Abstract
Enoxaparin is commonly used to prevent venous thromboembolism (VTE) [1, 2] but has not been well-studied in patients with extreme obesity, a population at high risk for VTE. We prospectively compared three enoxaparin dosing regimens for the achievement of goal peak anti-Factor Xa levels in medically-ill patients (n=31) with extreme obesity (body mass index (BMI) ≥ 40 kg/m2). Patients were assigned to receive fixed-dose (FD) enoxaparin 40mg daily (QDay, n=11), weight-based, lower-dose (LD) enoxaparin 0.4 mg/kg QDay (n=9), or weight-based, higher-dose (HD) enoxaparin 0.5 mg/kg QDay(n=11). The average BMI and weight of the entire cohort was 62.1 kg/m2 (range 40.5-82.4) and 176 kg (range 115-256 kg) and did not differ between groups. Peak anti-Factor Xa levels were significantly higher in the HD group compared to either LD or FD groups. Patients in the HD group achieved target anti-Factor Xa levels more frequently than the LD and FD groups (p<0.05). Peak anti-Factor Xa levels did not correlate with age, weight, BMI, or creatinine clearance, demonstrating the predictability of weight-based enoxaparin dosing. There were no adverse events (e.g. bleeding, thrombosis, thrombocytopenia). To our knowledge, this is the first prospective comparative study demonstrating that in extremely obese, medically-ill patients enoxaparin 0.5 mg/kg QDay is superior to fixed-dose and lower-dose enoxaparin for the achievement of target anti-Factor Xa levels.
Enoxaparin is a low-molecular weight heparin (LMWH) that effectively reduces the risk of venous thromboembolism (VTE) in both medically-ill and surgical patients[3, 4]. However, patients with extreme obesity (i.e. Body Mass Index >40 kg/m2), a major risk factor for VTE[1, 5, 6], were under-represented in VTE prophylaxis trials of enoxaparin (40 mg once daily (QDay)) in medically-ill patients [3, 7]. Obesity affects drug distribution and kinetics[1, 2, 8]. For example, in obese patients given fixed-dose enoxaparin 40 mg QDay anti-Factor Xa activity and actual body weight are inversely correlated[9, 10] and higher rates of VTE prophylaxis failure have been reported[11]. Although ACCP practice guidelines suggest increasing the dose of pharmacologic agents such as enoxaparin for the prevention of VTE in obese patients[4], optimal dose adjustments remain unclear. Given the striking rise in the prevalence of extreme obesity[12, 13], understanding how to best adjust the dose of enoxaparin in these patients is crucial in order to optimally prevent VTE in this high-risk population.
We prospectively enrolled hospitalized, consenting, medically-ill patients at risk for VTE (n=31) with extreme obesity (BMI≥40 kg/m2). Patients were sequentially assigned to one of three enoxaparin dosing groups, matched for age, weight, and BMI. The first was a control group who received fixed-dose (FD) enoxaparin 40mg daily (QDay, n=11), the current FDA approved dose of enoxaparin for the prevention of DVT in medically-ill patients. Groups two and three were intervention groups assigned to either weight-based, lower-dose (LD) enoxaparin 0.4 mg/kg QDay (n=9) or weight-based, higher-dose (HD) enoxaparin 0.5 mg/kg QDay (n=11). All patients had anti-Factor Xa levels drawn upon study enrollment and then daily, during their hospital stay, when feasible. Our primary outcome was the achievement of target peak anti-Factor Xa levels (defined as a peak anti-Factor Xa level between 0.2-0.5 IU/mL, measured 4-6 hours after enoxaparin administration).
The three groups were well matched on the pre-specified variables of age, weight, and BMI (Table 1). The average BMI exceeded 60 kg/m2 in all three groups, consistent with our goal of recruiting patients with extreme obesity. The average weight exceeded 170 kg in all three groups (range 115 to 256 kg) and the maximum weights were 254 kg, 238 kg, and 256 kg for the FD, LD, and HD groups, respectively (Table 1, p=NS). There was no difference in the time between enoxaparin administration and measurement of peak anti-Factor Xa levels between the three groups (Table 1). In addition to obesity, the most common VTE risk factors were sepsis (n=20/31, 64.5%) and acute respiratory failure (n=12/31, 38.7%). Overall, almost half of our subjects (n=15/31, 48.4%) had ≥2 major VTE risk factors (excluding the risk factor of morbid obesity).
Table 1.
