Abstract
Purpose
The anterior cruciate ligament (ACL) rarely heals spontaneously after rupture. Mesenchymal stem cells (MSCs) contribute to healing in various tissues, therefore, they may also have a key role in healing after ACL rupture. The purpose of this study was to investigate the properties of MSCs in ruptured ACLs.
Methods
Human ACL samples were harvested from patients undergoing primary ACL reconstruction, and samples were classified by the number of days post rupture (phase I <21 days; phase II 21–56 days; phase III 57–139 days phase IV ≥140 days). We evaluated the characteristics of MSCs, such as colony-forming capacity, differentiation potential and cell-surface markers.
Results
There was a tendency for high colony-forming capacity during phases I and II, which tended to decrease in phase III. Chondrogenic, adipogenic and osteogenic differentiation potential was maintained until phase II but decreased in phase III. Most surface-epitope expression was consistent from phase I to III: positive for CD44, CD73, CD90 and CD105; negative for CD11b, CD19, CD34, CD45 and human leukocyte antigen-D-related (HLA-DR). The presence of these surface markers proved the existence of MSCs in ruptured ACL tissue.
Conclusions
Our results suggest that colony-forming and differentiation potential decrease over time. It is important to consider changes in properties of MSCs and use ACL tissue in the acute phase of rupture when biological manipulation is required.
Introduction
The anterior cruciate ligament (ACL) is a major stabilizer of the knee joint and restrains tibial anterior translation and rotation [1]. If injured, it rarely heals spontaneously, even with suture repair [2]. However, the medial collateral ligament (MCL) heals with conservative, nonoperative treatment [3]. The reason why ACL fails to heal is unclear. Several possible theories have emerged, including lower vascularity, intra-articular location of the ligament, mechanical environment, lower proliferation and migration capacity of ligament fibroblasts, lower responsiveness to growth factors such as transforming growth factor (TGF)-β and basic fibroblast growth factor (bFGF) [2]. However, none of those theories fully explains why ACLs fail to heal.
Various changes occur in the ACL itself and surrounding structures after rupture. A ruptured ACL appears to retract initially, with no evidence of healing [2]. Four histological phases ensue: an inflammatory phase, an epiligamentous phase, a proliferative phase and a remodeling phase [4]. At levels of messenger RNA (mRNA), post injury changes and marked differences in extracellular matrices, growth factors and matrix metalloproteinases (MMPs) between the ACL and MCL are indicated [5–7]. Stress deprivation after rupture also affects ligament fibroblasts. Stress-deprived rabbit ligament fibroblasts decrease fibronectin synthesis and increase expression of β1 and α5 integrins [8]. Synovial fluid shows massive increases in inflammatory cytokines such as interleukin (IL)-6 and tumour necrosis factor (TNF)-α [9, 10].
Mesenchymal stem cells (MSCs) have a role in tissue healing [11–14] and have been characterised in vitro by their functional capacities to self-renew and to generate differentiated progeny, such as chondrogenic, osteogenic and adipogenic lineages. They are responsible for repair and regeneration of injured tissues by proliferation and differentiation and are also known to have strong immunomodulatory and trophic properties [15, 16]. They can reportedly be isolated from various tissue types, including bone marrow [17], adipose tissue [18] and synovium [19] and have been reported to be in intact and ruptured human ACL [20, 21]. The ability of MSCs to proliferate and differentiate may be a key factor in ACL rupture healing. However, changes in MSC properties in the ruptured ACL have not yet been elucidated.
We hypothesised that MSC properties in the human ACL declined after ligament rupture. This research aimed to verify changes in the MSC properties over time in ruptured human ACL, such as colony-forming capacity, differentiation potential and cell-surface markers.
