Voltage dependent anion channels (VDACs) are pore-forming proteins (approximately 30 kDa) associated with mitochondria, the membrane-bound organelles found in almost all eukaryotes. Commonly referred to as the “power-generators of cells”, mitochondria are composed of binary membranes, an outer membrane (OMM; outer mitochondrial membrane) and an inner membrane (IMM; inner mitochondrial membrane). While the latter is essentially impermeable to hydrophilic solutes of any kind, the OMM is made “porous” by the VDACs, which are integral membrane proteins that facilitate the free diffusion of molecules of approximately 5 kDa or less. Thus, the presence of VDACs (aptly named the “porins”),1 were used to explain the high solute permeability of the OMM, and its unresponsiveness to osmotic pressures. Electron micrographs of the OMM also display a porous appearance (reviewed by Mannella and Kinnaly, 2008)2 with a near-crystalline array of pores visible in lipid depleted OMMs. It should be noted that larger proteins encoded by nuclear DNA (and destined for the mitochondrion via an N-terminal signal sequence), bind to specialized “translocating protein complexes” on the OMM and are then transported across. The OMM also associates with the endoplasmic reticulum (ER) to facilitate transport of specific solutes, including lipids.
Discovered over three decades ago in mitochondrial extracts, the VDACs were first thought to be exclusively mitochondrial entities.3,4 However, at present, the VDACs have been characterized not only in the endoplasmic reticulum,5 but also in the plasma membrane as well (reviewed by Pinto, 2010).6 In fact, VDACs are the most abundant of proteins in the OMM.7 Currently, three isoforms of VDACs have been characterized primarily from mammalian tissues, with the VDAC-1 isoform being the most abundant by approximately an order of magnitude in comparison to the next most abundant VDAC isoform, VDAC-2 and over two orders of magnitude more abundant than the VDAC-3 (100-fold). VDACs 2- and 3-have been isolated primarily from testicular tissue and spermatozoa,8,9 while VDAC-1 is universally abundant across all other tissues types. Thus, it can be inferred that VDAC-1 is the most prevalent and physiologically and metabolically the most important isoform. In fact, almost all functional studies used to characterize VDACs have utilized VDAC-1 and form the basis for this commentary.
Functional Characterization of VDACs
The “pores” in the OMM were re-designated as VDACs primarily due to their electrophysiological properties when reconstituted in vitro within phospholipid bilayers or in liposomal vesicles. Although first thought to be “just a pore”, these in vitro functional studies indicated that the porins had the capacity to respond to an electrochemical potential.4 In essence, the OMM pores were found to be biased toward anion-selectivity among charged solutes (or metabolites), i.e., the VDAC channels display ion-selectivity that depends on the voltage across the membrane.10 In low-voltage states (~10 mV across the lipid bilayer) VDAC maintains an open-state, while at high-voltage states of either polarity (~40 mV or greater), VDAC manifests altered ion-selection and permeability levels.
Whether these in vitro phospholipid bilayer based studies faithfully relate to the function of VDACs in mitochondria (in their true physiological environment) is still being debated by various research groups. One possibility is that, at least at the “contact” sites between the OMM and IMM, the electrochemical potential generated across the inner-mitochondrial membrane (IMM) may directly induce a voltage potential across the OMM to regulate the “open” and “closed” states of the VDACs. Also, high cytoplasmic concentrations of NADH or a more reduced or bioenergetically sufficient state, have been found to promote VDAC closure, with the pore being more sensitive to closure at higher voltage and higher NADH levels.11,12
Which solutes are actually regulated by this pore? It is presumed that larger metabolites, i.e., nucleotides are regulated by the voltage-gated activity of VDAC. Which protein sub-structures facilitate this formation of a voltage-gated pore? Computer modeling and crystallography studies with bacterially expressed reconstitution systems indicate a barrel formed by 19 lipid bilayer spanning β-strands that form a membrane spanning β-barrel structure and one alpha-helical structure,13–15 while peptide loops that interconnect the β-strands and are exposed to the cytoplasm or the transmembrane space of mitochondria are thought to interact with other proteins or enzymes. A well-characterized example is the VDAC-hexokinase interaction on the cytoplasmic face of the OMM in tumor cell mitochondria.16,17
VDAC in Programmed Cell Death