Characteristics of medically-ill patients with extreme obesity (BMI ≥40kg/m2). Values represent the mean (±SD) unless otherwise specified. Time refers to the interval between administration of the enoxaparin dose and measurements of the anti-Factor Xa level. Creatinine clearance (CrCl) is based on adjusted body weight using the method of Cockcroft and Gault.
| Enoxaparin 40mg once daily (n=11) | Enoxaparin 0.4mg/kg once daily (n=9) | Enoxaparin 0.5mg/kg once daily (n=11) | p-value | |
|---|---|---|---|---|
| Age (yrs) | 45.5 (±7.2) | 43.8 (±15.7) | 42.7 (±12.3) | 0.86 |
| Male, n (%) | 2 (18.2) | 6 (66.7) | 3 (27.3) | 0.20 |
| Weight (kg) | 175.0 (±39.9) | 171.2 (±42.8) | 179.6 (±30.3) | 0.88 |
| BMI (kg/m2) | 63.4 (±11.6) | 60.7 (±12.4) | 61.3 (±12.2) | 0.81 |
| Time (min) | 279 (±45) | 278 (±62) | 272 (±29) | 0.93 |
| CrCl (mL/min) | 84 (±24) | 135 (±64) | 139 (±37) | 0.01 |
| Enoxaparin Dose [min-max] (mg/day) | 40 | 69.4 (±16.3), [50-90] | 91.8 (±15.4), [80-130] | <0.0001 |
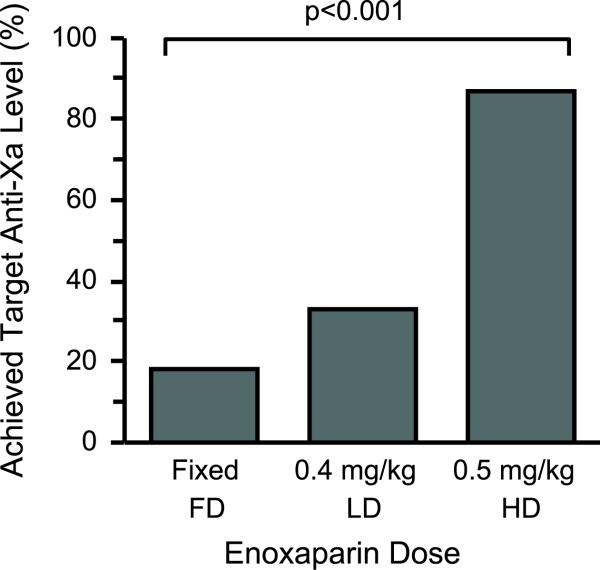
The primary outcome (anti-Factor Xa level between 0.20-0.50 IU/mL[2, 14]) was achieved significantly more often in the HD group compared to either the FD or LD group (Figure 2). Patients in the LD group achieved target anti-Factor Xa levels more often than the FD group but remained inferior to the HD group (Figure 2). Furthermore, 82% of patients in the FD group had anti-Factor Xa levels < 0.20 IU/mL while only 36% and 13% of patients in the LD and HD groups, respectively, had anti-Factor Xa levels < 0.20 IU/mL (p<0.001). Only one patient in our cohort had an anti-Factor Xa level above the upper limit of 0.50 IU/mL (peak anti-Factor Xa level = 0.55) and this patient was in the LD group. There were no significant differences in age, creatinine clearance, gender, actual body weight, BMI, or time between patients whose peak anti-Factor Xa levels were within or below target levels. Based on dosing in the HD group, using actual body weights up to 256 kg, we did not identify a weight above which the enoxaparin dose should be capped.
Figure 2.
Enoxaparin 0.5mg/kg once daily was significantly more effective in achieving appropriate peak anti-Factor Xa levels (defined as a peak anti-Factor Xa level between 0.20 and 0.50 IU/mL).
Patients in the FD group, which represented a control group, had only one anti-Factor Xa level drawn upon study entry. The median length of stay in these patients was 3 days (95% confidence interval (CI) 1-23 days). The majority of patients (75%) had a length of stay of 3 days or less, while 3 patients had an extended (> 20 days) hospitalization due to transfer to the intensive care unit or operating room and subsequent surgical service following study enrollment. Patients were not followed following hospital discharge or transfer.