Materials and methods
Tissue collection and isolation of mesenchymal stem cells
The study was approved by the Committee of Medical Ethics of Hirosaki University School of Medicine Institutional Review Board. Informed consent was obtained from all study participants. The clinical features of samples used in this study are shown in Table 1. Ruptured human ACL samples were harvested from 20 patients undergoing primary ACL reconstruction (age 23.7 ± 10.6 years). Samples were divided into four groups based on time between injury and surgery, according to a previous study [4]: phase I < 21 days; phase II 21–56 days; phase III 57–139 days; phase IV ≥ 140 days. Each group contained five samples. Harvested tissues were washed repeatedly in phosphate-buffered saline (PBS). Synovial sheath and fat tissues overlying the ligaments were carefully scraped off; only the core portions of the ligaments were used for cell isolation. The ACL tissue was minced into small pieces, which were then digested with 3 mg/ml collagenase (Type 5; Sigma-Aldrich, St. Louis, MO, USA) for three hours. Tissues were filtered with a 70-μm nylon filter (BD Biosciences, San Jose, CA, USA) to remove debris. Nucleated cells were resuspended after centrifugation and plated in 150-cm2 culture dishes (Nalge Nunc International, Rochester, NY, USA) with growth medium as passage zero. Growth medium consisted of α-minimum essential medium (MEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (JRH Bioscience, Lenexa, KS, USA), 10,000 U/ml penicillin, and 10,000 μg/ml streptomycin (Invitrogen). Culture dishes were incubated at 37°C in a humidified atmosphere of 5% carbon dioxide (CO2) and 95% air. Medium was changed twice a week. Cells at passage one or three were used for experiments.
Table 1.
Patient demographics
| No. | Age (years) | Gender | Time from rupture (days) | Phase |
|---|---|---|---|---|
| 1 | 13 | Female | 11 | I |
| 2 | 15 | Female | 15 | I |
| 3 | 27 | Female | 15 | I |
| 4 | 23 | Female | 17 | I |
| 5 | 24 | Male | 17 | I |
| 6 | 25 | Male | 24 | II |
| 7 | 17 | Female | 24 | II |
| 8 | 16 | Male | 25 | II |
| 9 | 12 | Female | 32 | II |
| 10 | 17 | Female | 37 | II |
| 11 | 20 | Female | 58 | III |
| 12 | 33 | Male | 64 | III |
| 13 | 19 | Female | 68 | III |
| 14 | 23 | Female | 95 | III |
| 15 | 14 | Female | 102 | III |
| 16 | 40 | Male | 156 | IV |
| 17 | 28 | Female | 9 months | IV |
| 18 | 22 | Male | 10 months | IV |
| 19 | 26 | Male | 6 years | IV |
| 20 | 58 | Male | 14 years | IV |
Colony-forming assay
One hundred cells at passage one were plated and cultured for 14 days in 56.7-cm2 dishes (Nalge Nunc International). Cells were subsequently fixed with 4% paraformaldehyde (Wako Pure Chemical Industries, Osaka, Japan), stained with 0.5% crystal violet (Wako Pure Chemical Industries) for five minutes and washed twice with distilled water. The number of colonies was then counted. Colonies <2 mm in diameter and faintly stained were ignored [22].
In vitro differentiation assay
In vitro multilineage mesenchymal differentiation was performed as previously described [22].
For chondrogenesis experiments, 250,000 cells at passage three were placed in a 15-ml polypropylene tube and centrifuged at 450 × g for ten minutes. Pellets were cultured in 400 μl chondrogenic media that contained 1,000 ng/ml bone morphogenetic protein two (BMP-2) (R&D Systems, Minneapolis, MN, USA), 10 ng/ml TGF-β3 (R&D Systems) and 100 nM dexamethasone (ICN Biomedicals Inc., Costa Mesa, CA, USA). It has been reported that BMP-2 initiates chondrogenic lineage development of adult human mesenchymal stem cells [23] and enhances chondrogenesis better than other BMP families, such as BMP-4 and -6 [24]. Pellets were harvested at day 21 and their sizes were measured using Canvas 11 (ACD Systems of America, USA). For microscopy, pellets were fixed with 4% formaldehyde, embedded in paraffin, cut into 5-μm sections and stained with Toluidine Blue (KANTO Chemical Co., Inc., Tokyo, Japan).
For adipogenesis experiments, 300 cells at passage three were plated in 56.7-cm2 dishes and cultured in complete medium for 14 days. Medium was then switched to adipogenic medium, which consisted of complete medium supplemented with 10-7 M dexamethasone, 0.5 mM isobutyl-1-methylxanthine (Wako Pure Chemical Industries) and 50 μM indomethacin (Sigma-Aldrich) for an additional 21 days. The adipogenic cultures were fixed in 4% paraformaldehyde and stained with fresh Oil Red O (Nacalai Tesque Inc, Kyoto, Japan) solution, and Oil Red O-positive colonies were counted. Colonies <2 mm in diameter or only faintly stained were ignored. The same adipogenic cultures were subsequently stained with crystal violet, and total cell colonies were counted. To evaluate adipogenic potential of cells from each phase, we compared Oil Red O-positive colony rates among phases.
