Abstract
Brain-derived neurotrophic factor (BDNF) modulates the synaptic transmission of several monoaminergic neuronal systems, including forebrain dopamine-containing neurons. Recent evidence shows a strong correlation between neuropsychiatric disorders and BDNF hypofunction. The aim of the present study was to characterize the effect of low endogenous levels of BDNF on dopamine system function in the caudate-putamen using heterozygous BDNF (BDNF+/−) mice. Apparent extracellular dopamine levels in the caudate-putamen, determined by quantitative microdialysis, were significantly elevated in BDNF+/− mice compared to wildtype controls (12 vs. 5 nM, respectively). BDNF+/− mice also had a potentiated increase in dopamine levels following potassium (120 mM)-stimulation (10-fold) relative to wildtype controls (6-fold). Slice fast-scan cyclic voltammetry revealed that BDNF+/− mice had reductions in both electrically-evoked dopamine release and dopamine uptake rates in the caudate-putamen. Superfusion of BDNF led to partial recovery of the electrically-stimulated dopamine release response in BDNF+/− mice. Conversely, tissue accumulation of L-3,4-dihydroxyphenylalanine, extracellular levels of dopamine metabolites, and spontaneous locomotor activity were unaltered. Together, this study indicates that endogenous BDNF influences dopamine system homeostasis by regulating the release and uptake dynamics of presynaptic dopamine transmission.
Keywords: microdialysis, fast scan cyclic voltammetry, locomotor activity, L-DOPA, dopamine transporter
INTRODUCTION
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors that regulate development, maintenance, and morphological plasticity of neuronal systems. In particular, numerous lines of evidence indicate BDNF interacts with striatal dopaminergic systems. The rodent striatum is rich in BDNF protein supplied from afferent midbrain dopamine and corticostriatal glutamate neurons (Altar et al. 1997, Conner et al. 1997, Kolbeck et al. 1999). Exogenous BDNF was shown to promote the survival and differentiation of cultured dopaminergic neurons (Hyman et al. 1991, Spina et al. 1992) associated with an overall increase in tyrosine hydroxylase (TH) activity, dopamine release, dopamine transporter (DAT) uptake capacity, and dopamine tissue content (Knusel et al. 1991, Beck et al. 1993, Hyman et al. 1994, Zhou et al. 1994, Blochl & Sirrenberg 1996, Hoover et al. 2007). Striatal in vivo infusions of BDNF locally augmented spontaneous electrical activity of midbrain dopamine neurons (Shen et al. 1994), enhanced dopamine turnover (Altar et al. 1992), and elevated activity-dependent release of dopamine (Goggi et al. 2002). Together, these studies suggest that BDNF plays a crucial role in regulating dopaminergic tone.
In line with the neuromodulatory effects of BDNF on dopaminergic function, alterations in central BDNF activity have been linked to mood-related disorders involving monoamine dysfunctions, including attention deficit-hyperactivity disorder (ADHD) (Tsai 2007) and depression (Martinowich et al. 2007). A role for BDNF in these disorders is supported by human genetic associations with BDNF polymorphisms (Kent et al. 2005, Tsai et al. 2010) and the finding that therapeutic agents, namely psychostimulants and antidepressants, modulate BDNF levels (Nibuya et al. 1996, Conti et al. 2002, Meredith et al. 2002, Molteni et al. 2006, Fumagalli et al. 2010). To understand the neuronal processes involved in this interaction, several studies have evaluated the effect of genetic reductions in BDNF on serotonin transmission. In the serotonin-rich region of the hippocampus, mice with low endogenous BDNF levels had a reduced capacity to clear serotonin (Daws et al. 2007), which was further associated with decreased serotonin transporter (SERT) function and increased extracellular serotonin levels (Guiard et al. 2008). Notably, the impact of BDNF deficiency on presynaptic dopamine dynamics in dopamine-rich regions, such as the striatum, has not similarly been characterized.
Heterozygote BDNF (BDNF+/−) mice that have a ~50% reduction in BDNF levels in several basal forebrain regions (Chourbaji et al. 2004, Szapacs et al. 2004) were utilized to investigate the role of endogenous BDNF in the regulation of striatal dopaminergic tone. Previous studies in the dorsal striatum found BDNF+/− mice had elevated tissue levels of dopamine (Dluzen et al. 1999, Dluzen et al. 2002, Joyce et al. 2004; but see Chourbaji et al. 2004), reduced dopamine D3 receptor binding (Joyce et al. 2004), and altered stimulated dopamine release (Dluzen et al. 2002, Boger et al. 2010). Other neurochemical markers of the nigrostriatal dopamine system, including TH, DAT, and the dopamine D2 receptor, were unchanged in BDNF+/− mice (Joyce et al. 2004, Boger et al. 2010). In light of these previous findings, the current study evaluated the presynaptic parameters of dopamine transmission in the caudate-putamen (CPu) of BDNF+/− mice. The complementary techniques of microdialysis and fast scan cyclic voltammetry (FSCV) were used to assess genotypic differences in extracellular concentrations of dopamine under basal and potassium-stimulated conditions as well as the dynamics of dopamine release and uptake. In addition, the effect of BDNF deficiency on dopamine biosynthesis and metabolism were evaluated by measuring the tissue content of L-3,4-dihydroxyphenylalanine (L-DOPA) and dialysate levels of dihydroxyphenylacetic acid (DOPAC) and homovallinic acid (HVA), respectively. Spontaneous locomotor activity was also assessed in light of previously conflicting results (Kernie et al. 2000, MacQueen et al. 2001, Boger et al. 2010), which were attributed to methodological and strain differences. Overall, our data demonstrate that constitutive reductions in BDNF lead to complex alterations in the dynamics of dopamine release and uptake in the CPu, which influence basal and stimulated dopamine transmission, with no apparent change in dopamine synthesis or metabolism.
MATERIALS AND METHODS
Animals
Wildtype and BDNF+/− mice were obtained from Jackson Laboratories (Bar Harbor, ME) and offspring were raised as a colony in-house. Genotype identification using PCR analysis of tail DNA was performed as previously described (Bosse & Mathews 2011). Mice were housed in groups of 3 - 4 per cage with food and water ad libitum (12-h light/dark cycle). Experiments were conducted during the light cycle (0700 – 1900 h) with 3 - 5 month old males. All procedures were designed and conducted to minimize pain and discomfort to the animals. Animal care and use was in accordance with the National Institutes of Health Animal Care guidelines and approved by the Wayne State University Institutional Animal Care and Use Committee.
In Vivo Microdialysis
The stereotaxic surgery for microdialysis was performed as previously described (Bosse & Mathews 2011). Briefly, a CMA/7 guide cannula was implanted under Avertin anesthesia (20 ml/kg, i.p.) in the CPu of mice using coordinates (in mm: A +0.8, L −1.3, V −2.5 from Bregma) determined from the mouse atlas (Paxinos & Franklin 2001) and empirical assessment. During surgery, mice were placed on a heating pad (~ 37°C) and lidocaine (0.5 mg/kg) was subcutaneously injected around the surgical site prior to incision to limit discomfort. Once implanted, the guide cannula was anchored with dental cement. Following recovery, a CMA/7 dialysis probe (2 mm membrane length, 0.24 mm membrane diameter, Cuprophane, 6 kDa cut-off) was inserted into the guide cannula and perfused overnight with artificial cerebrospinal fluid (aCSF; composition in mM: 145 NaCl, 3.5 KCl, 2 Na2HPO4, 1.0 CaCl2, 1.2 MgCl2; pH 7.4) at a flow rate of 0.4 μl/min. The next day, the flow rate was increased to 1.1 μl/min for a 1 h equilibration period and dialysate samples were collected from freely moving mice at 20 min intervals.
