Abstract
A key hallmark of many cancers, particularly the most aggressive, is the capacity to metabolize glucose at an elevated rate, a phenotype detected clinically using positron emission tomography (PET). This phenotype provides cancer cells, including those that participate in metastasis, a distinct competitive edge over normal cells. Specifically, after rapid entry of glucose into cancer cells on the glucose transporter, the highly glycolytic phenotype is supported by hexokinase (primarily HK II) that is overexpressed and bound to the outer mitochondrial membrane via the porin-like protein voltage-dependent anion channel (VDAC). This protein and the adenine nucleotide transporter move ATP, newly synthesized by the inner membrane located ATP synthase, to active sites on HK II. The abundant amounts of HK II bind both the ATP and the incoming glucose producing the product glucose-6-phosphate, also at an elevated rate. This critical metabolite then serves both as a biosynthetic precursor to support cell proliferation and as a precursor for lactic acid, the latter exiting cancer cells causing an unfavorable environment for normal cells. Although helping facilitate this chemical warfare, HK II via its mitochondrial location also suppresses the death of cancer cells, thus increasing their possibility for metastasis and the ultimate death of the human host. For these reasons, targeting this key enzyme is currently being investigated in several laboratories in a strategy to develop novel therapies that may turn the tide on the continuing struggle to find effective cures for cancer. One such candidate is 3-bromopyruvate that has been shown recently to eradicate advanced stage, PET positive hepatocellular carcinomas in an animal model without apparent harm to the animals.
Keywords: hexokinase II, glycolysis, mitochondria, cell death: 3-bromopyruvate
Glycolysis in cancers, a pivotal role for hexokinase
In humans and other mammals, glucose is essential both as an energy source to help sustain cell life and as a carbon source for most cell building blocks. Therefore, it is not surprising that glucose catabolism (glycolysis) is elevated in many cancers, particularly those with the most rapid growth rates (Warburg et al., 1930; Pedersen, 1978). It is also not surprising that the elevated consumption of glucose by such cancers is utilized clinically worldwide both as a diagnostic tool via 18F-deoxy glucose positron emission tomography (18FDG-PET) and as a prognostic marker. Critical to this highly glycolytic phenotype is the first enzymatic step of glucose phosphorylation (Bustamante and Pedersen, 1977; Bustamante et al., 1981). Here, glucose is entrapped by phosphorylation for the tumor’s utility, an event that involves specific isozymes of hexokinase (HK) and how they are expressed, regulated, and localized within the tumor in a manner that is highly advantageous to the tumor but destructive to the host (Pedersen, 1978; Mathupala et al., 1997B; Smith, 2000; Pedersen et al., 2002). Although the key hexokinase isozyme involved (HK II), and its associated ‘partners in cancer promotion’ (discussed below) represent ideal molecular targets for inhibition of cancer cell proliferation and therefore tumor destruction, this has yet to be fully exploited in a clinical setting.
Hexokinase II relative to other hexokinases (I, III, IV) in cancers
Hexokinases catalyse the essentially irreversible first step of the glycolytic pathway (below) where glucose is phosphorylated to glucose-6-phosphate with concomitant de-phosphorylation of ATP.
Four isoforms of hexokinase, denoted here as HK I, II, III and IV (glucokinase) have been characterized in mammalian tissue (Wilson, 1995, 1997, 2003). Among these, HK I, II and III have an approximately 250-fold higher affinity for the substrate glucose (Km = ~0.02 mM), relative to HK IV (Km = ~5 mM) (Wilson, 1995, 2003). Examination of the primary sequence of each of the four isoforms implicate those with a high affinity for glucose, that is, HK I, II, and III, as arising via duplication of an ancestral glucokinase gene similar to that which encodes HK IV (Tsai and Wilson, 1995, 1996, 1997; Ardehali et al., 1996; Printz et al., 1997). As a result, whereas HK IV has a molecular mass of approximately 50 kDa, the HK isoforms (I, II, and III) with a high affinity for glucose each have a molecular mass near 100 kDa.
One of the earliest adaptations observed during tumorigenesis in tissues such as liver and pancreas that express HK IV is a ‘switch-over’ to the expression of high-affinity isoforms of hexokinase, that is, HK II and to a lesser extent HK I. During this process, the expression of HK IV is silenced (Rempel et al., 1994b; Mathupala et al., 1997B; Mayer et al., 1997; Pedersen et al., 2002). This is clearly seen during tumorigenesis in the liver, where HK IV is expressed almost exclusively in adult hepatic tissue, whereas in highly malignant hepatomas HK IV is silenced and HK II, and to some extent HK I, are ‘switched-on’. There is also evidence that a similar set of events takes place, at least in part, when normal pancreatic islet cells become tumors (Vischer et al., 1987).
Interestingly, most normal mammalian tissues express very little HK II with those from muscle, adipocytes and lung expressing low but significant levels (Wilson, 1995, 2003).
Hexokinase II and its major partners in cancer promotion
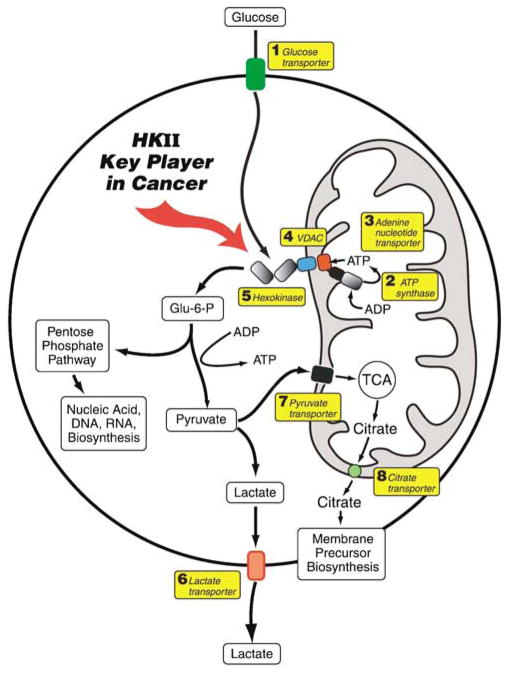
Although HK II is a major player in helping maintain the highly malignant state, it has four other key protein partners (Figure 1). These include a plasma membrane glucose transporter (Glut) that allows the entry of glucose into the cancer cell, with glucose serving as one of two substrates for HK II; the pore-like outer mitochondrial membrane protein voltage-dependent anion channel (VDAC) that binds HK II; the inner mitochondrial membrane protein ATP synthase that makes ATP, the second substrate of HK; and finally the adenine nucleotide translocator that transports the ATP to the VDAC-HK II complex (Figure 1). The net result of cooperation among these four proteins is the rapid and efficient production of glucose-6-phosphate that serves not only as the precursor for glycolysis but also for biosynthesis of key metabolites via the pentose-phosphate pathway and the mitochondrial tricarboxylic acid cycle, both essential for the growth and proliferation of cancer cells. But this is not all! In an ingenious example of the cancer cell’s efficiency in sustaining its own life long enough to proliferate and metastasize, it instructs the binding of HK II to VDAC (and likely other proteins) for another purpose. This is to inhibit mitochondrial-induced apoptosis and suppress cell death.