For osteogenesis experiments, 300 cells at passage three were plated in 56.7-cm2 dishes and cultured for 14 days. Medium was then switched to calcification medium consisting of complete medium supplemented with 10-7 M dexamethasone, 20 mM β-glycerol phosphate (Wako Pure Chemical Industries) and 50 μg/ml ascorbate-2-phosphate (Wako Pure Chemical Industries) for an additional 21 days. These dishes were stained with 0.5% Alizarin Red S (Sigma-Aldrich) solution, and Alizarin Red S-positive colonies were counted. The same calcification cultures were subsequently stained with crystal violet, and cell colonies were counted. To assess cell osteogenic potential, we compared Alizarin Red S-positive colony rates among phases.
Flow cytometry
One million cells in passage three were suspended in 50 μl PBS containing antibody. After incubation for 30 min at 4°C, cells were washed with PBS and then suspended in 300 μl of PBS for analysis. The following antihuman antibodies were used: phycoerythrin (PE)-coupled CD34 and CD73, V450-coupled CD44, fluorescein isothiocyanate (FITC)-coupled CD45 and human leukocyte antigen-D-related (HLA-DR), allophycocyanin (APC)-coupled CD11b and CD90 and peridinin chlorophyl protein (PerCP)-Cy5.5-coupled CD19 and CD105 (all from Becton Dickinson). As an isotype control, nonspecific mouse immunoglobulin (Ig)G (Becton Dickinson) was substituted for the primary antibody. Cell fluorescence was evaluated by flow cytometry using a fluorescence-activated cell-sorting (FACS) Calibur instrument (BD Biosciences), and data were analysed using Cell Quest software (BD Biosciences).
Statistical analysis
Comparison among three populations was made using a Bonferroni test. P < 0.05 was considered to denote statistical significance.
Results
Cells were obtained from all phases. However, the sample volume from phase IV was small, and cells did not proliferate enough to use in the experiment, so we excluded them in this study.
Colony-forming assay
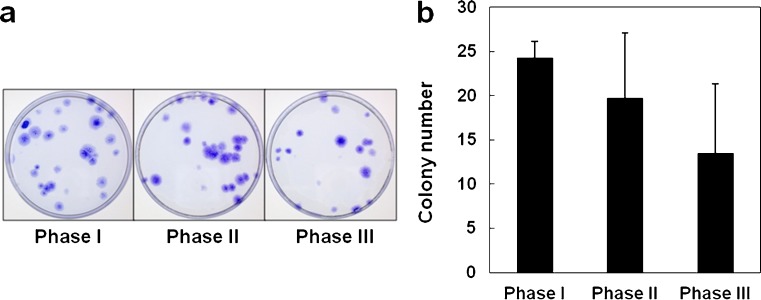
Cells derived from ruptured human ACL showed a high colony-forming tendency at phases I and II, which decreased at phase III (Fig. 1a). However, there was no statistically significant difference between phases (Fig. 1b). The maximum colony size was almost the same throughout phases; no obvious differences in morphology were noted.
Fig. 1.
Colony-forming capacity of mesenchymal stem cells obtained from ruptured human anterior cruciate ligament (ACL): a One hundred cells at passage 1 were seeded in 56.7-cm2 dishes and cultured for 14 days. Colonies were stained with crystal violet. b Cells derived from the ruptured human ACLs showed high colony-forming capacity at phases I and II, which decreased at phase III. Values are the mean and standard deviation
In vitro differentiation assay
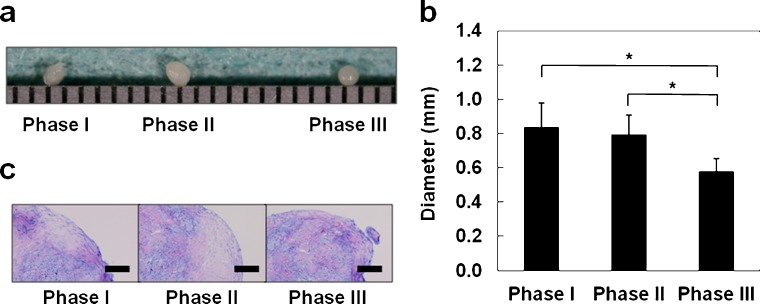
During chondrogenesis, the pellet size increased because of the production of extracellular matrix. We evaluated the largest pellet from each phase. Pellets were >1 mm in diameter. Macroscopically, pellets from phases I and II were larger than those from phase III (Fig. 2a). 0.84 ± 0.14, 0.80 ± 0.11, 0.58 ± 0.07 (mm) for phase I,s II and III, respectively, with phase III pellets being smaller than the other populations (P < 0.05) (Fig. 2b). In addition, pellets from phases I and II had greater amounts of Toluidine-Blue-staining cartilage matrix than pellets from phase III (Fig. 2c). These results indicate that the chondrogenic potential of MSCs derived from the ruptured human ACL is maintained until phase II but decreases at phase III.