The quantitative microdialysis technique of zero net flux was employed to determine basal extracellular dopamine levels as previously described (Lonnroth et al. 1987, Mathews et al. 2004, Khalid et al. 2011). After three baseline dialysis samples were collected with normal aCSF, perfusate containing 5, 10, and 20 nM dopamine (prepared in aCSF with 200 μM ascorbic acid) was delivered through the microdialysis probe for 90 min each using a CMA/402 programmable gradient infusion pump. The concentration of dopamine entering the probe (DAin) was empirically determined by in vitro calibration, in which dopamine-containing aCSF (5, 10, and 20 nM) was perfused through the dialysis system in the absence of an animal.
To assess depolarization-mediated release, isosmotic aCSF containing either 60 mM KCl (composition in mM: 90.5 NaCl, 60 KCl, 2.0 Na2HPO4, 1.0 CaCl2, 1.2 MgCl2; pH 7.4) or 120 mM KCl (composition in mM: 30.5 NaCl, 120 KCl, 2.0 Na2HPO4, 1.0 CaCl2, 1.2 MgCl2; pH 7.4) was perfused through the microdialysis probe for one 20 min fraction in separate cohorts of mice. Following collection of three baseline fractions with normal aCSF, one fraction (60 – 80 min) was collected with high-potassium aCSF before switching back to normal aCSF for collection of three subsequent dialysate fractions.
Liquid chromatography and electrochemical detection
Dopamine and metabolite (DOPAC and HVA) content from dialysate samples were measured using HPLC with electrochemical detection. Dialysis samples (20 μl) were manually injected onto a Phenomenex C18 (2)-HST HPLC column (100 mm × 3 mm, 2.5 μm) for separation followed by detection using an ESA 5014B microdialysis cell (E1 = −150 mV; E2 = +220 mV). A guard cell (ESA 5020) was placed in-line before the injection loop and set at a potential of +350 mV. The mobile phase (composition in mM: 75 NaH2PO4 monohydrate, 1.4 -1.8 1-octanesulfonic acid, 0.025 EDTA, 10% acetonitrile, and 0.002% triethylamine; pH 3.0, adjusted with 85% phosphoric acid) was delivered at a flow rate of 0.4 ml/min by an isocratic Shimadzu LC-20AD HPLC pump. Characteristic retention times of DOPAC, DA, and HVA were approximately 5, 6, and 12.5 min, respectively. Analyte peak areas were integrated and quantified against known standards using LC Solutions Shimadzu Software. Following dialysis, mice were euthanized and brains were sectioned for subsequent histological confirmation of probe placement.
L-DOPA tissue content
The activity of TH, the rate-limiting enzyme in dopamine biosynthesis, was evaluated indirectly by measuring L-DOPA accumulation in tissue samples. This was accomplished with an experimental procedure similar to that previously described (Galloway et al. 1986, Budygin et al. 2005) in which L-aromatic acid decarboxylase was inhibited with 3-(hydrazineomethyl)phenol dihydrochloride (NSD-1015) and γ-butyrolactone (GBL) was used to minimize the feedback effect of autoreceptor regulation on dopamine biosynthesis mediated by endogenous dopaminergic tone. Briefly, mice were first injected with GBL (750 mg/kg, i.p.) and then 5 min later with NSD-1015 (100 mg/kg, i.p.). Mice were sacrificed 40 min after administration with NSD-1015, at which time the CPu was rapidly dissected and immediately frozen in liquid nitrogen. Tissue samples were stored at −80°C until time of analysis. To measure L-DOPA content, the tissue samples were homogenized in 0.1 M HClO4 and centrifuged for 10 min at 9,000 × g. L-DOPA was quantified from the resulting supernatant using HPLC separation with a Phenomenex Luna C18 reverse phase column (50 × 2 mm, 3 μm) and electrochemical detection with an ESA 5011A analytical cell (E1 = −150 mV, E2 = +200 mV) and ESA Coulochem III detector. Mobile phase (composition in mM: 1 EDTA, 2.4 sodium octanesulfonate, 7.8 choloracetic acid, 10 Na2HPO4, 80 citric acid, and 3% acetonitrile; pH 3) was delivered by a Shimadzu LC-20 AD isocratic pump at a rate of 0.4 ml/min. The L-DOPA peak area was integrated and quantified against known standards using LC Solutions Shimadzu Software.
Slice FSCV
The slice FSCV procedure was similar to that described in Maina & Mathews 2010. Briefly, mice were anesthetized with CO2 and their brains were rapidly removed and cooled in pre-oxygenated (95% O2/5% CO2) high sucrose-aCSF buffer (composition in mM: 180 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 D-glucose; pH 7.4) for 10 min. The brain was sectioned with a Vibratome® into 400-μm-thick coronal slices containing the CPu. Voltammetric recordings were taken from a slice transferred to a Custom Scientific submersion chamber superfused with oxygenated aCSF (composition in mM: 108 NaCl, 5 KCl, 2 CaCl2, 8.2 MgCl2, 4 NaHCO3, 1 NaH2PO4, 11 D-glucose, 0.4 ascorbic acid; pH 7.4) at 32 °C at a flow rate of 1 ml/min. For the BDNF experiments, BDNF (PeproTech Inc.) was dissolved in oxygenated aCSF for a final concentration of 100 ng/ml (Goggi et al. 2002), which was then superfused over the slice for 30 min while dopamine recordings were made every 5 min.
Glass capillary carbon fiber microelectrodes (length of 50 - 200 μm) were fabricated in-house as previously described (Maina & Mathews 2010). For dopamine detection, the potential of the carbon fiber microelectrode was held at − 0.4 V versus a Ag/AgCl reference electrode, then ramped to + 1.2 V, and back to − 0.4 V (400 V/s) at a frequency of 10 Hz. (John & Jones 2007). A carbon fiber electrode was placed ~ 75 μm below the surface of the slice, approximately 100 – 200 μm away from a stimulating tungsten electrode placed directly on the slice. Dopamine release was evoked every 5 min by a one pulse stimulation (monophasic, 350 μA, 60 Hz, and 4 ms pulse width) delivered from the stimulating electrode. All electrode and stimulation parameters were controlled by TH software (ESA Inc.) through a ChemClamp potentiostat (Dagan Corporation). The peak oxidation current for dopamine was converted into concentration based on post-electrode calibration with 3 μM dopamine. The resulting current versus time plots were fit by nonlinear regression as described by John & Jones (2007) using LabVIEW National Instruments software. A Michaelis-Menten based kinetic model (Maina & Mathews 2010) was used to evaluate the peak amplitude of release ([DA]p), dopamine uptake kinetics (maximum velocity, Vmax), and affinity of dopamine for the dopamine transporter (apparent Km) by fitting dopamine concentration versus time FSCV traces. Km values were fixed to 0.16 μM, based on an average literature value (John & Jones 2007), permitting a non-linear fit of dopamine release and uptake.