Figure 1.
Delivery of glucose and ATP to hexokinase (HK) II bound to the outer mitochondrial membrane within a malignant cell and metabolic fates of the glucose-6-phosphate (G-6-P) formed. Glucose brought across the plasma membrane by glucose transporters (1) is rapidly phosphorylated by HK II (5) bound to voltage-dependent anion channel (VDAC) (4) located on the outer mitochondrial membrane. The VDAC allows direct access to HK II of ATP generated by the ATP synthase (2) and transported across the inner-mitochondrial membrane by the adenine nucleotide translocator (3). To maintain the highly glycolytic metabolic flux of such malignant cells, the product G-6-P is rapidly distributed across key metabolic routes. The primary routes are (a) direct entry of the G-6-P into the pentose-phosphate shunt for biosynthesis of nucleic-acid precursors and (b) conversion of the G-6-P via the glycolytic pathway to pyruvate and lactic acid. Here, whereas the lactic acid is transported out on lactate transporters (6) to provide an unfavorable environment for surrounding normal cells, some pyruvate is directed to mitochondria via the pyruvate transporter (7), to provide substrates for the tri-carboxylic acid (TCA) cycle. Citrate produced by this cycle then exits the mitochondria on the citrate transporter (8) to help synthesize membrane components (phospholipids and cholesterol) that are essential for tumor proliferation.
Discussed below are the key enzymes and transporters that help support the pivotal role of HK II in promoting the malignant phenotype.
The glucose transporter
In order to satisfy the enhanced glucose metabolism of malignant tumors, their plasma-membrane glucose uptake requirements need to be met. Glucose uptake in mammalian tissues is achieved by a set of five transmembrane transporters (Pauwels et al., 1998; Smith, 1999; Macheda et al., 2005), termed Glut (glucose transporter) 1–5, which are encoded by different Glut genes. Similar to the HK isoforms, the Glut isoforms also differ in their transport kinetics. Increased glucose transport in malignant tumors has been associated with increased and deregulated expression of these transporters, mostly with over-expression of the Glut-1 isoform. In human tumors, a high level of Glut-1 expression has been associated with poor prognosis (Macheda et al., 2005).
As Glut expression at the cell-surface is mediated by hormone-induced cycling of transporter vesicles between intracellular pools and the cell membrane, disregulated trafficking may contribute also to an enhanced display of Glut on malignant tumors thus facilitating enhanced glucose uptake (Smith, 1999). Finally, much more needs to be learned about the role of Gluts in highly malignant tumors as it relates to how the key transporter(s) involved deliver glucose to HK II bound to VDAC of the mitochondrial outer membrane. Figure 1 suggests that the glucose must diffuse through quite a distance, an unlikely scenario. Considering that highly malignant cancer cells and the mitochondria within them are not static, but likely dynamic, it does not seem unreasonable to suggest that the Glut on the cell membrane and HK II bound to VDAC on mitochondria may come into contact. Although several lines of evidence via confocal microscopy and immunostaining indicate that glyceraldehyde-3-phosphate dehydrogenase (GAPDH), aldolase (ALD), phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH) are membrane associated in oxygenated red-blood cells (RBCs; which lack mitochondria) (Campanella et al., 2005), it would appear that association of HK or Gluts in such a complex has not been examined in detail to date either in RBCs or cancer cell lines.
The voltage-dependent anion channel
The first detailed indications of a close relationship between mitochondria and HK were revealed over two decades ago (Rose and Warms, 1967, 1982; Bustamante and Pedersen, 1977; Bustamante et al., 1981; Nakashima et al., 1986). Significantly, the latter study by Nakashima et al. was the first to demonstrate an interaction between HK II and the pore-like protein VDAC located on the outer mitochondrial membrane. Voltage-dependent anion channel is now known to exist in several different isoforms that are abundantly expressed and localized in the outer membrane of eukaryotic mitochondria (Blachly-Dyson et al., 1993; Bay and Court, 2002; Cesar and Wilson, 2004; Colombini, 2004) where they allow for the movement of small (molecular weight <1500) metabolites across the membrane (Kropp and Wilson, 1970; Felgner et al., 1979; Wilson et al., 1983; Tedeschi et al., 1989; Arora et al., 1992; Sui and Wilson, 1997; Cesar and Wilson, 1998, 2004).
Voltage-dependent anion channels form the primary channel for movement of adenine nucleotides through the outer membrane, and serve as the mitochondrial binding site for both HK and glycerol kinase, among other mitochondria-bound proteins. Thus, the HK–VDAC binding interaction is crucial for the supply of ATP to the phosphorylation reaction, where mitochondrial oxidative phosphorylation is closely coupled to the glycolytic pathway via direct channeling of ATP through VDAC to the bound HK (Arora and Pedersen, 1988).
Enhanced expression of VDAC on tumor mitochondria in comparison to normal tissue has also been observed (Shinohara et al., 2000). Although several isoforms of VDAC are known, they do have similar kinetic characteristics, which indicate that the contribution of VDAC to enhanced HK binding and glucose phosphorylation is due to quantitative differences in binding site availability (Shinohara et al., 2000). The predominant form of VDAC expressed on mitochondria and known to interact with HK is VDAC-1. Most accessory proteins that modulate apoptosis via their interactions with mitochondria also use VDAC-1 as their anchor. Therefore, unless specified otherwise, the VDAC referred to within this review is VDAC-1.