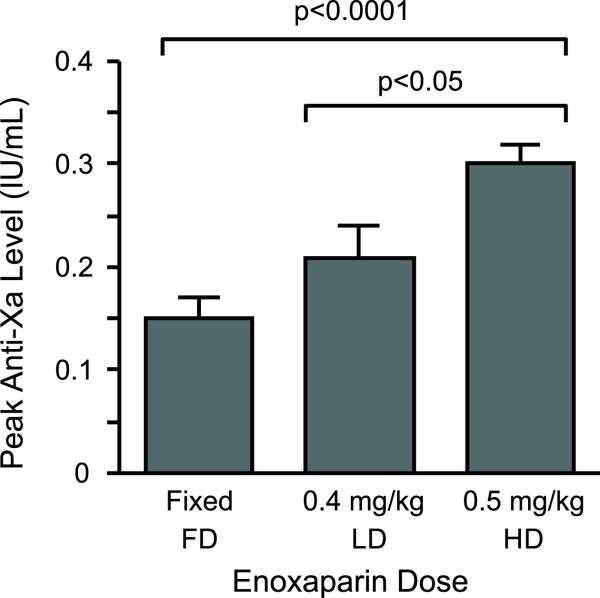
Overall, a total of 38 peak anti-Factor Xa levels in the LD and HD groups (on average, 1.9 anti-Xa levels per patient)were measured. This reflects the median length of stay of 3 days in these patients and includes 15 and 23 anti-Factor Xa measurements in the LD and HD groups, respectively. Only one anti-Factor Xa level was obtained in the FD group, as they represented a control group. Peak anti-Factor Xa levels in the HD group were significantly higher than levels in the FD and LD groups (Figure 1). Although anti-Factor Xa levels trended higher in LD group compared to the FD group, differences did not reach statistical significance (Figure 1). In stepwise, multiple regression analyses peak anti-Factor Xa levels were not correlated with any of the pre-specified variables of age, actual body weight, BMI, time (enoxaparin dose administration to anti-Factor Xa measurement), gender, or estimated creatinine clearance (based on the method of Cockroft and Gault, using adjusted body weight[15-17]).
Figure 1.
Peak anti-Factor Xa levels were significantly higher in patients with extreme obesity who received higher-dose enoxaparin 0.5mg/kg once daily. Patients with extreme obesity were assigned to one of three enoxaparin dosing groups (fixed-dose enoxaparin 40mg once daily (FD, n=11 patients), lower-dose enoxaparin 0.4mg/kg once daily (LD, n=9 patients), and higher-dose enoxaparin 0.5mg/kg once daily (HD, n=11 patients) groups). These data represent the mean(±SEM) values of each group.
Consistent with published data[18, 19], peak anti-Factor Xa levels were higher with repeated enoxaparin dosing, although the overall increase was not significant(enoxaparin dosing day 1: 0.23 (0.17, 0.34); enoxaparin dosing day 2: 0.28 (0.20, 0.36); enoxaparin dosing day 3: 0.28 (0.20, 0.31), p=0.69. Patients in the HD group were significantly more likely to achieve a goal anti-Factor Xa level on enoxaparin dosing day 2 compared to patients in the LD group (100% versus 25%, p<0.05). This difference was also seen on enoxaparin dosing day 3, although the sample size (n=6) was too small to reach statistical significance (100% versus 50%, p=0.30). There were no bleeding events, symptomatic DVT or PE, or episodes of heparin-induced thrombocytopenia (HIT) in any of the three groups.
Enoxaparin is a low-molecular weight heparin (LMWH) used commonly to prevent VTE in both medically-ill and surgical patients[3, 20]. Patients with extreme obesity, a recognized major risk factor for VTE[1, 5, 6], have not been adequately represented in clinical trials with enoxaparin. Obesity may alter the normal pharmacokinetics of LMWHs[1, 2] and cause higher rates of VTE prophylaxis failure[21]. As the prevalence of obesity is rising[12], understanding how to adjust the dose of enoxaparin in patients with obesity is necessary to optimally prevent thrombotic events in this high-risk population.