Locomotor activity
Wildtype and BDNF+/− mice were separated and singly housed the day before locomotor activity analysis. On the day of locomotor activity monitoring, animals were transported in their home-cage to the testing facility and the home-cage was immediately placed in the static exposure chambers. Animals were acclimated to the chambers for 60 min following transportation. Incorporation of this acclimation period provided an accurate measurement of baseline spontaneous locomotor activity, as it minimized the impact of any stress induced by the transportation process or novel environment. Food and water were removed from the home-cage immediately before it was placed into the chambers. Med Associates static exposure chambers monitored locomotor activity using three sets of 16-beam infrared emitter-detector arrays, two sets in the horizontal plane and one set in the vertical plane (Batis et al. 2010). Ambulatory distance was measured for a total of 120 min by the number of infrared beam breaks (binned into 10 min periods) in the horizontal plane using Open Field Activity Med Associates Software.
Data analysis
Data analysis was performed using GraphPad Prism software. All values were reported as mean ± standard error of the mean (SEM) and the criterion for statistical significance was p < 0.05. For the zero net flux regression analysis, the concentration of dopamine entering the probe (DAin) was plotted on the x-axis and the difference between the concentration of dopamine recovered from the probe (DAout) and DAin was plotted on the y-axis. The apparent interstitial concentration of dopamine (DAext) was determined from the x-intercept (Lonnroth et al. 1987) and the slope of the regression line was used to assess the relative in vivo recovery rate of dopamine, termed the dialysate extraction fraction (Ed, Smith & Justice 1994). Differences in DAext between genotypes were determined by a two-tailed Student’s t-test. The time course of potassium-stimulated extracellular dopamine was evaluated using separate two-way analysis of variance (ANOVA) with either genotype or concentration of potassium as the independent variable and time as the repeated measure. Bonferroni multiple comparison analysis was used for post-test. Area under the dopamine concentration curve (AUC ± SEM in arbitrary units) was calculated from the four 20-min samples following high-potassium perfusion (80 - 140 min) using the trapezoidal method and statistical significance for potassium treatment × genotype interaction was determined by two-way ANOVA with subsequent pair-wise comparisons by Student’s t-test (two-tailed). Differences in dopamine release and uptake measured by voltammetry were assessed with two-way ANOVA to determine the genotype X treatment (BDNF application) interaction and subsequent Student’s t-test for individual genotype or BDNF treatment comparisons. Levels of L-DOPA were normalized to milligram wet weight of brain tissue. Pair-wise comparisons using Student’s t-test (two-tailed) were made to evaluate genotypic differences on L-DOPA tissue accumulation and extracellular metabolite levels. Home cage locomotor activity was analyzed by two-way ANOVA to determine the interaction between genotype and time with time as the repeated measure.
RESULTS
Basal and stimulated extracellular concentrations of dopamine were elevated in the CPu of BDNF+/− mice
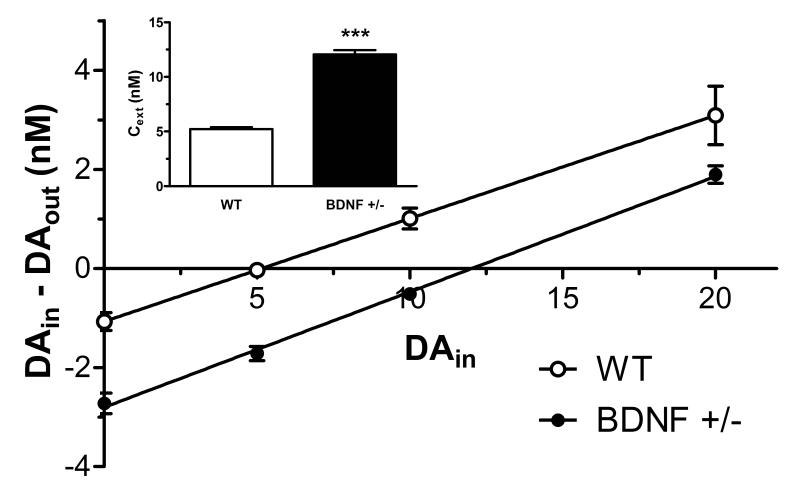
To determine the impact of low endogenous levels of BDNF on extracellular dopaminergic tone, basal and potassium-stimulated extracellular dopamine concentrations were evaluated using in vivo microdialysis in the CPu of BDNF+/− mice. Apparent basal concentrations of extracellular dopamine (DAext) and extraction fraction (Ed) values were determined from regression analysis using the zero net flux method (Lonnroth et al. 1987) for individual wildtype and BDNF+/− mice (n = 6/genotype, Fig. 1). DAext values, corrected for recovery, were significantly higher in BDNF+/− mice (12.0 ± 0.4 nM) compared to wildtype controls (5.0 ± 0.2 nM, p < 0.001), whose DAext value was consistent with that reported in literature (Jones et al. 1998, He & Shippenberg 2000) (Fig. 1, inset). However, average Ed values were similar for wildtype (0.21 ± 0.04) and BDNF+/− mice (0.23 ± 0.02).
Figure 1.
Linear regression analysis of dopamine (DA) levels in the CPu of wildtype (WT) and BDNF+/− mice determined by zero net flux. The x-intercept (point of zero net flux) represents an estimate of basal extracellular DA levels (DAext). Inset shows the mean ± SEM of apparent DAext (n = 6/group). ***p < 0.001 compared to WT mice (Student’s t-test).