Programmed cell death or apoptosis is primarily initiated via release of pro-apoptotic proteins from the inter-membrane space of the mitochondria to the cytosol, i.e., release of Cytochrome c from the inter-membrane space to the cytosol via permeabalization of the OMM.18 While the hexokinase-VDAC interaction described above facilitates preferential access to mitochondrial generated ATP to maintain the high glycolytic flux of such tumors,19,20 it also the prevents initiation of such apoptotic cascades.21 Here, hexokinase acts as a “damper” against formation of the yet-to-be fully characterized mitochondrial permeability transition pore (MPTP),22 thought to be necessary for induction of OMM permeablization. This and other similar molecular interactions of VDAC with OMM associated pro-and anti-apoptotic proteins are multi-faceted and help promote or prevent cell death. This phenotype is critical for the development of successful anti-cancer strategies. Thus, the mitochondrially expressed VDAC presents itself as a core component that can be targeted in tumors, which has the potential to debilitate the aberrant metabolic fluxes of tumors and initiate apoptotic signaling cascades.23
It should be noted that transgenic VDAC-1 knockout mice display normal behavior and functionality,24 while VDAC-3 “knockout” males are infertile.25 VDAC-2 “knockouts” appear to be embryonically lethal, as that isoform was found to manifest an anti-apoptotic effect by interacting with (and sequestering away) pro-apoptotic members of the Bcl-2 family of proteins.26
VDAC as an Anti-Cancer Target
Since several studies have indicated that the hexokinase-VDAC interaction on the OMM can inhibit apoptosis in several mammalian cell systems including tumor cells, an obvious cancer-targeting strategy would be to disrupt the complex or force the release of hexokinase from VDAC.21,27,28 In fact, several approaches to force the separation have been tested and shown to promote apoptosis in the targeted cells; in one, peptides that correspond to the N-terminal of hexokinase (necessary to anchor hexokinase to VDAC) or those that are on the putative hexokinase binding site on VDAC were used to interfere with the binding interaction.28 In another strategy, small-molecule drugs, i.e., methyl jasmonate (a stress hormone of botanicals)29 or clotrimazole30 were used, again to disrupt the hexokinase-VDAC binding. Expression analysis indicates that tumor cells highly overexpress VDAC, a fact borne by other indirect evidence, i.e., tumors display a much greater amount of mitochondrial-bound hexokinase than normal tissues. As such, targeting VDAC is an attractive modality to generate an anti-tumor response as many of the above in vitro studies have indicated. However, while either peptide-based or a small-molecule based strategies have been utilized in the past to test for inhibition of VDAC-1 and the effects of inhibition on tumor cell function, the effect of “silencing” VDAC-1 expression and its effects on the bioenergetic functions or apoptotic cascades in a tumor cell line has been tested only recently in vitro. These studies demonstrated that both mitochondrial bioenergetic functions and cell proliferation were affected by VDAC-1 silencing, at least in a non-tumorigenic recombinant cell line.31
The article by Koren, et al.32 in the current issue of Cancer Biology and Therapy now provides the first in vivo evidence of the effects of silencing VDAC-1 using a cervical cancer cell line (HeLa). To test the efficacy of such an “anti-VDAC” tumor targeting strategy, Koren et al. have utilized an inducible shRNA expression system (a doxycycline-based “Tet-On” system by Clontech) to first engineer cell-clones capable of regulated expression of RNAi against VDAC-1. After screening a series of RNAi templates (designed in silico) for the efficacy of VDAC-1 “knockdown” by western blotting, the authors then tested the same clonal cell isolates in a nude mouse model by sub-cutaneous implantation of the tumors followed by activation of RNAi via provision of “doxycycline-laced” water to the animals ad libitum.
The authors’ goals were to establish HeLa cell-lines that can express VDAC-1 in a regulated manner via exogenously applied doxycycline and test the cells for changes in proliferation rate in vitro, followed by implantation of the clonal isolates in the murine model to verify the effects of doxycycline-regulated silencing of VDAC-1 knockdown on tumor proliferation. Although the selected RNAi targets within VDAC-1 mRNA showed significant efficacy in reducing VDAC-1 expression, the “Tet-On” system employed also displayed the inherent difficulties sometimes encountered by researchers in getting this “molecular-switch” to work in a true “on/off” manner. While the authors were able to silence VDAC-1 expression by almost a magnitude, the test cells that were not exposed to doxycycline also displayed significant silencing of VDAC-1, most likely due to “leak-through” of the tet-regulated promoter elements employed by the system. Also surprising was the fact that doxycycline by itself silenced VDAC-1 expression by approximately 30% in control cells that harbored just the empty vector devoid of an shRNA template against VDAC-1; a facet of the authors’ results that need to be followed up in future studies. Perhaps the tetracycline mediated transcription silencing factors (Tts) that are utilized in the Tet-regulated system by themselves influence or alter the mitochondrial proteome (by as yet unrecognized methods).