In the current study, we demonstrate that enoxaparin 0.5mg/kg QDay in patients with an average BMI of > 60 kg/m2 is superior to both FDA-approved fixed enoxaparin dosing (40mg QDay) and lower-dose weight adjusted enoxaparin (0.4mg/kg QDay) for the achievement of target peak anti-Factor Xa levels (between 0.2-0.5 IU/mL). These data support studies in both medically-ill and surgical patients[1, 9, 11, 17] and are consistent with ACCP practice guidelines[4] suggesting that higher doses of pharmacologic thromboprophylaxis should be considered in patients with obesity. In the current study, we extend these published observations by prospectively comparing three enoxaparin dosing regimens in medically-ill patients with extreme obesity. By enrolling patients with an average BMI and weight exceeding 60 kg/m2 and 170kg, respectively, our findings provide new evidence of the predictability of weight-based enoxaparin (0.5mg/kg QDay) for DVT prophylaxis in patients weighing up to 256 kg. The findings of the current study also suggest that enoxaparin 0.5mg/kg effectively achieved recommended anti-Factor Xa levels without an observed increase in bleeding or thrombosis complications, although our study was not powered to determine clinical efficacy or safety. Similarly, a dosing regimen of enoxaparin 0.5 mg/kg did not result in therapeutic levels of anti-coagulation.
The primary limitations of the current study are the small sample size and the lack of clinical outcomes associated with the anti-Factor Xa measurements. A large, multicenter clinical trial is obviously necessary in order to demonstrate reductions in thrombotic events with a weight-based enoxaparin dosing algorithm for VTE prophylaxis. Nevertheless, until those clinical trials are completed, these data provide new pharmacokinetic evidence of the benefit of enoxaparin 0.5mg/kg over fixed-dose enoxaparin in patients with morbid obesity. Differences between groups were large enough that despite the small sample size, the absolute magnitude of difference in peak anti-Factor Xa levels between FD and HD enoxaparin (Figure 1) was sufficient to provide 99.9% power to avoid a type II error (using a two-sided, α=0.05).
Although the creatinine clearance was significantly lower in patients who received fixed-dose enoxaparin, there was no correlation between anti-Factor Xa levels and creatinine clearance and thus we do not believe this difference confounded our results. We also did not enroll any patients with a creatinine clearance < 30 mL/min. Enoxaparin undergoes renal elimination and dosing should be adjusted in patients with severe renal failure, in accordance with manufacturer's recommendations. Steady state levels with prophylactic-dose enoxaparin occurs on the second day of administration in healthy volunteers, with average exposure ratio about 15% higher than after a single dose. Consistent with these data, there was an increase of approximately 20% in peak anti-Factor Xa levels between enoxaparin dosing day 1 and day 2, suggesting that steady state in our cohort was also occurring on the second enoxaparin dosing day. Given the median length of stay of 3 days for most of our patients, we were unable to obtain anti-Factor Xa levels beyond three days in any meaningful fashion. In addition, anti-Factor Xa levels in the FD group were only measured following the first dose of enoxaparin and may not reflect a true steady state. Nevertheless, even if there was a 20% increase in peak anti-Factor Xa levels on dosing day 2, most patients in the FD may still have had anti-Factor levels below the target range. Enoxaparin accumulation has been reported occur during longer treatment periods[19] and in patients with moderate to severe renal failure[18] and thus close monitoring may be warranted when weight-based enoxaparin is prescribed for longer periods of time. Finally, we did not study anti-Factor Xa levels in non-obese, medically-ill patients and cannot exclude the possibility that non-obese patients would have similar variance in anti-Factor Xa levels.
In conclusion, these data demonstrate that enoxaparin 0.5mg/kg QDay is superior to either fixed-dose enoxaparin 40mg QDay or a lower weight-based regimen of 0.4mg/kg QDay for achieving target peak anti-Factor Xa levels in medically-ill patients with extreme obesity. This dosing regimen was not associated with adverse clinical events such as bleeding or thrombosis.
Materials and Methods
Patients
Eligible patients were hospitalized, medically ill patients ≥ 18 years of age with extreme obesity (WHO Class Obesity: body mass index (BMI) ≥ 40 kg/m2) and having ≥1 major VTE risk factor, including age > 70, heart failure, acute respiratory failure, previous VTE, cancer, stroke, sepsis, and immobility (defined as ≥3 days of bed-rest)[4]. Patients were excluded if they were pregnant, on therapeutic anticoagulation, had a bleeding disorder, platelet count of less than 100,000/mL, coagulopathy, active bleeding, estimated creatinine clearance < 30 mL/min (based on the method of Cockcroft and Gault using adjusted body weight[15-17]), or stroke, surgery or trauma within 14 days. This study was approved by the institutional Ethics Committee on human research and all patients provided informed consent.