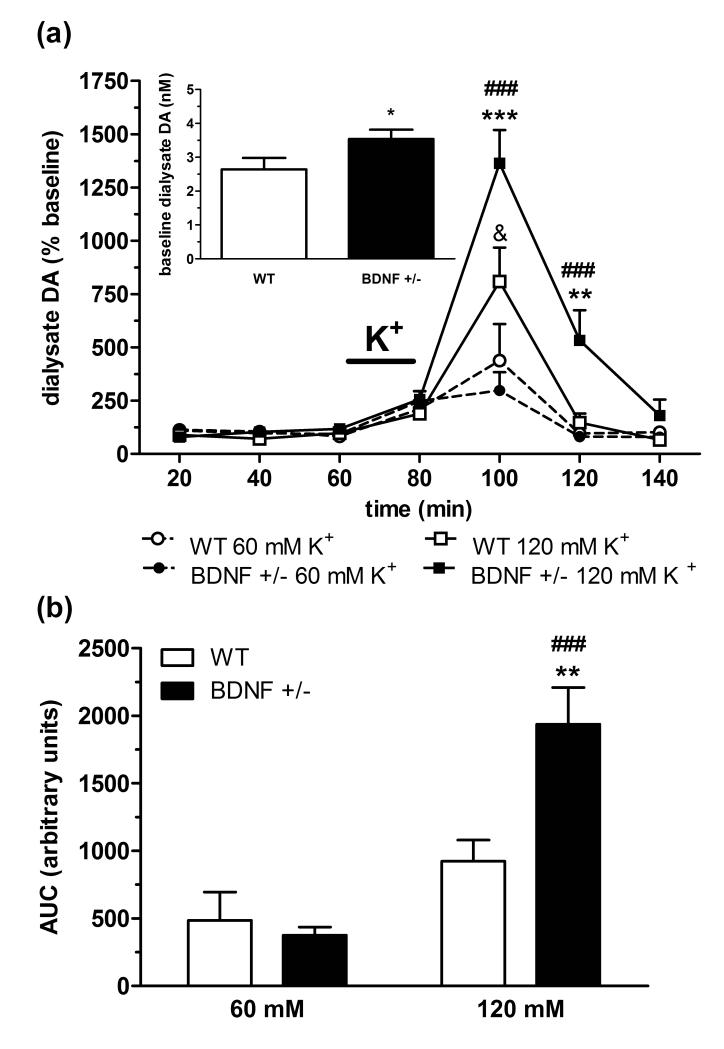
Genotypic differences in depolarization-mediated dopamine transmission induced by a moderate (60 mM) to high (120 mM) concentration of potassium were also assessed using microdialysis in wildtype and BDNF+/− mice (n = 6/group). In line with the zero net flux data (Fig. 1), the average uncorrected baseline concentration of extracellular dopamine (Fig. 2a, inset) was significantly elevated in BDNF+/− mice (3.5 ± 0.3 nM) compared to wildtype mice (2.6 ± 0.3 nM, p < 0.05). In both genotypes, there was a significant treatment × time interaction determined by two-way ANOVA analysis (wildtype: F(6,66) = 2.28, p < 0.05; BDNF+/−: F(6,68) = 16.70, p < 0.001) demonstrating that potassium produced concentration-dependent elevations in extracellular dopamine (Fig. 2a). Post-test analysis indicated that the maximal dopamine response (20-min fraction following potassium perfusion) was increased relative to baseline levels in a concentration-related manner in both wildtype (~ 480 ± 170% at 60 mM and ~810 ± 160% at 120 mM, p < 0.01) and BDNF+/− mice (~300 ± 85% at 60 mM and ~1365 ± 155% at 120 mM, p < 0.001). While increased potassium elevated dialysate dopamine levels in both genotypes, divergent genotypic differences were observed in the extent of increase at the two concentrations of potassium. With 60 mM potassium, the peak dopamine response in BDNF+/− mice was reduced relative to wildtype mice, though this was not significant by two-way ANOVA (F(6,68) = 0.49, p = 0.81). Conversely, dopamine stimulation with 120 mM potassium was significantly augmented in BDNF+/− mice compared to wildtype mice (F(6,66) = 3.77, p < 0.01). Dialysate levels of dopamine returned to baseline in most groups immediately after the peak potassium response. However, post-hoc test revealed a slower rate of decline in dopamine levels (100 – 120 min) in BDNF+/− mice (p < 0.001 vs. wildtype mice) following 120 mM potassium stimulation. Two-way ANOVA analysis of AUC values for the dopamine response following potassium stimulation also indicated a significant treatment × genotype interaction (F(1,20) = 8.72, p < 0.01) (Fig. 2b). Independent pair-wise comparisons showed that BDNF+/− mice had a significantly greater cumulative increase in dialysate dopamine following 120 mM potassium compared to wildtype mice (p < 0.01) or BDNF+/− mice after 60 mM potassium (p < 0.001), resulting from both an increased peak response and elongation of the descending phase to baseline.
Figure 2.
Potassium-evoked dopamine (DA) response in the CPu of wildtype (WT) and BDNF+/− mice. (a) Time-course of extracellular dopamine elevations following 20 min perfusion of moderate (60 mM K+, dashed line) to high (120 mM K+, solid line) potassium aCSF. Data represent mean baseline-corrected dialysate dopamine levels ± SEM (n = 6/group). &p < 0.05, compared to WT 60 mM K+ group; ###p < 0.001, compared to BDNF+/− 60 mM K+ group; **p < 0.01, ***p < 0.001, compared to WT 120 mM K+ group (two-way ANOVA). Inset shows the combined baseline values (uncorrected for probe recovery, mean ± SEM) for WT and BDNF+/− mice from both K+ treatment groups. (b) Area under the curve (AUC) of the cumulative increase in extracellular DA over four 20 min samples (80 - 140 min) following K+ perfusion. Data are mean AUC ± SEM. ###p < 0.01 compared to BDNF+/− 60 mM K+ group; **p < 0.05 compared to WT 120 mM K+ group (Student’s t-test).
Electrically-evoked striatal dopamine release and uptake are attenuated in BDNF+/− mice
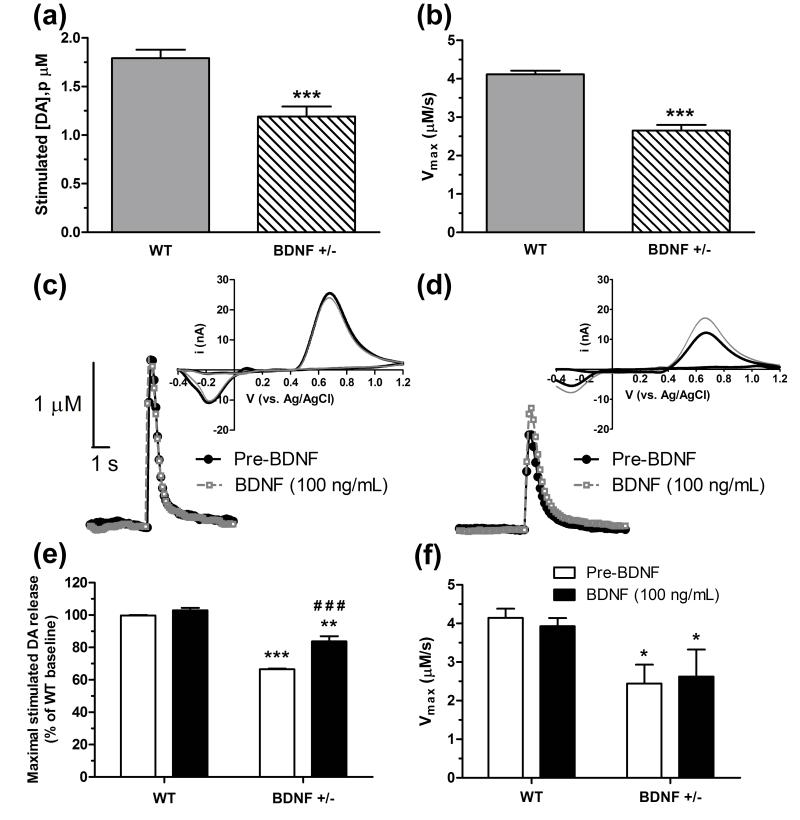
FSCV was used to examine the dynamics of single-pulse, electrically-stimulated dopamine release ([DA]p) and uptake kinetics (Vmax) in the CPu. FSCV recordings from wildtype and BDNF+/− mice revealed a genotypic alteration in both evoked release (Fig. 3a) and uptake rates (Fig. 3b) (refer to supplemental Fig. 1 for representative false color plots and corresponding concentration versus time traces from wildtype and BDNF+/− mice). Electrically-stimulated dopamine release was reduced by ~33% in BDNF+/− mice (1.2 ± 0.1 μM, n = 24) compared to wildtype mice (1.8 ± 0.1 μM, n = 22, p < 0.0001). Similarly, dopamine Vmax rate was attenuated by ~34% in BDNF+/− mice (2.7 ± 0.1 μM/s, n = 24) relative to wildtype mice (4.1 ± 0.1 μM/s, n = 22, p < 0.0001).