Fig. 2.
Chondrogenic differentiation potentials of mesenchymal stem cells obtained from ruptured human anterior cruciate ligament: a Representative gross appearance with a 1-mm-scaled ruler, by phase. b Pellets from phases I and II showed significantly larger size than pellets from phase III. Values are the mean and standard deviation. *P < 0.05. c Histological appearance with Toluidine Blue staining. Bars = 100 μm
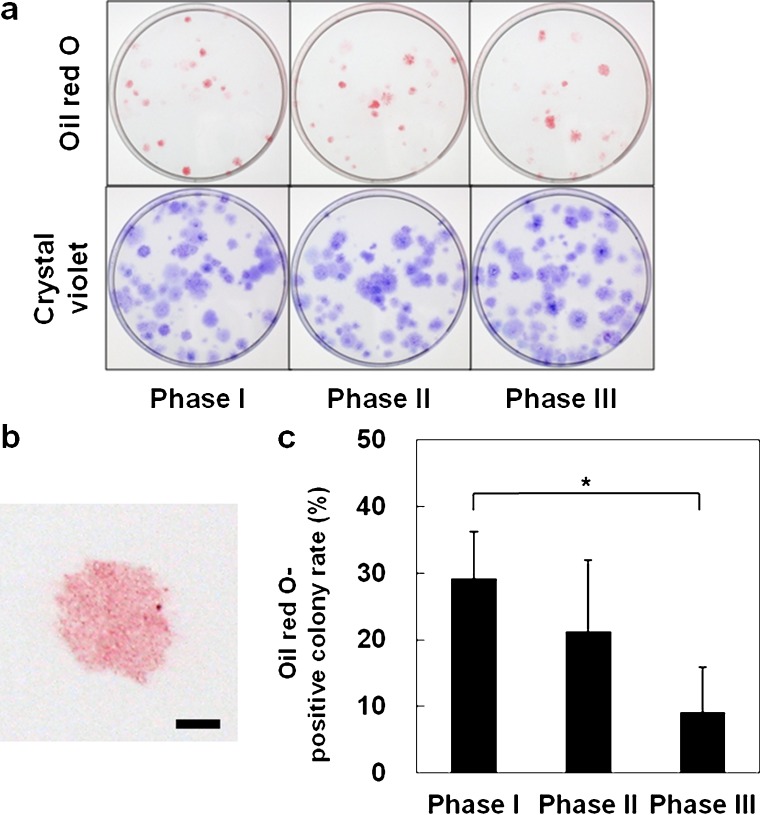
Lipid droplets, which stained with Oil Red O, were observed in cells from each phase. The sizes of the colonies positive for Oil Red O were approximately the same (Fig. 3a, b), but the rate of Oil Red O-positive colonies varied. The rate of Oil Red O-positive colonies was significantly lower at phase III (P < 0.05) (Fig. 3c). From a total of five donors, the number in whom the rate of Oil Red O-positive colonies was larger than 30% was three for phase I, one for phase II and zero for phase III. These results indicate that adipogenic ability of MSCs derived from ruptured human ACL decrease as phase progresses.
Fig. 3.
