Abstract
Purpose
We used quantitative CT in conjunction with finite element analysis to provide a new tool for assessment of bone quality after total hip arthroplasty in vivo. The hypothesis of this prospective five-year study is that the combination of the two modalities allows 3D patient-specific imaging of cortical and cancellous bone changes and stress shielding.
Method
We tested quantitative CT in conjunction with finite elements on a cohort of 29 patients (31 hips) who have been scanned postoperatively and at one year, two years and five years follow-up. The method uses cubic Hermite finite element interpolation for efficient mesh generation directly from qCT datasets. The element Gauss points that are used for the geometric interpolation functions are also used for interpolation of osteodensitometry data.
Results
The study showed changes of bone density suggestive of proximal femur diaphysis load transfer with osteointegration and moderate metaphyseal stress shielding. Our model revealed that cortical bone initially became porous in the greater trochanter, but this phenomenon progressed to the cortex of the lesser trochanter and the posterior aspect of the metaphysis. The diaphyseal area did not experience major change in bone density for either cortical or cancellous bone.
Conclusion
The combination of quantitative CT with finite element analysis allows visualization of changes to bone density and architecture. It also provides correlation of bone density/architectural changes with stress patterns enabling the study of the effects of stress shielding on bone remodelling in vivo. This technology can be useful in predicting bone remodeling and the quality of implant fixation using prostheses with different design and/or biomaterials.
Introduction
Numerous clinical studies have investigated bone remodelling patterns around the femoral component in total hip arthroplasty (THA) [1–4]. These studies have used various imaging modalities such as radiographs, DEXA or quantitative CT (qCT) scans. However, it is now well established that bone density (BD) alone may not properly measure bone quality [5]. Micro-architecture and structural aspects must be considered too. Finite element (FE) analysis provides a platform to integrate these influences along with external loads to elucidate bone quality and its remodelling response [6, 7].
We have used geometric and density information from qCT datasets of previous osteodensitometry studies [8–10] to create patient-specific finite element (FE) models. The hypothesis of this study is that the combination of these two modalities, qCT and FE, allows three dimensional patient specific imaging of cortical and cancellous bone changes over time as well as the consequences of stress shielding. Specifically, we hypothesize that the initial stress pattern computed from FE simulation is strongly correlated with BD loss patterns from qCT at five-year follow-up.
Materials and methods
Twenty-nine consecutive patients (31 hips) with degenerative joint disease and without deformity of the proximal femur were operated by one surgeon (RP) in one institution. The patients received an uncemented THA with a taper-design femoral component coated with hydroxyapatite (Summit; DePuy International, Leeds, UK), and a press-fit titanium cup (Duraloc; DePuy) with ceramic–ceramic pairing (Biolox Delta, CeramTec, Plochingen, Germany). The average age of the patients at the index operation was 58 years (range, 30–81 years, 16 men and 13 women). Computed tomography scans were carried out on all patients postoperatively, one year, two and five years after the index operation. The method used for qCT assessment has been already described in the one and two-year follow-up reports [8, 9].