Figure 3.
FSCV measurements following single-pulse stimulation in dorsal CPu slices from wildtype (WT) and BDNF+/− mice. Mean ± SEM of (a) maximum electrically-evoked DA release and (b) DA uptake rates measured from untreated mice (n = 22-24/group). Representative concentration versus time traces and corresponding cyclic voltammograms (inset) are shown for (c) WT and (d) BDNF +/− mice (n = 5/group) prior to (pre-BDNF baseline, solid black line) and following 30 min perfusion with BDNF (100 ng/ml, dashed gray line). The effect of exogenous BDNF application on (e) DA release (normalized to % of WT baseline) and (f) DA uptake rates are shown as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared to WT (baseline or pre-BDNF); ###p < 0.001 compared to BDNF+/− pre-BDNF (Student’s t-test).
Numerous reports suggest that exogenously applied BDNF is able to enhance dopamine release (Altar et al. 1994, Siuciak et al. 1996, Goggi et al. 2002). To understand how exogenous BDNF influences dopamine dynamics, FSCV measurements were made before (pre-BDNF) and after local BDNF application (100 ng/ml) to CPu slices from wildtype and BDNF+/− mice (n = 5/genotype). An overlay of representative traces recorded during pre- and post-BDNF conditions are shown for wildtype (Fig. 3c) and BDNF+/− mice (Fig. 3d). Two-way ANOVA analysis of electrically-stimulated dopamine release (Fig. 3e) showed a significant main effect of treatment (F(1,36) = 33.01, p < 0.0001), genotype (F(1,36) = 218.5, p < 0.0001), and a genotype × treatment interaction (F(1,36) = 15.56, p < 0.001). Independent pair-wise comparisons revealed that exogenous application of BDNF did not alter dopamine release from pre-BDNF levels in wildtype mice (p = 0.06), but did significantly enhance the baseline release response in BDNF+/− mice by ~17% (p < 0.001). Since baseline dopamine release in BDNF+/− mice was reduced relative to wildtype values (p < 0.001), the increase observed following BDNF treatment partially restored the release response in BDNF+/− mice, as this response was still significantly attenuated compared to wildtype mice either before or after BDNF perfusion (p < 0.01). In contrast, two-way ANOVA analysis indicated that there was no differences in the main effect of BDNF treatment (F(1,36) = 0.01) or genotype X treatment interaction (F(1,36) = 0.19) on dopamine uptake rates (Fig. 3f). A significant main effect for genotype was found (F(1,36) = 10.94), confirming that the kinetics of uptake were similarly reduced in BDNF+/− mice compared to wildtype mice before and after BDNF treatment (p < 0.05).
Dopamine synthesis rates and metabolite concentrations were unchanged in BDNF+/− mice
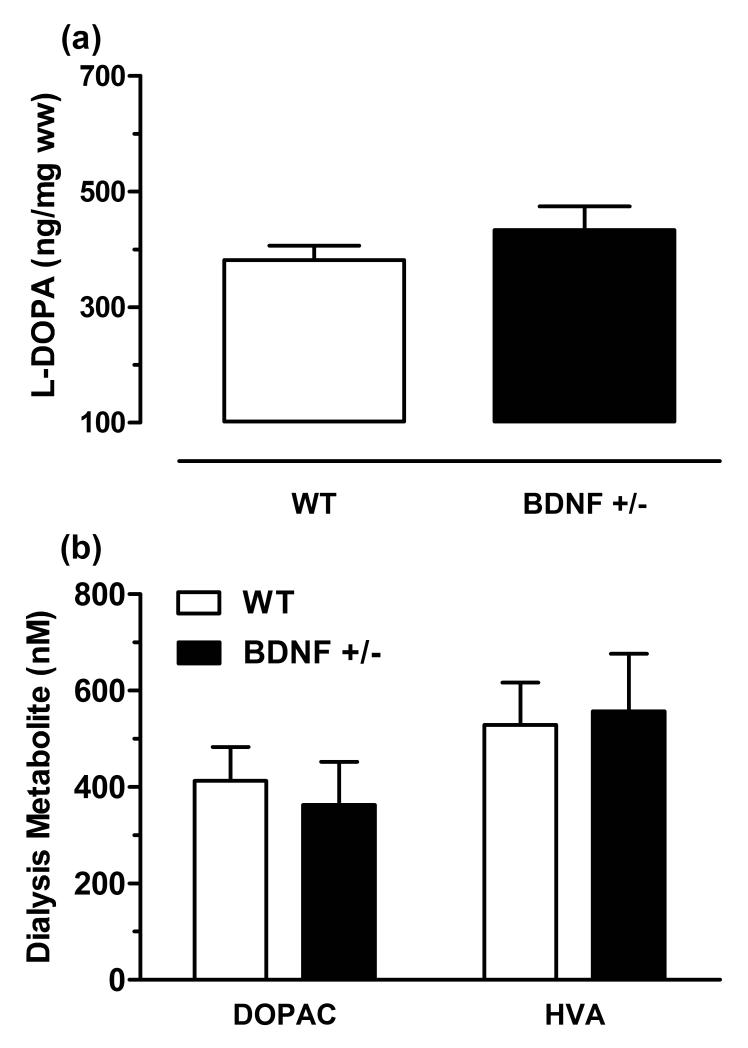
Dopamine synthesis was determined from the tissue accumulation of L-DOPA in the CPu following inhibition of aromatic acid decarboxylase (Fig. 4a). No statistical difference was detected between the average striatal tissue levels of L-DOPA in wildtype mice (380 ± 25 ng/mg wet weight, n = 13) and BDNF+/− mice (430 ± 40 ng/mg wet weight, n = 11, p = 0.28). Dopamine metabolism was evaluated by measuring the extraneuronal concentration of the dopamine metabolites, DOPAC and HVA, from baseline dialysis samples (Fig. 4b). The mean extracellular concentrations (average of three samples), determined from triplicate analysis, for both DOPAC (wildtype: 410 ± 70 nM, n = 16; BDNF+/−: 330 ± 90 nM, n = 10, p = 0.664) and HVA (wildtype: 465 ± 65 nM, n = 16; BDNF+/−: 560 ± 120 nM, n = 10, p = 0.47) were also comparable across the two genotypes. Together, these data indicate that constitutive reductions in BDNF do not result in altered rates of dopamine synthesis or metabolism.
Figure 4.
Dopamine synthesis and metabolism in the CPu of wildtype (WT) and BDNF+/− mice. (a) L-DOPA tissue accumulation following treatment with NSD-1015 and GBL. Data are means ± SEM and expressed as ng L-DOPA/mg wet weight of tissue (ww) (n = 11-13/group). (b) Extracellular levels of the dopamine metabolites, DOPAC and HVA as measured by microdialysis. Data are means ± SEM of uncorrected baseline values (n = 10-16/group).