Adipogenic differentiation potentials of mesenchymal stem cells obtained from ruptured human anterior cruciate ligament: a Gross appearance of culture dishes stained with Oil Red O and crystal violet, by phase. b Colonies stained positively with Oil RedO. Bar = 2 mm. c Proportion of Oil Red O-positive colonies in relation to the total number of colonies. Valuesare the mean and standard deviation. *P < 0.05
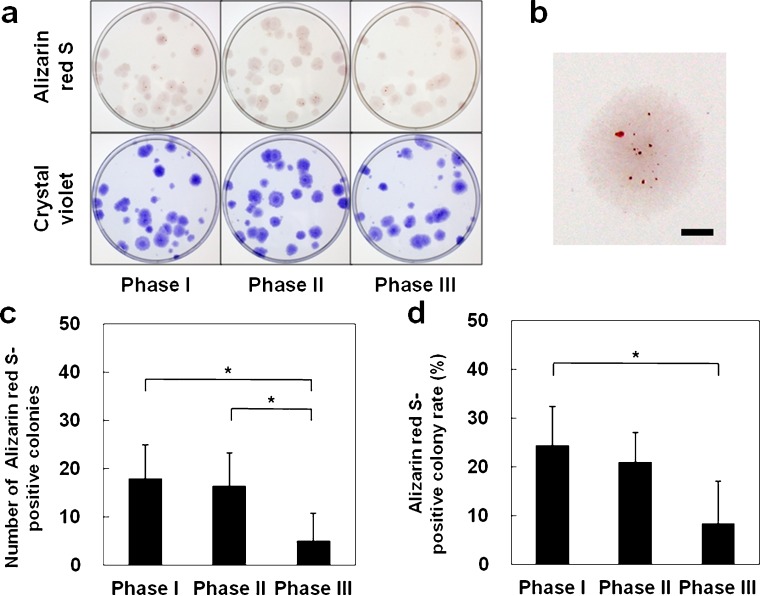
In each phase, Alizarin Red S-positive area was observed at the colony center (Fig. 4a, b). The number and rate of Alizarin Red S-positive colonies was significantly lower at phase III (P < 0.05) (Fig. 4c, d). Among the five donors from whom samples were tested, the number in whom the rate of Alizarin Red S-positive colonies was larger than 20% was three for phase I, two for phase II and zero for phase III. These findings indicate that osteogenic potential of MSCs derived from ruptured human ACL is maintained until phase II but decreases at phase III.
Fig. 4.
Osteogenic differentiation potentials of mesenchymal stem cells obtained from ruptured human anterior cruciate ligament: a Gross appearanceof culture dishes stained with Alizarin Red S and crystal violet, by phase. b Colonies stained positively withAlizarin Red S. Bar = 2 mm. c Number of Alizarin Red S-positive colonies. d Proportion of Alizarin Red S-positivecolonies in relation to total number of colonies. Values are the mean and standard deviation. *P < 0.05
Flow cytometry
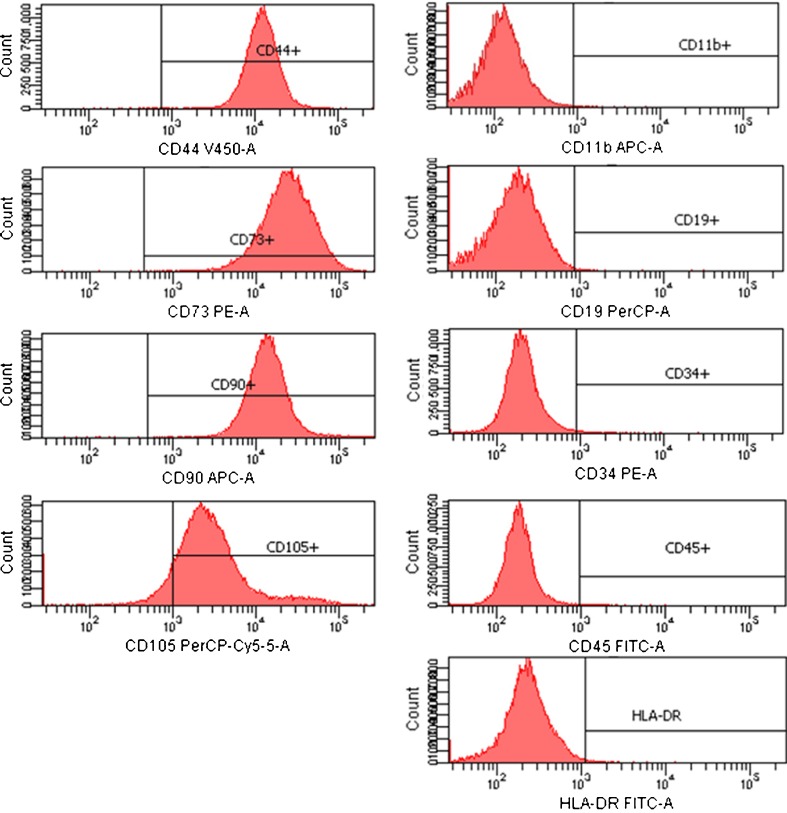
Flow cytometric analysis demonstrated that most surface epitope expression was consistent through phase I to III positive for CD44, CD73, CD90 and CD105, and negative for CD11b, CD19, CD34, CD45 and HLA-DR (Fig. 5), confirming that the cells being cultured were MSCs. However, the rate of positivity for CD34 varied, with phase I at 10.2 ± 15.0%, phase II at 3.4 ± 2.5%, and phase III at 8.3 ± 8.2%. There was no statistically significant difference between the phases (Table 2).