Treatment Protocol
Patients were consecutively identified and assigned sequentially to one of three groups. The first was a control group assigned to fixed-dose (FD) enoxaparin 40mg daily (QDay, n=11). Groups two and three were intervention groups assigned to either weight-based, lower-dose (LD) enoxaparin 0.4 mg/kg QDay (n=9) or weight- based, higher-dose (HD) enoxaparin 0.5 mg/kg QDay (n=11), in random fashion. Peak anti-Factor Xa levels were measured daily, 4-6 hours after the enoxaparin dose for up to three days in patients assigned to either LD or HD groups, while patients remained hospitalized on a medicine service. For safety, when patients were transferred to the ICU, a surgical team, or a non-medicine service, VTE prophylaxis was chosen at the discretion of the primary team.
Our primary outcome was the achievement of target peak anti-Factor Xa levels (defined as a peak anti-Factor Xa level between 0.2-0.5 IU/mL, measured 4-6 hours after enoxaparin administration).
Enoxaparin dosing for the HD group was based on previous studies in medically-ill obese patients[17]. Enoxaparin dosing for the LD group was chosen with consideration of published investigations[17, 22]. We estimated that the average weight of enrolled patients in the current study would be 150kg. A weight-based enoxaparin dose of 0.4mg/kg would provide a conservative 50% dose increase on average over fixed-dosing (e.g. 40mg daily). This dosing also was chosen to allow comparisons of safety and efficacy between a more moderate (e.g 0.4mg/kg) and more aggressive (e.g. 0.5mg/kg) prophylactic dosing regimen.
Our study protocol specified that patients would have Group 1 (n=11) received fixed-dose (FD) enoxaparin 40mg once daily (QDay), Group 2 (n=9) received lower-dose (LD) enoxaparin 0.4 mg/kg QDay, and Group 3 (n=11) received higher-dose (HD) enoxaparin 0.5 mg/kg QDay. Actual body weight was determined upon hospital admission with a standardized, calibrated scale. The dose of enoxaparin was not capped and was rounded to the nearest 5 mg unit in accordance with standard procedures to ensure the precise administration of each dose, as we have done previously[17]. Medication administration times were reconciled with laboratory records to ensure the precise capture of the interval between enoxaparin dosing and peak anti-Factor Xa level measurements. No patients received other types of heparinoids during the study period that would have interfered with anti-Factor Xa measurements.
Patients were monitored daily during their hospitalization with routine clinical laboratory tests (e.g. complete blood count) and clinical assessments for the development of any adverse events such as symptomatic VTE (including DVT and PE), majoror non-major clinically significant bleeding (defined as fatal bleeding, bleeding resulting in the transfusion of ≥ 2 units of packed red blood cells, bleeding in a critical site, such as intracranial hemorrhage, retroperitoneal bleeding, or bleeding that required medical intervention (e.g. gastrointestinal bleeding requiring endoscopy, epistaxis requiring nasal packing, etc), and thrombocytopenia concerning for heparin-induced thrombocytopenia in accordance with current guidelines[23]).
Anti-Factor Xa Level Measurements
Peak anti-Factor Xa levels were obtained through venipuncture 4-6 hours after the dose of enoxaparin was given, as described before[17] and briefly reviewed here. A pilot tube was drawn first and an exact ratio of 9 volumes of blood to 1 volume of anticoagulant (32 g/L citrate) was maintained. The Rotachrom® assay using the STA-Compact instrument (Diagnostica Stago, Parsippany, NJ) was used to quantify anti-Factor Xa (LMWH) activity for enoxaparin. The sensitivity of this assay is 0.2 U/mL and within run imprecision is 5.5 (% CV) at 1 U/mL. The assay is linear between 0.2-2.0 U/mL. All anti-Factor Xa levels were analyzed by technicians blinded to the assigned treatment regimen. The target range for peak anti-Factor Xa levels was chosen to be 0.2-0.5 IU/mL[14].
Statistical Analyses
The sample size for this pilot study was chosen to provide a cohort large enough to identify differences between groups, based on previous studies[17]. Descriptive statistics were used to calculate summary data. Data are represented as the mean ± SD, unless otherwise indicated. For all analyses, continuous variables were assessed for normality visually and with skewness and kurtosis tests (version 11.0, StataCorp, College Station, TX 77845). Parametric, two-tailed t-tests or ANOVA were used for continuous variables and the chi-square and Fisher's exact test for categorical variables with a predetermined alpha level of p<0.05. Stepwise, multiple regression analyses were used to identify any pre-specified variables potentially affecting peak anti-Factor Xa levels, including age, actual body weight, BMI, time (enoxaparin dose administration to anti-Factor Xa measurements), gender, or estimated creatinine clearance.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (Grant K23HL092161). We thank all the patients that agreed to participate in this study, Ms. Pam Proctor and Linda Kelly for their research oversight, Dr. Chris Lehman for his assistance with anti-Factor Xa measurements, and Diana Lim for her assistance with illustrations and tables.