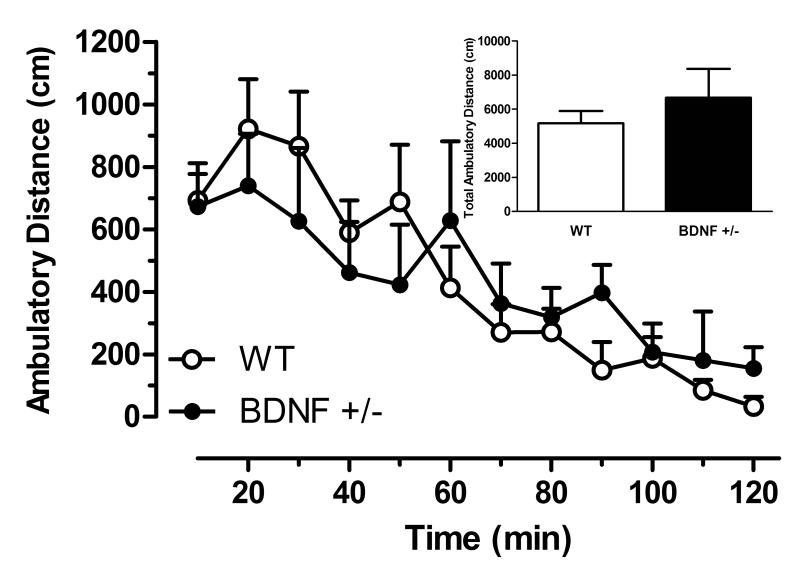
Baseline locomotor activity was comparable between wildtype and BDNF+/− mice
The behavioral impact of BDNF deficiency on spontaneous home-cage locomotor activity was determined in naïve wildtype and BDNF+/− mice using activity monitors that recorded ambulatory distance every 10 min for a total of 120 min (Fig. 5). Two-way ANOVA revealed that the genotype × time interaction was not significant (F(11,167)=0.91, n = 7-8/group, p =0.54), indicating that the time course of spontaneous locomotor activity was not different between BDNF+/− mice and wildtype littermates.
Figure 5.
Home cage locomotor activity measured in BDNF+/− and wildtype (WT) mice as distance traveled (cm) with respect to time. Data are means ± SEM (n = 7-8/group). Inset shows the total distance traveled over 120 min for each genotype.
DISCUSSION
Previous studies demonstrate that BDNF is important for maintaining the function of striatal dopamine neurons (Hyman et al. 1991, Spina et al. 1992, Dluzen et al. 2002, Joyce et al. 2004, Boger et al. 2010). Since these reports suggest that BDNF plays a crucial role in regulating dopaminergic tone, the present study utilized two complementary neurochemical techniques, in vivo microdialysis and slice voltammetry, to evaluate the influence of BDNF haplodeficiency on nigrostriatal dopamine transmission. Reductions in BDNF expression resulted in elevated basal and high (120 mM)-potassium stimulated levels of extracellular dopamine in the CPu of adult BDNF+/− mice (3 - 5 months old). Voltammetric assessment of the rapid dynamics of electrically-evoked release and uptake revealed similar reductions in both parameters in BDNF+/− mice and that exogenous addition of BDNF selectively restored the release response in BDNF+/− mice. These neurochemical changes do not appear to involve alterations in either dopamine synthesis, measured by L-DOPA accumulation, or metabolism, evident by similar extracellular levels of DOPAC and HVA. Together, these findings indicate that endogenous BDNF tonically regulates presynaptic dopamine homeostasis through apparent functional alterations in dopamine release and uptake.
The current study is the first to evaluate basal levels of dopamine in BDNF+/− mice with quantitative (zero net flux) microdialysis, revealing that BDNF-deficient mice have elevated extraneuronal dopamine levels in the CPu. A prior study in BDNF+/− mice reported that baseline dialysate levels of striatal dopamine, not corrected for probe recovery, were decreased (Boger et al. 2010). Since several factors can affect analyte recovery through the probe (e.g. perfusion rate, probe material and surface area, temperature, etc.) (Lonnroth et al. 1987, Benveniste & Hansen 1991, Parsons et al. 1991), the concentration of dopamine at steady state, determined by zero net flux, may differ from the relative concentration measured with conventional dialysis. Indeed, in the present study, the marked elevation in basal dopamine levels in BDNF+/− mice determined with zero net flux was less detectable by conventional methods. Strain differences may also contribute to this discrepancy, as the Boger et al. (2010) study used mice with a C57BL/6 background, while the mice in the current study were generated on a mixed B6/SV129 background. Further, our study indicates that BDNF+/− mice have a two- to three-fold increase in extracellular dopamine levels that corresponded with decreased DAT uptake measured by FSCV. Previous evidence that the striatal density of DAT binding was unchanged in BDNF+/− mice (Joyce et al. 2004, Boger et al. 2010) suggests that DAT function, but not expression, is altered. Accordingly, the activity of DAT is regulated by protein kinases (Carvelli et al. 2002, Lin et al. 2003, Morón et al. 2003) that are activated by the cognate receptor for BDNF, TrkB, which is also located on mesencephalic dopamine neurons (Numan & Seroogy 1999). In comparison to the measurable reductions in dopamine uptake observed with FSCV, no genotypic differences in in vivo dialysate extraction fraction (Ed) were detected with quantitative microdialysis. Although transporter-mediated uptake has been identified as the primary contributor to alterations in Ed (Smith & Justice 1994), both SERT and DAT-deficient mice were shown to have similarly elevated extracellular monoamine levels in the striatum without a significant change in Ed values (Jones et al. 1998, Mathews et al. 2004).
Slice FSCV also demonstrated that electrically-evoked dopamine release was attenuated in BDNF+/− mice, which may be a homeostatic response to compensate for a deficit in DAT function and/or elevated levels of extracellular dopamine. Alternatively, the present observation that exogenous addition of BDNF selectively restored evoked release, but not uptake, suggests that reductions in release are the intrinsic alteration. However, additional studies are warranted to determine if the uptake rates would also normalize following longer treatment times and/or higher concentrations of BDNF. This hypothesis is consistent with the finding that BDNF+/− mice have impaired hippocampal presynaptic transmitter release, associated with fewer docked vesicles in the active zone and lower synaptosomal levels of the vesicle-associated proteins synaptobrevin and synaptophysin, which were reversed with BDNF treatment (Pozzo-Miller et al. 1999). In the striatum, BDNF+/− mice were shown to have reduced vesicular monoamine (VMAT2) activity (Boger et al. 2010), which suggest that the vesicular storage of dopamine is also altered. Since extracellular levels of neurotransmitters assessed by microdialysis represent a balance between release and uptake processes, the increase in basal levels of dopamine in young adult BDNF+/− mice suggest that the reductions in uptake overcompensate for impaired release, at least to some extent. Furthermore, an impairment in vesicular-mediated release could lead to an accumulation of dopamine containing vesicles in the nerve terminal, evidenced by enhanced striatal tissue levels of dopamine (Dluzen et al. 1999, Dluzen et al. 2002, Joyce et al. 2004).
