi: 10.1161/01.cir.100.suppl_2.ii-216. [DOI] [PubMed] [Google Scholar]
- 76.Morawietz H, Szibor M, Goettsch W, Bartling B, Barton M, Shaw S, Koerfer R, Zerkowski HR, Holtz J. Deloading of the left ventricle by ventricular assist device normalizes increased expression of endothelin ETA receptors but not endothelin-converting enzyme-1 in patients with end-stage heart failure. Circulation. 2000;102(suppl III):III188–III193. doi: 10.1161/01.cir.102.suppl_3.iii-188. [DOI] [PubMed] [Google Scholar]
- 77.Razeghi P, Young ME, Ying J, Depre C, Uray IP, Kolesar J, Shipley GL, Moravec CS, Davies PJ, Frazier OH, Taegtmeyer H. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology. 2002;97:203–309. doi: 10.1159/000063122. [DOI] [PubMed] [Google Scholar]
- 78.Razeghi P, Bruckner BA, Sharma S, Youker KA, Frazier OH, Taegtmeyer H. Mechanical unloading of the failing human heart fails to activate the protein kinase B/Akt/Glycogen synthase kinase-3β survival pathway. Cardiology. 2003;100:17–22. doi: 10.1159/000072387. [DOI] [PubMed] [Google Scholar]
- 79.Birks EJ, Hall JL, Barton PJ, Grindle S, Latif N, Hardy JP, Rider JE, Banner NR, Khaghani A, Miller LW, Yacoub MH. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular-assist device support. Circulation. 2005;112(suppl I):I57–I64. doi: 10.1161/CIRCULATIONAHA.104.526137. [DOI] [PubMed] [Google Scholar]
- 80.Rodrigue-way A, Burkhoff D, Geesaman BJ, Golden S, Xu J, Pollman MJ, Donoghue M, Jeyaseelan R, Houser S, Breitbart RE, Marks A, Acton S. Sarcomeric genes involved in reverse remodeling of the heart during left ventricular assist device support. J Heart Lung Transplant. 2005;24:73–80. doi: 10.1016/j.healun.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 81.Cullen ME, Yuen AH, Felkin LE, Smolenski RT, Hall JL, Grindle S, Miller LW, Birks EJ, Yacoub MH, Barton PJ. Myocardial expression of the arginine:glycine amidinotransferase gene is elevated in heart failure and normalized after recovery: Potential implications for local creatine synthesis. Circulation. 2006;114(suppl):I16–I20. doi: 10.1161/CIRCULATIONAHA.105.000448. [DOI] [PubMed] [Google Scholar]
- 82.Hall JL, Birks EJ, Grindle S, Cullen ME, Barton PJ, Rider JE, Lee S, Harwalker S, Mariash A, Adhikari N, Charles NJ, Felkin LE, Polster S, George RS, Miller LW, Yacoub MH. Molecular signature of recovery following combination left ventricular assist device (LVAD) support and pharmacologic therapy. Eur Heart J. 2007;28:613–627. doi: 10.1093/eurheartj/ehl365. [DOI] [PubMed] [Google Scholar]
- 83.Lowes BD, Zolty R, Shakar SF, Brieke A, Gray N, Reed M, Calalb M, Minobe W, Lindenfeld J, Wolfel EE, Geraci M, Bristow MR, Cleveland J. Assist devices fail to reverse patterns of fetal gene expression despite β-blockers. J Heart Lung Transplant. 2007;26:1170–1176. doi: 10.1016/j.healun.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Margulies KB, Matiwala S, Cornejo C, Olsen H, Craven WA, Bednarik D. Mixed messages: Transcription patterns in failing and recovering human myocardium. Circ Res. 2005;96:592–599. doi: 10.1161/01.RES.0000159390.03503.c3. [DOI] [PubMed] [Google Scholar]
- 85.Schipper MEI, van Kuik J, de Jonge N, Dullens HFJ, de Weger RA. Changes in regulatory microRNA expression in myocardium of heart failure patients on left ventricular assist device support. J Heart Lung Transplant. 2008;27:1282–1285. doi: 10.1016/j.healun.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA, Margulies KB, Dorn GW. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009;119:1263–1271. doi: 10.1161/CIRCULATIONAHA.108.813576. [DOI] [PMC free article] [PubMed] [Google Scholar]