Fig. 5.
Representative immunophenotype of mesenchymal stem cells (MSCs) derived from the postinjured human anterior cruciate ligament (ACL). MSCs werelabeled with antibodies against human antigens CD11b, CD19, CD34, CD44, CD45, CD73, CD90, CD105 and human leukocyte antigen-D-related (HLA-DR), as indicated and analysed by fluorescence-activated cell-sorting
Table 2.
Average values of surface immunophenotypes of mesenchymal stem cells derived from ruptured anterior cruciate ligaments of five different donors (in percent)
| Phase I | Phase II | Phase III | |
|---|---|---|---|
| CD11b | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.1 |
| CD19 | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.2 |
| CD34 | 10.2 ± 15.0 | 3.4 ± 2.5 | 8.3 ± 8.2 |
| CD44 | 99.9 ± 0.1 | 99.8 ± 0.3 | 99.9 ± 0.3 |
| CD45 | 0.5 ± 0.4 | 0.5 ± 0.5 | 0.3 ± 0.1 |
| CD73 | 100 ± 0.1 | 99.9 ± 0.1 | 100 ± 0.1 |
| CD90 | 100 ± 0.1 | 99.7 ± 0.1 | 99.9 ± 0.2 |
| CD105 | 93.9 ± 4.4 | 94.9 ± 3.5 | 95.2 ± 4.7 |
| HLA-DR | 0.5 ± 0.3 | 0.3 ± 0.2 | 0.3 ± 0.2 |
HLA-DR human leukocyte antigen D-related
Discussion
This study showed changes in colony-forming capacity, differentiation properties and cell-surface markers of MSCs derived from ruptured ACL in patients undergoing ACL reconstruction. There have been studies on the existence [20, 21] and properties [25] of MSCs in human ACL, including quantitative evaluation of their colony-forming capacity and differentiation potential. Our data concerning colony-forming capacity and differentiation potential corresponded generally to earlier reports, as did our results for most cell-surface markers. Although our results for the CD34+ rate also correspond to past studies, such reports vary (1.4–10%) [20, 21, 25]. We hypothesised that MSC properties decline over time. Colony-forming capacity and differentiation potential of stem cells derived from ruptured human ACL decreased as the phase advanced, and these results confirmed our hypothesis. However, it is unclear whether this decline in properties is a reflection of an actual change in cell nature a decrease in its numbers. We could not evaluate the numbers of nucleated cells per volume of samples or changes of localization and distribution of MSCs in the ruptured human ACL. However, change in differentiated colony rates is independent of a decrease in MSCs, so postinjury changes in properties of MSCs may be due to both a changed nature and decreased MSCs in the ruptured human ACL.
In colony-forming capacity evaluation, error bars [standard deviation (SD)] in phases II and III were high. One possible factor for this result is individual difference. We performed the assay three times per sample. Error bars (SD) within each sample were not high, but those among samples were. Individual difference is an element that cannot be disregarded as long as human sample is being used. Another possible factor is variation among samples in the number of days when the ACL was harvested. We defined each phase by the number of days post rupture. The length of period in phases II and III were longer than that of phase I, and scattering in the number of days post rupture among samples was largest in descending order of phases III, II and I. This scattering might have contributed to the large error bars. However, a tendency for colony-forming capacity to decrease over time was observed.
Two factors might account for the changes in MSC properties observed in this study: mechanical stress deprivation and inflammatory cytokines. Ligaments suffer mechanical stress constantly; stress deprivation is known to cause various changes. Kawabata et al. reported that stress deprivation on a patellar tendon induced fibroblast apoptosis with activation of mitogen-activated protein kinase [26]. Inflammatory cytokines may affect differentiation potential. Massive increases in inflammatory cytokines, such as IL-6 and TNF-α, occur immediately after ACL rupture [9, 10]. Lacey et al. reported that TNF-α and IL-1β can compromise bone development from primary MSC population [27]. However, TNF-α reportedly stimulates osteogenic differentiation of MSCs, which could be blocked by the presence of anti-inflammatory agents such as dexamethasone [28]. Further studies are required to investigate relationships between differentiation potential of MSCs and inflammatory cytokines.