Despite the above difficulties in employing these “cutting-edge” molecular biology tools to better elucidate the phenotypic changes due to VDAC-1 silencing of tumors, both the in vitro and in vivo data presented in the article display a clear correlation between silencing of VDAC-1 expression and inhibition of tumor proliferation. The in vivo data indicate an approximately 40-fold reduction in tumor size (as measured by tumor volume) in targeted cells, in comparison to that in controls. Interestingly, the studies also demonstrate that while tumors initially became established in both control and test animals, further development of the tumors were retarded in the test animals. This indicates that continued silencing of VDAC-1 inhibited growth of the tumors.
It would be interesting to continue these in vivo studies with longer-term analyses of the results obtained. For example, since both pro-and anti-apoptotic phenotypes have been associated with VDAC-1, does silencing of VDAC-1 expression (which should deprive hexokinase of a mitochondrial anchor and negate the anti-apoptotic effects of the hexokinase-VDAC binding) promote apoptosis? Or, would the strategy suppress apoptosis in tumors (as the latter is possible if pro-apoptotic members of the Bcl-2 family of proteins are prevented from forming hetero-oligomers with VDAC-1)? Also, does silencing of VDAC-1 prevent the formation of the still-elusive MPTP complex (as recent data in ref. 33 indicate that VDAC-1 may form multi-mers in order to form or support the MPTP)?
With the RNAi templates that were developed by Koren et al. to target VDAC-1 and displayed near total silencing, the above questions can now be answered at the pre-clinical level. This should reveal whether targeted-therapy against VDAC-1 will be an effective strategy to pursue in continuing efforts to harness the aberrant metabolic flux pathways of tumors and debilitate them.
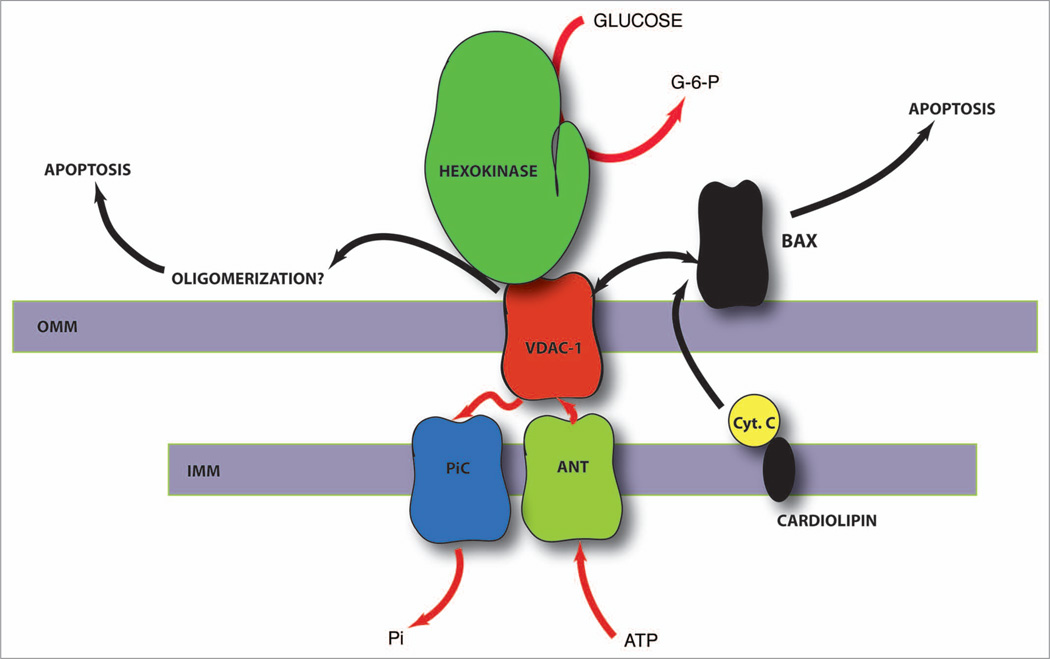
Figure 1.
Silencing of VDAC-1 expression via RNAi may result in opposing physiological effects on the tumor cell. Dispossessing of an “anchor” for hexokinase on the outer mitochondrial membrane may deprive the tumor of a high flux rate of glycolysis, thus inhibiting tumor proliferation; Pro-apoptotic—the same can relieve the anti-apoptotic effects that result from the hexokinase-VDAC interaction. Anti-apoptotic—if oligomers of VDAC are involved in forming the mitochondrial permeability transition pore complex (MPTP), silencing VDAC may inhibit apoptosis; the same may occur if VDAC is involved in hetero-multimer formation with Bax or other pro-apoptotic members of the Bcl-2 family of proteins. Despite the opposing effects on apoptosis, the data presented by Koren et al. indicate that silencing VDAC-1 inhibits tumor metabolism significantly, most likely via disruption of both the enhanced glycolytic flux and the nucleotide/metabolite/ion shuttles across mitochondria.