Recent studies show that the HK–VDAC interaction is critical for preventing induction of apoptosis in tumors (Pastorino and Hoek, 2003; Vyssokikh and Brdiczka, 2004). In contrast, overexpression of VDAC, or disruption of HK–VDAC binding via mutagenesis of key amino acids on VDAC, significantly enhances induction of apoptosis in tumors (Zaid et al., 2005). Presumably, in its antiapoptotic role, HK binding to VDAC alters the latter’s conformation to a ‘closed’ state which prevents Ca2+ dependent induction of the mitochondrial permeability transition (Azoulay-Zohar et al., 2004) that enhances the permeability of the inner mitochondrial membrane. However, published evidence to date indicate contradictory hypotheses for the involvement of VDAC in promoting apoptosis, where either the ‘closed’ (Shimizu et al., 1999, 2000a) or the ‘open’ conformations of VDAC are reported to be involved. In the latter case, the opened VDAC is proposed to restore metabolic exchange across the outer mitochondrial membrane while preventing the release of cytochrome c (Vander Heiden et al., 1999, 2001; Majewski et al., 2004a).
With regard to the enhanced glycolysis in tumors, an alternate view has also been put forward, where suppression of mitochondrial function owing to closure of porins is proposed to be responsible (Lemasters and Holmuhamedov, 2006). According to this hypothesis, HK binding to VDAC inhibits its conductance and thus suppresses mitochondrial function while stimulating glycolysis. Additionally, the glucose-6-phosphate mediated release of HK from VDACs is proposed to be an escape mechanism to prevent ‘runaway’ glycolysis (Lemasters and Holmuhamedov, 2006). However, this view makes it difficult to explain the well-observed phenomenon of direct access of mitochondrial generated ATP to the VDAC-bound HK (Arora and Pedersen, 1988), and thus needs further examination.
The mitochondrial ATP synthase and adenine nucleotide transporter
Hexokinase bound to VDAC preferentially accesses and utilizes mitochondrial generated ATP and not ATP generated by substrate-level phosphorylation in the cytosol (Arora and Pedersen, 1988; Shinohara et al., 1997; Cesar and Wilson, 1998). Therefore, for ATP to be delivered to HK II bound to VDAC on the outer membrane of mitochondria within cancer cells, the ATP must be made on the ATP synthase facing the matrix side of the inner membrane, transported out of the mitochondria via the adenine nucleotide transporter ANT, and finally delivered to VDAC on the outer membrane (Figure 1). Here, the ATP must channel through this pore-like protein to the active site of bound HK II, and perhaps in some tumors also to the active site of HK I. Ironically, the ‘substrate channeling’ path taken by ATP is completely unlike that of glucose, the other HK II substrate that accesses the active site of HK II bound to VDAC from the cytoplasm. It is likely that the mitochondrial ‘substrate channeling’ path is facilitated by the close association of ANT with the ATP synthase, a recent discovery made in work conducted in our laboratories (Ko et al., 2003; Chen et al., 2004). As is the case for the ATP synthase, ANT is also present in abundant amounts in the inner mitochondrial membrane (Chevrollier et al., 2005). In fact, three ANT isoforms (ANT-1–3) are known to be present in human tissues (Chevrollier et al., 2005) with ANT-2 being the predominantly expressed isoform in malignant tissues (Chevrollier et al., 2005). Perhaps ANT-2 has the optimal kinetic properties for ATP/ADP translocation across the inner mitochondrial membrane, an issue that needs further investigation.
Genetic and epigenetic events related to hexokinase II and its partners in cancer promotion
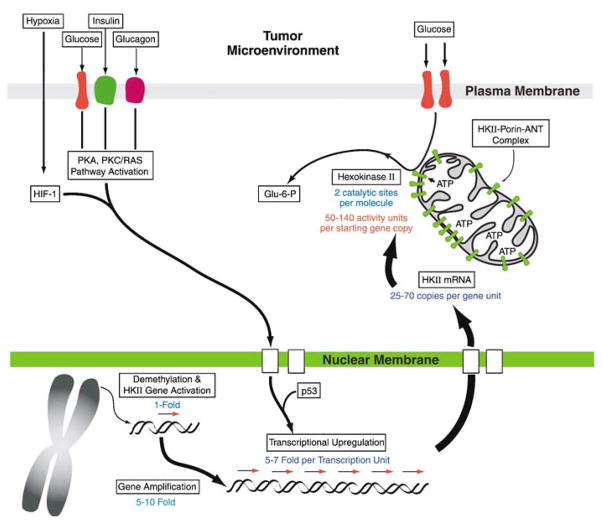
The respective HK isozymes and transporter isoforms that are implicated in enhancing glucose phosphorylation with concomitant upregulation of glycolysis in malignant tumors are encoded at different chromosomal loci. For example, the human hexokinase isoforms HK I, II, III and IV are located on chromosome arms 10q22, 2p13, 5q35, and 7p15, respectively. None of the protein isoforms, that is, HKs, Gluts, VDACs, or adenine nucleotide translocators appears to result from alternate exon splicing events or chromosomal rearrangements/deletions. Therefore, these finding indicate that genetic events, for example, gene duplication, and/or epigenetic events, for example, demethylation, may play a role in activating that sets of genes of interest here, in particular HK II that is ‘switched-on’ during tumorigenesis and tumor progression (Figure 2).
Figure 2.
Tumors harness a multitude of genetic, epigenetic, transcriptional and post-translational strategies for enhanced expression and function of hexokinase (HK) II. During tumorigenesis of tissues where HK II is absent, the gene may be first brought out of its hibernation by demethylation, and then amplified 5–10-fold. Subsequently, the highly promiscuous promoter of the gene, which is activated by HIF-1, p53, glucose, and by both insulin and glucagon, further facilitates the tumor’s requirements regardless of the nutritional status of the tumor-bearing host, and fuels the enhanced and continued synthesis of the gene product. In contrast to other hexokinase isoforms, HK II harbors two active sites per enzyme moiety. As much as a 100-fold amplification of the enzyme may be observed in malignant tumors owing to these different processes. The mitochondrial voltage-dependent anion channel (VDAC)-bound localization of HK II further facilitates enzyme activity by allowing HK II to escape product inhibition, likely via conformational constraints.