Footnotes
Conflict of Interest Statement: The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Freeman AL, Pendleton RC, Rondina MT. Prevention of venous thromboembolism in obesity. Expert review of cardiovascular therapy. 2010;8:1711–1721. doi: 10.1586/erc.10.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rondina MT, Pendleton RC, Wheeler M, Rodgers GM. The treatment of venous thromboembolism in special populations. Thrombosis research. 2007;119:391–402. doi: 10.1016/j.thromres.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. The New England journal of medicine. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 4.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 5.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–980. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Ageno W, Squizzato A, Garcia D, Imberti D. Epidemiology and risk factors of venous thromboembolism. Semin Thromb Hemost. 2006;32:651–658. doi: 10.1055/s-2006-951293. [DOI] [PubMed] [Google Scholar]
- 7.Kanaan AO, Silva MA, Donovan JL, Roy T, Al-Homsi AS. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395–2405. doi: 10.1016/j.clinthera.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39:215–231. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Frederiksen SG, Hedenbro JL, Norgren L. Enoxaparin effect depends on body-weight and current doses may be inadequate in obese patients. Br J Surg. 2003;90:547–548. doi: 10.1002/bjs.4068. [DOI] [PubMed] [Google Scholar]
- 10.Priglinger U, Delle Karth G, Geppert A, Joukhadar C, Graf S, Berger R, et al. Prophylactic anticoagulation with enoxaparin: Is the subcutaneous route appropriate in the critically ill? Crit Care Med. 2003;31:1405–1409. doi: 10.1097/01.CCM.0000059725.60509.A0. [DOI] [PubMed] [Google Scholar]
- 11.Hamad GG, Choban PS. Enoxaparin for thromboprophylaxis in morbidly obese patients undergoing bariatric surgery: findings of the prophylaxis against VTE outcomes in bariatric surgery patients receiving enoxaparin (PROBE) study. Obes Surg. 2005;15:1368–1374. doi: 10.1381/096089205774859245. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008;93:S1–8. doi: 10.1210/jc.2008-1356. [DOI] [PubMed] [Google Scholar]
- 13.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32(Suppl 7):S120–126. doi: 10.1038/ijo.2008.247. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig KP, Simons HJ, Mone M, Barton RG, Kimball EJ. Implementation of an enoxaparin protocol for venous thromboembolism prophylaxis in obese surgical intensive care unit patients. Ann Pharmacother. 2011;45:1356–1362. doi: 10.1345/aph.1Q313. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Martinez L, Burnett A, Borrego M, Streeter JC, Townsend K, Garcia D. Effect of fondaparinux prophylaxis on anti-factor Xa concentrations in patients with morbid obesity. Am J Health Syst Pharm. 2011;68:1716–1722. doi: 10.2146/ajhp110010. [DOI] [PubMed] [Google Scholar]
- 17.Rondina MT, Wheeler M, Rodgers GM, Draper L, Pendleton RC. Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-Ill patients. Thrombosis research. 2010;125:220–223. doi: 10.1016/j.thromres.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanderink GJ, Guimart CG, Ozoux ML, Jariwala NU, Shukla UA, Boutouyrie BX. Pharmacokinetics and pharmacodynamics of the prophylactic dose of enoxaparin once daily over 4 days in patients with renal impairment. Thromb Res. 2002;105:225–231. doi: 10.1016/s0049-3848(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 19.Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26(Suppl 2):24–38. doi: 10.1159/000217270. [DOI] [PubMed] [Google Scholar]
- 20.Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 21.Rocha AT, de Vasconcellos AG, da Luz Neto ER, Araujo DM, Alves ES, Lopes AA. Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obes Surg. 2006;16:1645–1655. doi: 10.1381/096089206779319383. [DOI] [PubMed] [Google Scholar]
- 22.Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19–24. doi: 10.1381/096089202321144522. [DOI] [PubMed] [Google Scholar]
- 23.Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, et al. Treatment and Prevention of Heparin-Induced Thrombocytopenia. Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e495S–e530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]