The complexity of BDNF-mediated regulation of dopamine transmission is further evidenced by the present finding that the effect of BDNF-deficiency on depolarization-stimulated dopamine varied based on the concentration of potassium perfused. The present microdialysis results showed a slight, but not significant, decrease in evoked extracellular dopamine levels induced by a moderate (60 mM) concentration of potassium in BDNF+/− mice when these data were corrected for their higher baseline dopamine levels. Previous studies also reported a lack of genotypic differences in dopamine stimulation above basal levels with the same (60 mM; Boger et al. 2010) or lower (30 mM; Dluzen et al. 2004) potassium concentrations. Conversely, BDNF+/− mice in the current study had an increase in the peak stimulated dopamine response and a slower rate of return to pre-stimulus levels following a high (120 mM) concentration of potassium. A similar attenuation in the return rate for dialysate dopamine was previously reported in BDNF+/− mice following stimulation with 60 mM potassium (Boger et al. 2010), though this effect was not observed in the present study, perhaps due to a slower rate of dialysate sampling (i.e. 20 versus 15 min). Combined with the present FSCV results, these findings suggest that the complex alterations in dopamine dynamics present in BDNF+/− mice result in differential effects on transmission depending on the level of stimulation. Therefore, the shift in potassium-stimulated dopamine responses in BDNF+/− mice, from no effect at 30 mM potassium (Dluzen et al. 2004), to only a slower rate of decline with 60 mM potassium (Boger et al. 2010), to a greater cumulative increase (enhancement in both the peak and rate of decline) at 120 mM potassium (current study), may indicate that decreased DAT activity gradually dominates and/or deficiencies in exocytotic release are rectified with increasing stimulation. Further investigation of these possibilities is not permissible with microdialysis due to the low temporal resolution of this method. Future studies to evaluate real-time release and uptake dynamics with different stimulation durations and frequencies are planned with slice FSCV.
Compared to the profound alterations observed in the dynamics of release and uptake, the activities of both the dopamine synthetic and metabolic pathways did not differ between BDNF+/− and wildtype mice. Our data demonstrating unaltered TH activity (assessed by L-DOPA accumulation in striatal tissue) coincides with the finding that TH expression was unchanged in BDNF-deficient mice (Joyce et al. 2004, Boger et al. 2010). Together, these observations suggest that the increased levels of both intracellular (Dluzen et al. 1999, Dluzen et al. 2002, Joyce et al. 2004) and extracellular dopamine (present study) in BDNF+/− mice are not related to adaptive changes in the cytosolic biosynthesis of dopamine. Previous evaluation of dopamine metabolism in BDNF+/− mice revealed unchanged tissue levels of HVA (Chourbaji et al. 2004) and increased levels of DOPAC, though this was not accompanied by increased DOPAC/dopamine ratios (Joyce et al. 2004). The present finding that dialysate levels of DOPAC and HVA were similar in BDNF+/− and wildtype mice supports the conclusion that constitutive reductions in BDNF do not impact the enzymatic degradation of dopamine.
Similar to other reports in young adult (3 – 5 months) BDNF+/− mice (Dlunzen et al. 2001, MacQueen et al. 2001, Chourbaji et al. 2004, Boger et al. 2010), the present study found no genotypic differences in spontaneous locomotor activity. In light of evidence that BDNF-deficient mice are behaviorally sensitive to external stressors (Rios et al. 2001), our procedure allowed for a one hour acclimation period following placement of the home-cage in the activity chamber, to ensure that the activity measured was a reliable representation of baseline spontaneous behavior without the caveat of novelty-induced effects. The finding that locomotor activity, as well as several emotional behaviors associated with monoamine regulation (e.g. exploration, anxiety, fear-associated learning, and depressive-like behavior; MacQueen et al. 2001, Chourbaji et al. 2004), were unchanged in BDNF+/− mice is perplexing considering the measurable alterations in dopamine (present study, Dluzen et al. 2002, Joyce et al. 2004, Boger et al. 2010) and serotonin (Daws et al. 2007, Guiard et al. 2008) transmission reported in young BDNF+/− mice. However, evidence that BDNF+/− mice have an altered response in beam walking (Dlunzen et al. 2001) and rotarod assessment (Boger et al. 2010) may indicate that the behavioral impact of striatal dopamine dysregulation in these mice is more evident in the presence of increased behavioral demands associated with performance of a complex motor task. Additionally, gross motor deficits were more pronounced in BDNF+/− mice with aging (Kernie et al. 2000, Dluzen et al. 2001, Boger et al. 2010), which were associated with further age-related alterations in dopamine system function (Dluzen et al. 2004, Boger et al. 2010)
In conclusion, the dynamic alterations in stimulated release and transporter-mediated uptake observed in BDNF+/− mice provide insight into the putative mechanisms underlying BDNF-mediated regulation of dopamine function. Nevertheless, future studies are warranted to further elucidate the influence of BDNF on different dopaminergic states to address if BDNF+/− mice have elevated resting/basal/tonic dopamine levels but have impaired phasic dopamine release. Interestingly, our findings largely correspond with serotonin system alterations reported in the hippocampus of BDNF-deficient mice (Daws et al. 2007, Guiard et al. 2008), perhaps indicating a conserved function for BDNF in regulating monoaminergic transmission. Overall, the ability of BDNF to regulate these functions supports prior evidence for its essential role as a synaptic modulator important in neuronal plasticity (Schuman 1999, Poo 2001, Tyler et al. 2002) and has mechanistic implications in ADHD and antidepressant therapies for which BDNF is a molecular target (Nibuya et al. 1996, Conti et al. 2002, Meredith et al. 2002, Molteni et al. 2006, Fumagalli et al. 2010).
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Parvej Khan, Natasha Bohin, and Andrzej Czaja for technical support and Dr. Scott Bowen for the use of the activity chambers. The authors would like to thank Drs. Alana C. Conti, Tamara L. Hendrickson, and Anne M. Andrews for insightful comments on the manuscript. Funding provided by the National Institute on Alcohol Abuse and Alcoholism (NIAAA; AA-016967 and AA016967-01S1; TAM) and Wayne State University. The content is solely the responsibility of the authors and does not represent the official views of NIAAA or the National Institutes of Health.
Footnotes
The authors have no conflict of interest to disclose.