Recently, epigenetic events leading to activation or silencing of alternate HK gene isoforms have been described during hepatocarcinogenesis. As noted before, HK IV (glucokinase) is exclusively expressed in normal liver, whereas the HK II, and to a lesser extent HK I are highly expressed in malignant hepatomas (Pedersen, 1978; Pedersen et al., 2002). Methylation restriction endonuclease analysis of normal hepatocytes and hepatoma cells has indicated differential methylation patterns in the HK II gene promoter during tumorigenesis where the HK isozyme expression shifts from HK IV, that exhibits a low affinity for glucose, to the HK II and HK I that exhibit a high affinity for glucose (Goel et al., 2003). Thus, bisulfite methylation footprint analysis revealed 18 methylated CpG sites within a CpG island (−350 to +781 bp) in the hepatocyte HKII gene, but none in the hepatoma. In addition, when a hepatocyte cell line was treated with DNA methyl-transferase inhibitors, that is, 5′-azacytidine and 5′-aza-2′-deoxycytidine, or, were transfected with a DNA demethylase, basal levels of expression of HK II mRNA and protein were observed (Goel et al., 2003). Thus, these observations indicate that one of the initial events in activating the HK II gene during either transformation or tumor progression may reside at the epigenetic level.
A first indication that gene amplification plays a role in enhanced expression of HK isozymes with a low Km for glucose (high apparent affinity) during tumorigenesis was demonstrated with Southern blot analysis and fluorescence in situ hybridization of hepatocytes and a hepatoma cell line. Here, enhanced HK II expression was observed to be associated with at least a fivefold amplification of the HK II gene relative to that of normal hepatocytes (Rempel et al., 1996a). This gene amplification was located intra-chromosomally, and most likely occurs at the site of the resident gene. No rearrangement of the gene was detected. Thus, these findings revealed that gene amplification plays a key role in overexpression of the low Km (high apparent affinity) HK II isoform in a highly malignant tumor expressing the high glycolytic phenotype. Whether this will prove to be the case in other highly malignant tumors, or whether such tumors have devised multiple strategies for assuring enhanced glucose utilization, remains to be established.
Transcriptional and post-transcriptional events related to hexokinase II in cancer
Evidence for the involvement of enhanced transcription of HK II in a malignant tumor expressing the high glycolytic phenotype came first from mRNA analysis via Northern blots (Johansson et al., 1985; Rempel et al., 1994a, b; Mathupala et al., 1995). Here, an approximately 100-fold enhancement of the message was observed over the background mRNA signal. Thus, these studies strongly implicated HK II promoter activation and upregulation during tumorigenesis.
Sequence analysis of the HK II promoter revealed well-defined cis-elements for transcription initiation (TATA and CAAT elements), and cis-elements for activation by protein kinase-A (PKA) and protein kinase-C (PKC/RAS) pathways (Mathupala et al., 1995; Rempel et al., 1996b; Lee and Pedersen, 2003). Significantly, functional response elements for hypoxia (HIF-1), and p53 were located on the distal region of the promoter with the proximal region also being signficantly implicated in the hypoxic response (Mathupala et al., 1997a, 2001). Reporter gene analysis also revealed an activation of the HK II promoter by glucose and the opposing metabolic hormones insulin and glucagon as well as cAMP analogs, the latter implicating the involvement of PKA and PKC pathways (Mathupala et al., 1995; Rempel et al., 1996b). As the exact cis-elements for glucose and insulin response are unknown, their precise locations on the HK II promoter remain to be identified.
Activation of the HK II gene by glucagon at first appears paradoxical based on our knowledge of the role of this hormone in normal tissues where it opposes the action of insulin. However, from the point-of-view of tumor survival the preferred over expression of the HK II isoform makes ‘metabolic sense’, as now, the tumor can continue to synthesize HK regardless of the metabolic status of the host. Had the expression of a low Km HK been the only requirement of cancer cells, expression of HK I and HK III isozymes likely would have been observed as well, a phenotype that has not been encountered in studies to date. Therefore, it can be inferred that even at the terminal stages of cancer progression in the cachexic patient, the tumor will continue to harness glucose from the patient’s body and thrive whereas the rest of the body systematically shuts down.
Finally, from the above discussion it seems clear that the presence of a multitude of cis-elements, or the lack thereof, between HK II and HK I promoters provides an explanation for the predominant expression of HK II in malignant tumors that exhibit the high glycolytic phenotype. This view is further supported by alignment of the sequence of the proximal promoter region of rat hepatoma HK II with that of HK I (Lee and Pedersen, 2003). Therefore, the HK II promoter seems ideally tailored to provide an enhanced response to microenvironmental stimuli, resulting in greater HK II synthesis.
A key role of hexokinase II in cancer cell survival by suppressing cell death
In a given highly glycolytic tumor, a predominant fraction of the HK II is localized on the mitochondria with the enzyme anchored to the VDAC protein via an N-terminal-binding domain. A variety of stimuli, both metabolic and signal-transduction related, have been implicated in regulating this HK II or HK I/VDAC interaction, including intracellular lactate, ATP/ADP and glucose/glucose-6-phosphate metabolite couples, pH, and the signaling cascades involving protein kinase-B (PKB/Akt) (Graham et al., 1985; Gauthier et al., 1990; Miccoli et al., 1996). Although once viewed only as a reason for metabolites like glucose to obtain preferred access to mitochondrial generated ATP, this HK–mitochondrial interaction is now believed to be a key component that regulates the cellular apoptotic signaling cascades that ultimately decide the fate of a tumor, as well as that of the host.