Murray et al. reported that ACL evinced four histological phases after rupture: an inflammatory phase, an epiligamentous phase, a proliferative phase and a remodeling phase [4]. Within an inflammatory phase, ligament remnants are populated by fibroblasts, polymorphonuclear neutrophils, lymphocytes and macrophages. Phase III (the period between eight and 20 weeks after rupture), was characterized by increasing cell number and density and blood vessel density in and among collagen fascicles of the ligament remnant. We observed that the CD34+ rate differed according to phase: i.e. higher at phases I and III; however, rates of CD11b, CD19, CD44, CD45, CD73, CD90, CD105 and HLA-DR were consistently the same throughout phases. Increased CD34 positivity might reflect increases in other cells, such as hematopoietic and vascular endothelial cells. Matsumoto et al. reported a higher number of CD34 and CD146-positive cells in the ACL septum region compared with the midsubstance and a population of vascular-derived stem cells in the ACL septum region [29]. Their findings might support our results.
There are some limitations to this study. Firstly, we could not investigate the properties of MSCs derived from uninjured human ACL because of the ethical issues involved in harvesting healthy human ACL. Secondly, we did not evaluate the proliferation potential of MSCs obtained from ruptured human ACL. Although stem cells are defined as having infinite proliferative potential, in the culture system used in this study, the proliferation potential of MSCs was not maintained [30]. In addition, as fast proliferation reportedly comes with high multilineage capacity, we thought it unnecessary to evaluate proliferation potential [31]. Thirdly, we did not evaluate the glycosaminoglycan (GAG) in chondrogenesis and alkaline phosphatase (ALP) activity in osteogenesis quantitatively. However, we demonstrated that pellets were positively stained with Toluidine Blue. Toluidine Blue dyes form complexes with anionic glycoconjugates, such as proteoglycans and GAG. That is, the pellets had extracellular matrix rich in GAG. Of course, simply staining is insufficient for quantitative evaluation. Instead of quantitatively evaluating GAG, we measured pellet size. For osteogenesis, even if ALP activity is high, it is important in osteogenesis whether calcification eventually arises. Alizarin Red S staining is used to identify calcium in tissue sections. In this study, we did not focus on the process but on the end result of differentiation. From this standpoint, we used Alizarin Red S to evaluate osteogenic differentiation. Finally, we were concerned stem cells may have been contaminated by other tissue, such as synovium or adipose tissue. We therefore removed synovial sheath and fat tissues overlying the ligament macroscopically, so the possibility of stem cell contamination from such origins could not be excluded. In addition, Morito et al. reported that MSCs increase in the joint fluid after ACL rupture, so contamination of the cells might occur [32].
In conclusion, we demonstrated property changes in MSCs from ruptured human ACL. Our results suggest that colony-forming capacity and differentiation properties of MSCs in ruptured ACL tissue decrease over time. It is important to consider such changes and use ACL tissue in the acute phase of rupture when biological manipulation is required.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 2.Murray MM, Spindler KP. Anteiror cruciate ligament healing and repair. Sports Med Arthrosc Rev. 2005;13(3):151–155. doi: 10.1097/01.jsa.0000173243.92319.da. [DOI] [Google Scholar]
- 3.Woo SL, Vogrin TM, Abramowitch SD. Healing and repair of ligament injuries in the knee. J Am Acad Orthop Surg. 2000;8(6):364–372. doi: 10.5435/00124635-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82(10):1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Lo IK, Marchuk L, Hart DA, Frank CB. Messenger ribonucleic acid levels in disrupted human anterior cruciate ligaments. Clin Orthop Relat Res. 2003;407:249–258. doi: 10.1097/00003086-200302000-00034. [DOI] [PubMed] [Google Scholar]
- 6.Bramono DS, Richmond JC, Weitzel PP, Chernoff H, Martin I, Volloch V, Jakuba CM, Diaz F, Gandhi JS, Kaplan DL, Altman GH. Characterization of transcript levels for matrix molecules and proteases in ruptured human anterior cruciate ligaments. Connect Tissue Res. 2005;46(1):53–65. doi: 10.1080/03008200590935556. [DOI] [PubMed] [Google Scholar]
- 7.Beye JA, Hart DA, Bray RC, McDougall JJ, Salo PT. Injury-induced changes in mRNA levels differ widely between anterior cruciate ligament and medial collateral ligament. Am J Sports Med. 2008;36(7):1337–1346. doi: 10.1177/0363546508316283. [DOI] [PubMed] [Google Scholar]