Acknowledgements
S.P.M. is supported by NIH grant R01CA116257 and an endowment by Marvin E. Klein, M.D. Charitable Trust and P.L.P. by NIH grants R01CA08018 and R01CA010951.
Abbreviations
- Tet
tetracycline
- RNAi
interference RNA
- shRNA
short-hairpin RNA
- NADH
reduced nicotinamide adenine dinucleotide
References
- 1.Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit Rev Biochem. 1985;19:145–190. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- 2.Mannella CA, Kinnally KW. Reflections on VDAC as a voltage-gated channel and a mitochondrial regulator. J Bioenerg Biomembr. 2008;40:149–155. doi: 10.1007/s10863-008-9143-0. [DOI] [PubMed] [Google Scholar]
- 3.Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- 4.Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979;279:643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- 5.Shoshan-Barmatz V, Zalk R, Gincel D, Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim Biophys Acta. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 6.De Pinto V, Messina A, Lane DJ, Lawen A. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett. 584:1793–1799. doi: 10.1016/j.febslet.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Yamada A, Watanabe M, Yoshimura Y, Yamazaki N, Yamauchi T, et al. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS-polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues. J Proteome Res. 2006;5:3336–3344. doi: 10.1021/pr060291w. [DOI] [PubMed] [Google Scholar]
- 8.Menzel VA, Cassara MC, Benz R, de Pinto V, Messina A, Cunsolo V, et al. Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci Rep. 2009;29:351–362. doi: 10.1042/BSR20080123. [DOI] [PubMed] [Google Scholar]
- 9.Hinsch KD, De Pinto V, Aires VA, Schneider X, Messina A, Hinsch E. Voltage-dependent anion-selective channels VDAC2 and VDAC3 are abundant proteins in bovine outer dense fibers, a cytoskeletal component of the sperm flagellum. J Biol Chem. 2004;279:15281–15288. doi: 10.1074/jbc.M313433200. [DOI] [PubMed] [Google Scholar]
- 10.Hodge T, Colombini M. Regulation of metabolite flux through voltage-gating of VDAC channels. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 11.Zizi M, Forte M, Blachly-Dyson E, Colombini M. NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J Biol Chem. 1994;269:1614–1616. [PubMed] [Google Scholar]
- 12.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator—thinking outside the box. Biochim Biophys Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, et al. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, et al. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakashima RA, Mangan PS, Colombini M, Pedersen PL. Hexokinase receptor complex in hepatoma mitochondria: evidence from N,N’-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry. 1986;25:1015–1021. doi: 10.1021/bi00353a010. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen PL. Voltage dependent anion channels (VDACs): a brief introduction with a focus on the outer mitochondrial compartment’s roles together with hexokinase-2 in the “Warburg effect” in cancer. J Bioenerg Biomembr. 2008;40:123–126. doi: 10.1007/s10863-008-9165-7. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G. Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun. 2003;304:433–435. doi: 10.1016/s0006-291x(03)00614-4. [DOI] [PubMed] [Google Scholar]
- 19.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40:171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic Cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 24.Weeber EJ, Levy M, Sampson MJ, Anflous K, Armstrong DL, Brown SE, et al. The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J Biol Chem. 2002;277:18891–18897. doi: 10.1074/jbc.M201649200. [DOI] [PubMed] [Google Scholar]
- 25.Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, et al. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J Biol Chem. 2001;276:39206–39212. doi: 10.1074/jbc.M104724200. [DOI] [PubMed] [Google Scholar]
- 26.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 27.Pastorino JG, Hoek JB. Hexokinase II: the integration of energy metabolism and control of apoptosis. Curr Med Chem. 2003;10:1535–1551. doi: 10.2174/0929867033457269. [DOI] [PubMed] [Google Scholar]
- 28.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced Cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 29.Goldin N, Arzoine L, Heyfets A, Israelson A, Zaslavsky Z, Bravman T, et al. Methyl jasmonate binds to and detaches mitochondria-bound hexokinase. Oncogene. 2008;27:4636–4643. doi: 10.1038/onc.2008.108. [DOI] [PubMed] [Google Scholar]
- 30.Penso J, Beitner R. Clotrimazole and bifonazole detach hexokinase from mitochondria of melanoma cells. Eur J Pharmacol. 1998;342:113–117. doi: 10.1016/s0014-2999(97)01507-0. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Hamad S, Sivan S, Shoshan-Barmatz V. The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc Natl Acad Sci USA. 2006;103:5787–5792. doi: 10.1073/pnas.0600103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koren I, Raviv Z, Shoshan-Barmatz V. Downregulation of voltage-dependent anion channel-1 expression by RNA interference prevents cancer cell growth in vivo. Cancer Biol Ther. 2010 doi: 10.4161/cbt.9.12.11879. [DOI] [PubMed] [Google Scholar]
- 33.Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, et al. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]