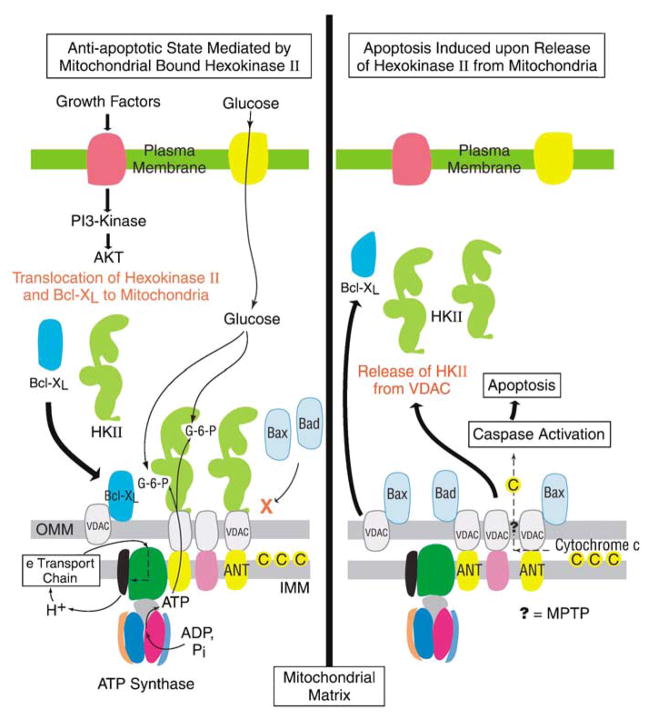
Akt (protein kinase B), a serine/threonine kinase, is a key mediator of various metabolic pathways including glycolysis (Gottlob et al., 2001; Elstrom et al., 2004; Plas and Thompson, 2005), where it imparts its effects via protein–protein interactions. Akt in turn is activated by the upstream phosphoinositide 3-kinase (PI 3-kinase) pathway, which is stimulated by growth factor signaling. As illustrated in Figure 3, Akt is also known to be a potent effector of antiapoptotic stimuli in tumors (Gottlob et al., 2001), which are modulated by two mechanisms; (1) induction of HK–VDAC binding to enhance the mitochondrial-bound HK fraction and (2) mobilization of the antiapoptotic Bcl2 member Bcl-XL also to VDAC on mitochondria (Zhou et al., 2005).
Figure 3.
Mitochondrial-bound hexokinase (HK) II plays a major role in preventing tumor apoptosis. Right: Without control mechanisms in place to prevent it, cell death would be highly likely within the unfavorable conditions that exist in a tumor microenvironment. Thus, caspase-mediated induction of apoptosis would be facilitated first by activation of the mitochondrial permeability transition pore complex (MPTP), indicated on the right by a question mark (?), that in turn would facilitate the release to the cytoplasm of the caspase activator cytochrome c (located within the inter-membrane space). Bcl-2-related proteins (Bax and Bad) would likely overcome effects of the MPTP inhibiting protein Bcl-XL and help facilitate release of cytochrome c. Left: By populating mitochondrial voltage-dependent anion channels (VDACs) with HK II and by persistent channeling of adenine nucleotides, opening of the MPTP is inhibited. This in turn inhibits access of VDACs to Bax and Bad, and most likely maintains cytochrome c in a state favorable for its mitochondrial retention in the inter-membrane space. Thus, HK II helps assure a highly malignant tumor’s proliferation, and its escape from cell death, under conditions that would otherwise favor this process. (The authors recognize that some aspects of this figure remain open to discussion and will necessitate additional studies to verify, modify or negate.)
Voltage-dependent anion channel-bound HK (predominantly HK II in cancers) is thought to prevent apoptosis via several mechanisms whereby the formation of the mitochondrial permeability transition pore complex (MPTP) is inhibited. Binding of HK to VDAC reduces the availability of free VDAC sites that can interact with activated proapoptotic molecules such as Bax (Pastorino et al., 2002; Capano and Crompton, 2002). It prevents localization of other activated proapoptotic molecules such as Bad to the outer mitochondrial membrane, and their oligomerization necessary for activation of the MPTP, and finally, its interaction with VDAC prevents formation of a MPTP by altering the conformation of the VDAC-ANT protein complex.
The efficacy of Akt as an antiapoptotic signaling molecule arises mainly due to its effect on HK-VDAC binding. In fact, secondary disruption of the HK–VDAC interaction via non-Akt involved pathways, even in the absence of activation of proapoptotic factors such as Bax and Bak, induces apoptosis (Majewski et al., 2004a). Although the precise mechanism is unknown, this is believed to occur via modulation of the ‘HK-free’ VDAC-ANT channel (the purported mitochondrial permeability transition pore) with concomitant oligomerization of proapoptotic proteins in the outer mitochondrial membrane resulting in release of mitochondrial cytochrome c to the cytoplasm with subsequent activation of cellular caspases (Figure 3). Thus, maintenance of the HK–VDAC interaction is critical for the antiapoptotic phenotype of these tumor cells.
The proapoptotic factors Bax and Bak are activated by their upstream regulator tBid, a proteolytically processed truncated form of Bid, another of the apoptotic family of proteins (Wei et al., 2000, 2001; Majewski et al., 2004b). Opening of the MPTP by activated Bax or Bak has been variously described in the literature as due to either oligomerization of these factors within the mitochondrial membranes, or due to their interactions with VDAC-1 or VDAC-2, respectively (Cheng et al., 2003; Granville and Gottlieb, 2003). Regardless of the actual type of interaction, the VDAC-bound HK is now known to prevent induction of apoptosis by Bax and Bad by preventing their activation/oligomerization by tBID (Majewski et al., 2004a, b). Finally, ectopic expression of only the N-terminal domain of HK II, which alone can maintain its catalytic activity and contains the mitochondrial-binding domain, can still antagonize tBID (Majewski et al., 2004b). Thus, all these studies point to a key role of VDAC-bound HK in malignant cells in promoting the remodeling of the MPTP and the suppression of apoptosis.
A recent study has also indicated that in liver cells a complex between glucokinase (the high Km isozyme among HKs that is predominantly expressed in the liver, and long known to be cytoplasmic) and Bad exists that is bound to the mitochondria (Danial et al., 2003). Thus a glucokinase–Bad–VDAC interaction may indicate a role for Bad in integrating pathways of glucose metabolism and apoptosis in liver tissue. However, a more recent report challenges this view of a glucokinase-mitochondrial interaction, as the latter authors have been unable to identify mitochondria-bound glucokinase (Bustamante et al., 2005). Thus, more studies are needed to clarify this novel association and its implied metabolic consequences.