- 8.AbiEzzi SS, Foulk RA, Harwood FL, Akeson WH, Amiel D. Decrease in fibronectin occurs coincident with the increased expression of its integrin receptor α5β1 in stress-deprived ligaments. Iowa Orthop J. 1997;17:102–109. [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH. The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25(6):751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi H, Shirakura K, Kimura M, Terauchi M, Shinozaki T, Watanabe H, Takagishi K. Changes in biochemical parameters after anterior cruciate ligament injury. Int Orthop. 2006;30(1):43–47. doi: 10.1007/s00264-005-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivkovic A, Marijanovic I, Hudetz D, Porter RM, Pecina M, Evans CH (2011) Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 3:923–944 [DOI] [PubMed]
- 12.Gao J, Caplan AI. Mesenchymal stem cells and tissue engineering for orthopaedic surgery. Chir Organi Mov. 2003;88(3):305–316. [PubMed] [Google Scholar]
- 13.Zhi L, Chen C, Pang X, Uludag H, Jiang H. Synergistic effect of recombinant human bone morphogenic protein-7 and osteogenic differentiation medium on human bone-marrow-derived mesenchymal stem cells in vitro. Int Orthop. 2011;35(12):1889–1895. doi: 10.1007/s00264-011-1247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rui YF, Lui PP, Lee YW, Chan KM (2011) Higher BMP receptor expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared with bone-marrow-derived mesenchymal stem cells. Int Orthop. doi:10.1007/s00264-011-1417-1 [DOI] [PMC free article] [PubMed]
- 15.Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 2011;10(7):410–415. doi: 10.1016/j.autrev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Leijten JC, Georgi N, Post JN, Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17(9–10):1425–1436. doi: 10.1089/ten.tea.2010.0517. [DOI] [PubMed] [Google Scholar]
- 17.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 18.Zuk PA, Zhu M, Ashjian P, Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Cheng MT, Yang HW, Chen TH, Lee OK. Isolation and characterization of multipotent stem cells from human cruciate ligaments. Cell Prolif. 2009;42(4):448–460. doi: 10.1111/j.1365-2184.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, Ezura Y, Umezawa A, Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27(4):435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt B, Ringe J, Häupl T, Notter M, Manz R, Burmester GR, Sittinger M, Kaps C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation. 2003;71(9–10):567–577. doi: 10.1111/j.1432-0436.2003.07109003.x. [DOI] [PubMed] [Google Scholar]
- 24.Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320(2):269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 25.Steinert AF, Kunz M, Prager P, Barthel T, Jakob F, Nöth U, Murray MM, Evans CH, Porter RM. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A. 2011;17(9–10):1375–1388. doi: 10.1089/ten.tea.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawabata H, Katsura T, Kondo E, Kitamura N, Miyatake S, Tanabe Y, Setoguchi T, Komiya S, Yasuda K. Stress deprivation from the patellar tendon induces apoptosis of fibroblasts in vivo with activation of mitogen-activated protein kinases. J Biomech. 2009;42(15):2611–2615. doi: 10.1016/j.jbiomech.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthr Cartil. 2009;17(6):735–742. doi: 10.1016/j.joca.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-alpha on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010;31(7):1666–1675. doi: 10.1016/j.biomaterials.2009.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto T, Ingham SM, Mifune Y, Osawa A, Logar A, Usas A, Kuroda R, Kurosaka M, Fu FH, Huard J (2011) Isolation and characterization of human anterior cruciate ligament-derived vascular stem cells. Stem Cells Dev. doi:10.1089/scd.2010.0528 [DOI] [PMC free article] [PubMed]
- 30.Shibata KR, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M, Kohno Y, Ito K, Fujibayashi S, Neo M, Nakayama T, Nakamura T, Toguchida J. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007;25(9):2371–2382. doi: 10.1634/stemcells.2007-0225. [DOI] [PubMed] [Google Scholar]
- 31.Dexheimer V, Mueller S, Braatz F, Richter W (2011) Reduced reactivation from dormancy but maintained lineage choice of human mesenchymal stem cells with donor age. PLoS One. doi:10.1371/journal.pone.0022980 [DOI] [PMC free article] [PubMed]
- 32.Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford) 2008;47(8):1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]