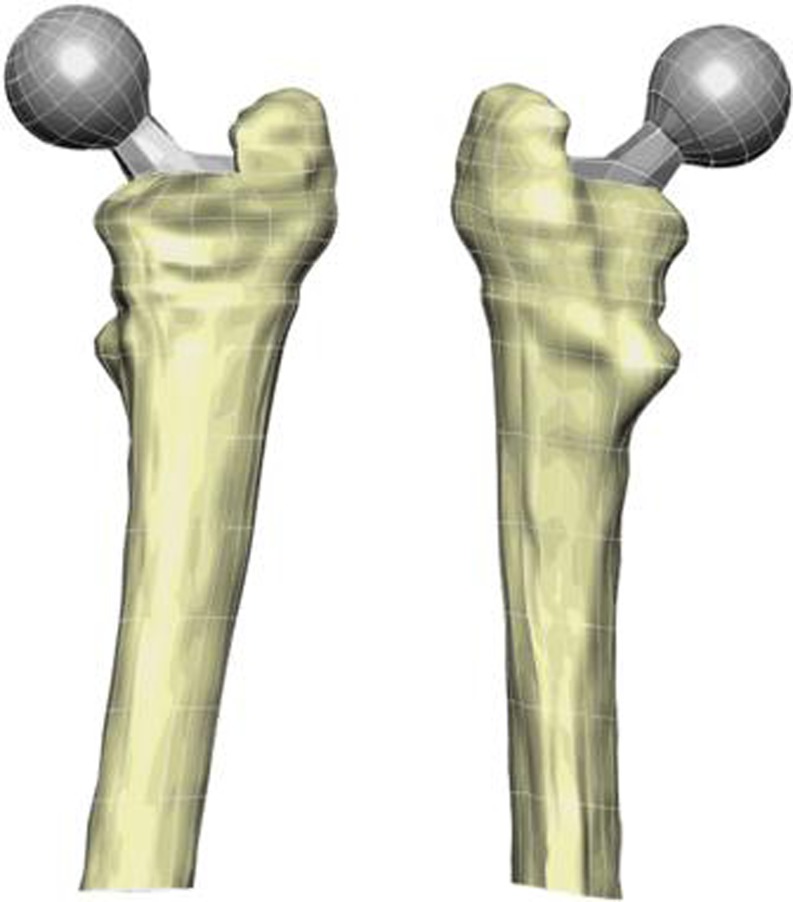
Five of the 31 qCT datasets were used. These were judged by the surgeon (RP) to present with the least preoperative deformity (average age 60 years, three males, two females, five right hips). FE models were combined with qCT imaging to generate patient-specific models. The method involved the use of cubic Hermite interpolation functions that can capture the complex bone geometry with fewer degrees of freedom than would be needed if a linear interpolation was used [11]. Bone surface data points were extracted from each scan. A least squares algorithm was then used to fit element boundaries to the bone data. The method is described fully in references [12] and [13]. FE models of both the femur and implant were generated from CT scans preserving their relative positions to each other. The model is depicted in Fig. 1.
Fig. 1.
Anterior and posterior views of a patient-specific quantitative CT (qCT) / finite element (FE) model
We used geometric and density information from qCT datasets to create patient-specific finite element (FE) models. These models were analysed in two different ways. First, they were used to reveal three-dimensional patterns in BD changes that cannot be easily seen in two-dimensional scans. Second, mechanical simulation results were used to correlate stress transfer pattern and BD changes in order to investigate stress shielding effects.