The MPTP and the hexokinase-Voltage-dependent anion channel complex in cancer
The primary initializing event during induction of cellular apoptosis is the alteration in permeability of the mitochondrial membranes, which cause the release of cytochrome c from the apical surface of the mitochondrial inner membrane into the cytoplasm. Released cytochrome c activates cellular caspases resulting in apoptotic cell death. Members of the BcL-2 family of proteins, which are either antiapoptotic (e.g. Bcl, Bcl-XL) or proapoptotic (e.g. Bax, Bak, Bad), regulate this process by their interactions with the complex of proteins/protein channels that control the mitochondrial membrane permeability. Current literature points to the involvement of ANT and VDAC in this process (Crompton, 1999; Vyssokikh et al., 2002; Vyssokikh and Brdiczka, 2003), where various models have been proposed to explain the phenomenon. In one, the opening of both VDAC and ANT is proposed to precede the mitochondrial permeability transition and release of cytochrome c. Here, Bax and Bak interact with VDAC to accelerate its opening, whereas Bcl-XL interacts with VDAC directly to close it (Figure 3). Thus, whereas Bax and Bak facilitate cytochrome c release through VDAC, the passage is prevented by BCL-XL. For example, in a yeast model, VDAC-1-deficient mitochondria did not exhibit Bax or Bak-induced MPT and cytochrome c release, indicating that these proapoptotic proteins bind to VDAC in order to regulate the MPT and the release of cytochrome c during apoptosis (Shimizu et al., 1999, 2000b). When HK is released from VDAC via manipulation of glucose-6-phosphate levels or with compounds that disrupt the VDAC–HK interaction, tumor cells rapidly undergo apoptosis under a variety of stimuli which were previously ineffective in inducing apoptosis (Pastorino et al., 2002; Pastorino and Hoek, 2003; Azoulay-Zohar et al., 2004). Thus, these studies support the role that HK plays in inhibiting apoptosis by binding to VDAC.
How does the binding of HK to VDAC inhibit opening of the MPTP? One possibility is the interaction between VDAC and ANT, where changes in the conformation of VDAC also alters the conformation of the ANT channel between two states, of which one is conducive to specific sequestration of cytochrome c and release (Crompton, 1999; Vyssokikh and Brdiczka, 2003). Thus, binding of HK to VDAC may inhibit the MPTP formation in at least two ways; (1) HK binding first changes the conformation of VDAC, which in turn alters the conformation of ANT that is not conducive for formation of a MPTP; (2) VDAC occupied by HK prevents Bax and other proapoptotic proteins binding to it, which in turn prevents conformational changes in ANT that favors formation of the MPTP. Thus, occupation of VDAC by HK may initiate a series of molecular changes in key proteins in the inner mitochondrial membrane that, in turn, prevent the creation of a MPT pore complex.
Not examined to date is whether the continuous and enhanced flux of ATP and ADP through ANT and VDAC during HK-mediated glucose phosphorylation on the outer mitochondrial membrane also plays a role in maintaining ANT-VDAC conformations in a state that prevents initiation of a MPT pore. Under such conditions, the ANT pore will oscillate between states that are necessary for ATP/ADP flux, but not conducive for cytochrome c release. However, recent studies with ANT knockout mice have indicated that MPT can occur even in the absence of ANTs. Thus, the exact role of these translocators in facilitating apoptosis remains to be elucidated (Kokoszka et al., 2004).
Hexokinase–mitochondrial interactions as a future anticancer drug target
As implied above, malignant tumors are defined by their uncontrolled proliferative capacity and their resistance to apoptosis. Nevertheless, such tumors will not be able to prevent apoptotic cell death once permeabilization of the mitochondrial inner membrane is initiated as this will result in disruption of the HKII–VDAC (or HK I–VDAC) complex. This in turn will result in altered conformations in the channel proteins as well as disruption of optimal glycolytic energy generation. Therefore, this new knowledge derived from basic science studies is likely to lead to many new strategies in the future for targeting highly glycolytic/PET scan-positive human tumors (Don and Hogg, 2004).
Such strategies may involve direct inhibition of the synthesis of HK isoforms. This can be accomplished via siRNA-mediated gene silencing techniques (Sui and Wilson, 2004). Additionally, what might be employed are small-molecule drugs such as Lonidamine (Fanciulli et al., 1996; Floridi et al., 1998) that inhibit HK activity, or analogs of the azole derivatives Clotrimazole [1-(alpha-2-chlorotrityl) imidazole] and Bifonazole [1-(alpha-biphenyl-4-ylbenzyl) imidazole] that act by interfering with HK–VDAC binding (Penso and Beitner, 1998), releasing the enzyme from mitochondria to the cytosol. Thus, apoptotic events may be promoted by either direct inhibition of HK activity, or via disruption of the ‘guardian-role’ mitochondria-bound HK plays in inhibiting induction of apoptosis.
An alternative strategy to target highly glycolytic/PET scan-positive human tumors is via disruption of the HK–mitochondrial energy flux with small molecule reactive substrate or product analogs. In animal models, the use of a halogenated pyruvate derivative (3-bromopyruvic acid), has shown high efficacy against advanced stage malignant tumors (Ko et al., 2001; Geschwind et al., 2002, 2004; Ko et al., 2004) by inhibiting both glycolysis and mitochondrial energy generation, without any adverse side effects on the animals. In fact, in the most recent report, advanced stage cancers were eradicated by 3-bromopyruvate in 19 out of 19 animals without any recurrence (Ko et al., 2004).
Conclusions
Understanding at a molecular level both metabolic and signaling events related to mitochondrial-bound HK (HK II and to some extent HK I) has provided compelling evidence for how highly glycolytic/PET scan-positive cancers have become immortalized. Hexokinase–mitochondrial interactions, once considered only necessary to facilitate and maintain a highly glycolytic rate in malignant tumors, have now been shown to be crucial also for tumor survival. Thus, mitochondrial-bound HK, now recognized as acting as both a facilitator and gatekeeper of the malignant state in many cancers will by necessity become one of the primary targets of those focused on eradicating not one or even a few cancer types, but all cancers that are shown clinically to be PET scan positive.