REFERENCES
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Altar CA, Siuciak JA, Wright P, Ip NY, Lindsay RM, Wiegand SJ. In situ hybridization of trkB and trkC receptor mRNA in rat forebrain and association with high-affinity binding of [125I]BDNF, [125I]NT-4/5 and [125I]NT-3. Eur J Neurosci. 1994;6:1389–1405. doi: 10.1111/j.1460-9568.1994.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Batis JC, Hannigan JH, Bowen SE. Differential effects of inhaled toluene on locomotor activity in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:438–448. doi: 10.1016/j.pbb.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Knusel B, Hefti F. The nature of the trophic action of brain-derived neurotrophic factor, des(1-3)-insulin-like growth factor-1, and basic fibroblast growth factor on mesencephalic dopaminergic neurons developing in culture. Neuroscience. 1993;52:855–866. doi: 10.1016/0306-4522(93)90534-m. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Hansen AJ. Practical aspects of using microdialysis for determination of brain interstitial concentrations. In: Robinson TE, Justice JB Jr., editors. Microdialysis in the Neurosciences. Elsevier Science; Amsterdam: 1991. pp. 81–100. [Google Scholar]
- Blochl A, Sirrenberg C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J Biol Chem. 1996;271:21100–21107. doi: 10.1074/jbc.271.35.21100. [DOI] [PubMed] [Google Scholar]
- Boger HA, Mannangatti P, Samuvel DJ, et al. Effects of brain-derived neurotrophic factor on dopaminergic function and motor behavior during aging. Genes Brain Behav. 2010;10:186–198. doi: 10.1111/j.1601-183X.2010.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse KE, Mathews TA. Ethanol-induced increases in extracellular dopamine are blunted in brain-derived neurotrophic factor heterozygous mice. Neurosci Lett. 2011;489:172–176. doi: 10.1016/j.neulet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Mathews TA, Lapa GB, Jones SR. Local effects of acute ethanol on dopamine neurotransmission in the ventral striatum in C57BL/6 mice. Eur J Pharmacol. 2005;523:40–45. doi: 10.1016/j.ejphar.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Carvelli L, Moron JA, Kahlig KM, et al. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Hellweg R, Brandis D, Zorner B, Zacher C, Lang UE, Henn FA, Hortnagl H, Gass P. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res. 2004;121:28–36. doi: 10.1016/j.molbrainres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Munn JL, Valdez MF, Frosto-Burke T, Hensler JG. Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J Neurochem. 2007;101:641–651. doi: 10.1111/j.1471-4159.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Anderson LI, McDermott JL, Kucera J, Walro JM. Striatal dopamine output is compromised within BDNF +/− mice. Synapse. 2002;43:112–117. doi: 10.1002/syn.10027. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Gao X, Story GM, Anderson LI, Kucera J, Walro JM. Evaluation of nigrostriatal dopaminergic function in adult +/+ and +/− BDNF mutant mice. Exp Neurol. 2001;170:121–128. doi: 10.1006/exnr.2001.7698. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL, Anderson LI, Kucera J, Joyce JN, Osredkar T, Walro JM. Age-related changes in nigrostriatal dopaminergic function are accentuated in +/− brain-derived neurotrophic factor mice. Neuroscience. 2004;128:201–208. doi: 10.1016/j.neuroscience.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Story GM, Xu K, Kucera J, Walro JM. Alterations in nigrostriatal dopaminergic function within BDNF mutant mice. Exp Neurol. 1999;160:500–507. doi: 10.1006/exnr.1999.7225. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Cattaneo A, Caffino L, Ibba M, Racagni G, Carboni E, Gennarelli M, Riva MA. Sub-chronic exposure to atomoxetine up-regulates BDNF expression and signalling in the brain of adolescent spontaneously hypertensive rats: comparison with methylphenidate. Pharmacol Res. 2010;62:523–529. doi: 10.1016/j.phrs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Galloway MP, Wolf ME, Roth RH. Regulation of dopamine synthesis in the medial prefrontal cortex is mediated by release modulating autoreceptors: studies in vivo. J Pharmacol Exp Ther. 1986;236:689–698. [PubMed] [Google Scholar]
- Goggi J, Pullar IA, Carney SL, Bradford HF. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro. Brain Res. 2002;941:34–42. doi: 10.1016/s0006-8993(02)02505-2. [DOI] [PubMed] [Google Scholar]
- Guiard BP, David DJ, Deltheil T, et al. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol. 2008;11:79–92. doi: 10.1017/S1461145707007857. [DOI] [PubMed] [Google Scholar]
- He M, Shippenberg TS. Strain differences in basal and cocaine-evoked dopamine dynamics in mouse striatum. J Pharmacol Exp Ther. 2000;293:121–127. [PubMed] [Google Scholar]
- Hoover BR, Everett CV, Sorkin A, Zahniser NR. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem. 2007;101:1258–1271. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN, Renish L, Osredkar T, Walro JM, Kucera J, Dluzen DE. Methamphetamine-induced loss of striatal dopamine innervation in BDNF heterozygote mice does not further reduce D3 receptor concentrations. Synapse. 2004;52:11–19. doi: 10.1002/syn.10309. [DOI] [PubMed] [Google Scholar]
- Kent L, Green E, Hawi Z, et al. Association of the paternally transmitted copy of common Valine allele of the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene with susceptibility to ADHD. Mol Psychiatry. 2005;10:939–943. doi: 10.1038/sj.mp.4001696. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M, Aoun RA, Mathews TA. Altered striatal dopamine release following a sub-acute exposure to manganese. J Neurosci Methods. 2011 doi: 10.1016/j.jneumeth.2011.06.019. doi:10.1016/j.jneumeth.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Knusel B, Winslow JW, Rosenthal A, Burton LE, Seid DP, Nikolics K, Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci U S A. 1991;88:961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbeck R, Bartke I, Eberle W, Barde YA. Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem. 1999;72:1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhang PW, Zhu X, Melgari JM, Huff R, Spieldoch RL, Uhl GR. Phosphatidylinositol 3-kinase, protein kinase C, and MEK1/2 kinase regulation of dopamine transporters (DAT) require N-terminal DAT phosphoacceptor sites. J Biol Chem. 2003;278:20162–20170. doi: 10.1074/jbc.M209584200. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253:E228–231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Maina FK, Mathews TA. Functional Fast Scan Cyclic Voltammetry Assay to Characterize Dopamine D2 and D3 Autoreceptors in the Mouse Striatum. ACS Chemical Neuroscience. 2010;1:450–462. doi: 10.1021/cn100003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Bedogni F, Tongiorgi E, Fumagalli F, Racagni G, Riva MA. Chronic treatment with fluoxetine up-regulates cellular BDNF mRNA expression in rat dopaminergic regions. Int J Neuropsychopharmacol. 2006;9:307–317. doi: 10.1017/S1461145705005766. [DOI] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan S, Seroogy KB. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: a double-label in situ hybridization study. J Comp Neurol. 1999;403:295–308. doi: 10.1002/(sici)1096-9861(19990118)403:3<295::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr. The in vivo microdialysis recovery of dopamine is altered independently of basal level by 6-hydroxydopamine lesions to the nucleus accumbens. J Neurosci Methods. 1991;40:139–147. doi: 10.1016/0165-0270(91)90063-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain: In Stereotaxic Coordinates. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Schuman EM. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Shen RY, Altar CA, Chiodo LA. Brain-derived neurotrophic factor increases the electrical activity of pars compacta dopamine neurons in vivo. Proc Natl Acad Sci U S A. 1994;91:8920–8924. doi: 10.1073/pnas.91.19.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Boylan C, Fritsche M, Altar CA, Lindsay RM. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J Neurosci Methods. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Spina MB, Squinto SP, Miller J, Lindsay RM, Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J Neurochem. 1992;59:99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- Szapacs ME, Mathews TA, Tessarollo L, Lyons W. Ernest, Mamounas LA, Andrews AM. Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Methods. 2004;140:81–92. doi: 10.1016/j.jneumeth.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Liou YJ. Effects of BDNF polymorphisms on antidepressant action. Psychiatry Investig. 2010;7:236–242. doi: 10.4306/pi.2010.7.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ. Attention-deficit hyperactivity disorder may be associated with decreased central brain-derived neurotrophic factor activity: clinical and therapeutic implications. Med Hypotheses. 2007;68:896–899. doi: 10.1016/j.mehy.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Perrett SP, Pozzo-Miller LD. The role of neurotrophins in neurotransmitter release. Neuroscientist. 2002;8:524–531. doi: 10.1177/1073858402238511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Bradford HF, Stern GM. The stimulatory effect of brain-derived neurotrophic factor on dopaminergic phenotype expression of embryonic rat cortical neurons in vitro. Brain Res Dev Brain Res. 1994;81:318–324. doi: 10.1016/0165-3806(94)90318-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.