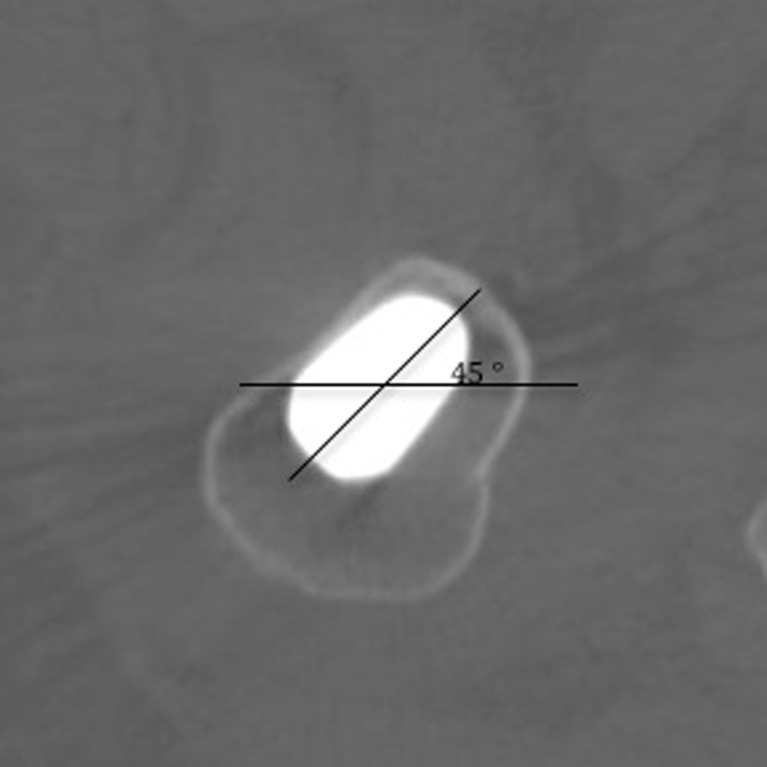
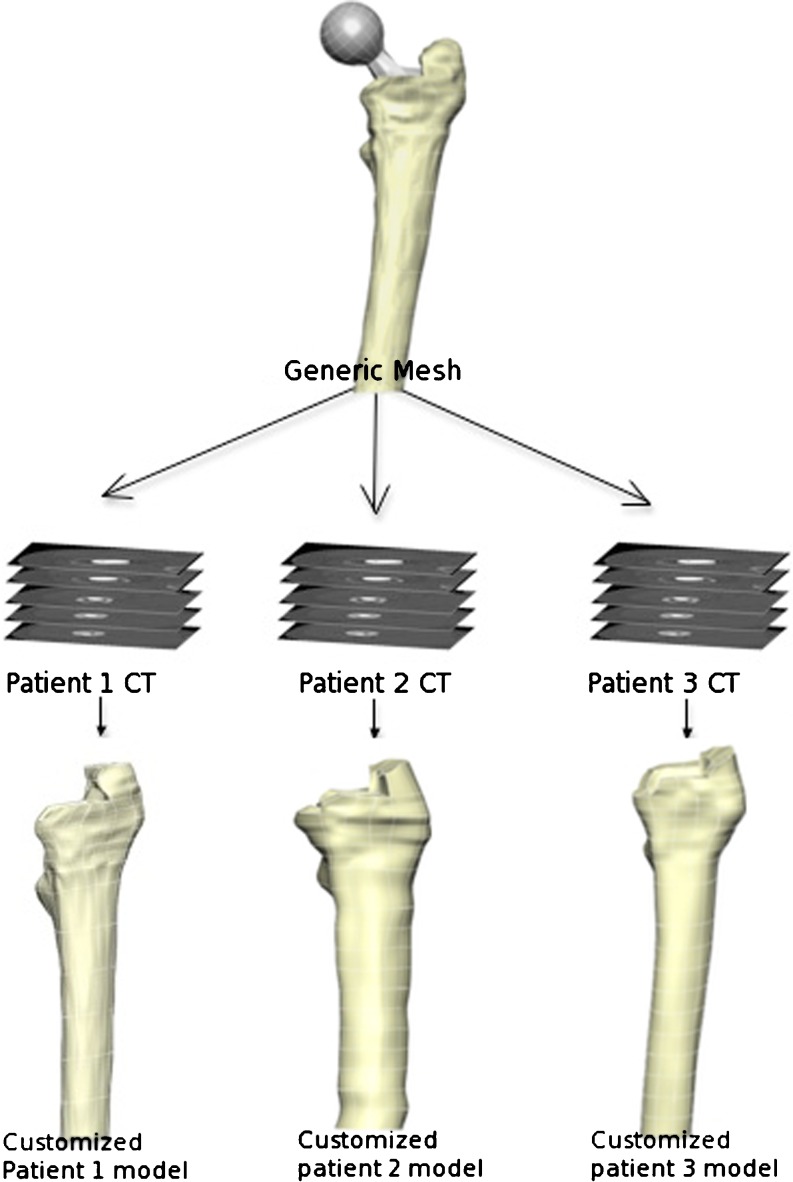
Two types of comparison were made. The first between patients (inter-patient) and the other between different follow-up CT scans (intra-patient). In order to achieve precise and detailed inter and intra patient comparisons, we aligned CT scans so that the most proximal part of the prosthesis was at the centre of the image with an angle of 45° (Fig. 2) and then extracted boundaries creating a dataset for each scan (total of 20 datasets: five patients x four follow-up scans [postoperative, one, two and five year follow-up]). Then we produced a generic mesh and then customized to each patient using the Free Form Deformation method [13] (Fig. 3), ensuring that nodal positions occupied the same anatomical landmarks. We utilized Gauss points inside the mesh for inter and intra patient comparison as Gauss points always have predetermined locations in the local coordinate system of the element. We then took calibrated qCT density data and assigned it to Gauss points [12] and then turned it into a modulus using the following formula [14]:
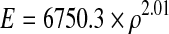 |
where E is in MPa and ρ is in g/cm3.