Acknowledgments
SPM is supported by Grant IRG-85-003-14 from the American Cancer Society and a grant from the LEARN Foundation, Michigan, YHK by Grant BCTR0402523 from the Susan Komen Breast Cancer Foundation, and PLP by NIH Grants R01CA08018 and R01CA010951.
References
- Ardehali H, Yano Y, Printz RL, Koch S, Whitesell RR, May JM, et al. J Biol Chem. 1996;271:1849–1852. doi: 10.1074/jbc.271.4.1849. [DOI] [PubMed] [Google Scholar]
- Arora KK, Parry DM, Pedersen PL. J Bioenerg Biomembr. 1992;24:47–53. doi: 10.1007/BF00769530. [DOI] [PubMed] [Google Scholar]
- Arora KK, Pedersen PL. J Biol Chem. 1988;263:17422–17428. [PubMed] [Google Scholar]
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. Biochem J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay DC, Court DA. Biochem Cell Biol. 2002;80:551–562. doi: 10.1139/o02-149. [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ER, Adelman J, et al. J Biol Chem. 1993;268:1835–1841. [PubMed] [Google Scholar]
- Bustamante E, Morris HP, Pedersen PL. J Biol Chem. 1981;256:8699–8704. [PubMed] [Google Scholar]
- Bustamante E, Pedersen PL. Proc Natl Acad Sci USA. 1977;74:3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante E, Pediaditakis P, He L, Lemasters JJ. Biochem Biophys Res Commun. 2005;334:907–910. doi: 10.1016/j.bbrc.2005.06.174. [DOI] [PubMed] [Google Scholar]
- Campanella ME, Chu H, Low PS. Proc Natl Acad Sci USA. 2005;102:2402–2407. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capano M, Crompton M. Biochem J. 2002;367:169–178. doi: 10.1042/BJ20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesar MC, Wilson JE. Arch Biochem Biophys. 1998;350:109–117. doi: 10.1006/abbi.1997.0497. [DOI] [PubMed] [Google Scholar]
- Cesar MC, Wilson JE. Arch Biochem Biophys. 2004;422:191–196. doi: 10.1016/j.abb.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Chen C, Ko Y, Delannoy M, Ludtke SJ, Chiu W, Pedersen PL. J Biol Chem. 2004;279:31761–31768. doi: 10.1074/jbc.M401353200. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Chevrollier A, Loiseau D, Chabi B, Renier G, Douay O, Malthiery Y, et al. J Bioenerg Biomembr. 2005;37:307–317. doi: 10.1007/s10863-005-8642-5. [DOI] [PubMed] [Google Scholar]
- Colombini M. Mol Cell Biochem. 2004;256–257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- Crompton M. Biochem J. 1999;341(Part 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, et al. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Don AS, Hogg PJ. Trends Mol Med. 2004;10:372–378. doi: 10.1016/j.molmed.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Fanciulli M, Valentini A, Bruno T, Citro G, Zupi G, Floridi A. Oncol Res. 1996;8:111–120. [PubMed] [Google Scholar]
- Felgner PL, Messer JL, Wilson JE. J Biol Chem. 1979;254:4946–4949. [PubMed] [Google Scholar]
- Floridi A, Bruno T, Miccadei S, Fanciulli M, Federico A, Paggi MG. Biochem Pharmacol. 1998;56:841–849. doi: 10.1016/s0006-2952(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Gauthier T, Denis-Pouxviel C, Murat JC. Int J Biochem. 1990;22:419–423. doi: 10.1016/0020-711x(90)90146-t. [DOI] [PubMed] [Google Scholar]
- Geschwind JF, Georgiades CS, Ko YH, Pedersen PL. Expert Rev Anticancer Ther. 2004;4:449–457. doi: 10.1586/14737140.4.3.449. [DOI] [PubMed] [Google Scholar]
- Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Cancer Res. 2002;62:3909–3913. [PubMed] [Google Scholar]
- Goel A, Mathupala SP, Pedersen PL. J Biol Chem. 2003;278:15333–15340. doi: 10.1074/jbc.M300608200. [DOI] [PubMed] [Google Scholar]
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JF, Cummins CJ, Smith BH, Kornblith PL. Neurosurgery. 1985;17:537–542. doi: 10.1227/00006123-198510000-00001. [DOI] [PubMed] [Google Scholar]
- Granville DJ, Gottlieb RA. Curr Med Chem. 2003;10:1527–1533. doi: 10.2174/0929867033457214. [DOI] [PubMed] [Google Scholar]
- Johansson T, Berrez JM, Nelson BD. Biochem Biophys Res Commun. 1985;133:608–613. doi: 10.1016/0006-291x(85)90948-9. [DOI] [PubMed] [Google Scholar]
- Ko YH, Delannoy M, Hullihen J, Chiu W, Pedersen PL. J Biol Chem. 2003;278:12305–12309. doi: 10.1074/jbc.C200703200. [DOI] [PubMed] [Google Scholar]
- Ko YH, Pedersen PL, Geschwind JF. Cancer Lett. 2001;173:83–91. doi: 10.1016/s0304-3835(01)00667-x. [DOI] [PubMed] [Google Scholar]
- Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Biochem Biophys Res Commun. 2004;324:269–275. doi: 10.1016/j.bbrc.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp ES, Wilson JE. Biochem Biophys Res Commun. 1970;38:74–79. doi: 10.1016/0006-291x(70)91085-5. [DOI] [PubMed] [Google Scholar]
- Lee MG, Pedersen PL. J Biol Chem. 2003;278:41047–41058. doi: 10.1074/jbc.M307031200. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Holmuhamedov E. Biochim Biophys Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Macheda ML, Rogers S, Best JD. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, et al. Mol Cell. 2004a;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Robey RB, Hay N. Mol Cell Biol. 2004b;24:730–740. doi: 10.1128/MCB.24.2.730-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathupala SP, Heese C, Pedersen PL. J Biol Chem. 1997a;272:22776–22780. doi: 10.1074/jbc.272.36.22776. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Rempel A, Pedersen PL. J Bioenerg Biomembr. 1997b;29:339–343. doi: 10.1023/a:1022494613613. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Rempel A, Pedersen PL. J Biol Chem. 1995;270:16918–16925. doi: 10.1074/jbc.270.28.16918. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Rempel A, Pedersen PL. J Biol Chem. 2001;276:43407–43412. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- Mayer D, Klimek F, Rempel A, Bannasch P. Biochem Soc Trans. 1997;25:122–127. doi: 10.1042/bst0250122. [DOI] [PubMed] [Google Scholar]
- Miccoli L, Oudard S, Sureau F, Poirson F, Dutrillaux B, Poupon MF. Biochem J. 1996;313(Part 3):957–962. doi: 10.1042/bj3130957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima RA, Mangan PS, Colombini M, Pedersen PL. Biochemistry. 1986;25:1015–1021. doi: 10.1021/bi00353a010. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Hoek JB. Curr Med Chem. 2003;10:1535–1551. doi: 10.2174/0929867033457269. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Shulga N, Hoek JB. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- Pauwels EK, Ribeiro MJ, Stoot JH, McCready VR, Bourguignon M, Maziere B. Nucl Med Biol. 1998;25:317–322. doi: 10.1016/s0969-8051(97)00226-6. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Biochim Biophys Acta. 2002;1555:14–20. doi: 10.1016/s0005-2728(02)00248-7. [DOI] [PubMed] [Google Scholar]
- Penso J, Beitner R. Eur J Pharmacol. 1998;342:113–117. doi: 10.1016/s0014-2999(97)01507-0. [DOI] [PubMed] [Google Scholar]
- Plas DR, Thompson CB. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- Printz RL, Osawa H, Ardehali H, Koch S, Granner DK. Biochem Soc Trans. 1997;25:107–112. doi: 10.1042/bst0250107. [DOI] [PubMed] [Google Scholar]
- Rempel A, Bannasch P, Mayer D. Biochem J. 1994a;303 (Part 1):269–274. doi: 10.1042/bj3030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel A, Bannasch P, Mayer D. Biochim Biophys Acta. 1994b;1219:660–668. doi: 10.1016/0167-4781(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Rempel A, Mathupala SP, Griffin CA, Hawkins AL, Pedersen PL. Cancer Res. 1996a;56:2468–2471. [PubMed] [Google Scholar]
- Rempel A, Mathupala SP, Pedersen PL. FEBS Lett. 1996b;385:233–237. doi: 10.1016/0014-5793(96)00399-7. [DOI] [PubMed] [Google Scholar]
- Rose IA, Warms JV. J Biol Chem. 1967;242:1635–1645. [PubMed] [Google Scholar]
- Rose IA, Warms JV. Arch Biochem Biophys. 1982;213:625–634. doi: 10.1016/0003-9861(82)90592-6. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Konishi A, Kodama T, Tsujimoto Y. Proc Natl Acad Sci USA. 2000a;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Shinohara Y, Tsujimoto Y. Oncogene. 2000b;19:4309–4318. doi: 10.1038/sj.onc.1203788. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Ishida T, Hino M, Yamazaki N, Baba Y, Terada H. Eur J Biochem. 2000;267:6067–6073. doi: 10.1046/j.1432-1327.2000.01687.x. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Sagawa I, Ichihara J, Yamamoto K, Terao K, Terada H. Biochim Biophys Acta. 1997;1319:319–330. doi: 10.1016/s0005-2728(97)00002-9. [DOI] [PubMed] [Google Scholar]
- Smith TA. Br J Biomed Sci. 1999;56:285–292. [PubMed] [Google Scholar]
- Smith TA. Br J Biomed Sci. 2000;57:170–178. [PubMed] [Google Scholar]
- Sui D, Wilson JE. Arch Biochem Biophys. 1997;345:111–125. doi: 10.1006/abbi.1997.0241. [DOI] [PubMed] [Google Scholar]
- Sui D, Wilson JE. Biochem Biophys Res Commun. 2004;319:768–773. doi: 10.1016/j.bbrc.2004.04.198. [DOI] [PubMed] [Google Scholar]
- Tedeschi H, Kinnally KW, Mannella CA. J Bioenerg Biomembr. 1989;21:451–459. doi: 10.1007/BF00762517. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Wilson JE. Arch Biochem Biophys. 1995;316:206–214. doi: 10.1006/abbi.1995.1029. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Wilson JE. Arch Biochem Biophys. 1996;329:17–23. doi: 10.1006/abbi.1996.0186. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Wilson JE. Arch Biochem Biophys. 1997;338:183–192. doi: 10.1006/abbi.1996.9850. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- Vischer U, Blondel B, Wollheim CB, Hoppner W, Seitz HJ, Iynedjian PB. Biochem J. 1987;241:249–255. doi: 10.1042/bj2410249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyssokikh M, Brdiczka D. Mol Cell Biochem. 2004;256–257:117–126. doi: 10.1023/b:mcbi.0000009863.69249.d9. [DOI] [PubMed] [Google Scholar]
- Vyssokikh MY, Brdiczka D. Acta Biochim Pol. 2003;50:389–404. [PubMed] [Google Scholar]
- Vyssokikh MY, Zorova L, Zorov D, Heimlich G, Jurgensmeier JJ, Brdiczka D. Mol Biol Rep. 2002;29:93–96. doi: 10.1023/a:1020383108620. [DOI] [PubMed] [Google Scholar]
- Warburg O, Dickens F Kaiser Wilhelm-Institut für Biologie B. The Metabolism of Tumours: Investigations. Kaiser-Wilhelm Institute for Biology; Berlin-Dahlem: Constable; London: 1930. [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, et al. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE. Rev Physiol Biochem Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- Wilson JE. Biochem Soc Trans. 1997;25:103–107. doi: 10.1042/bst0250103. [DOI] [PubMed] [Google Scholar]
- Wilson JE. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Messer JL, Felgner PL. Methods Enzymol. 1983;97:469–475. doi: 10.1016/0076-6879(83)97155-0. [DOI] [PubMed] [Google Scholar]
- Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V. Cell Death Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- Zhou H, Hou Q, Chai Y, Hsu YT. Exp Cell Res. 2005;309:316–328. doi: 10.1016/j.yexcr.2005.06.014. [DOI] [PubMed] [Google Scholar]