Fig. 2.
The method to achieve reproducible alignment of images in serial CT scans. The main diagonal of the stem axial section was rotated to obtain a 45° angle to the horizontal line
Fig. 3.
Mesh generation and customization. We used a generic mesh and customized it to individual patient CT scans. Therefore all the meshes have the same number of elements and nodes but the shape and material properties are specific to the patient
This enabled us to perform much more detailed comparisons than similar studies which were based on either Gruen Zones or some arbitrary regions of interest [15–17]. This approach has been validated with in-vitro mechanical experiment [12, 18].
The boundary conditions included hip joint force and abductor muscle force. These two forces were found to be the most significant ones for in-vivo simulation of THA patients [19]. We used the maximum values of hip joint force and abductor muscle force during the normal gait cycle of an average subject from Bergmann’s study [20]. The contact between bone and implant was simulated as frictional contact with a coefficient of 0.5 [21].
The bone remodeling pattern around the uncemented taper-design stem was analysed in three ways: (1) Density change over time was visualized using the 3D FE mesh to examine the major pattern of bone loss and identify the regions with maximum bone loss; (2) Gauss points with the most dramatic density changes were monitored, focusing on Gauss points that occupied positions where bone transformed from cortical to porous cortical bone; (3) stress (von Mises and principal stresses) pattern changes calculated from FE simulations were compared with bone density changes to examine the role of mechanical loading in bone remodeling. In particular, the initial stress transfer patterns from the post-op CT of three patients were compared with their BD loss pattern at five-year follow-up to find out if there is any quantitative relationship between the two.
Results
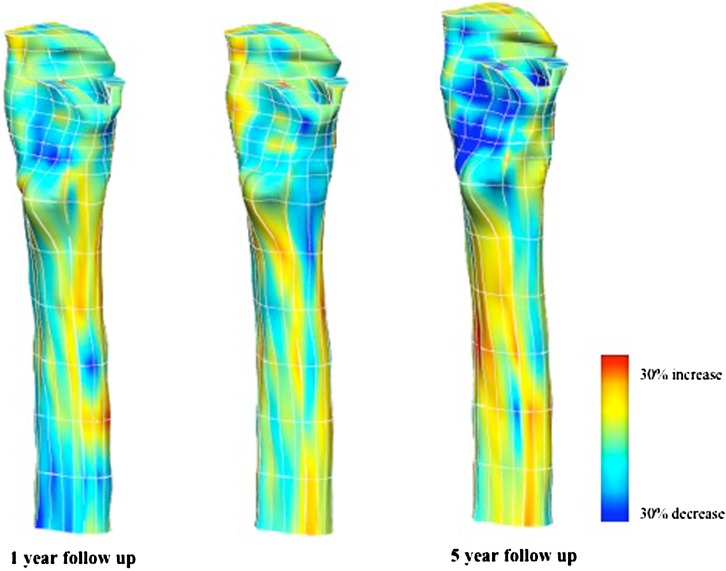
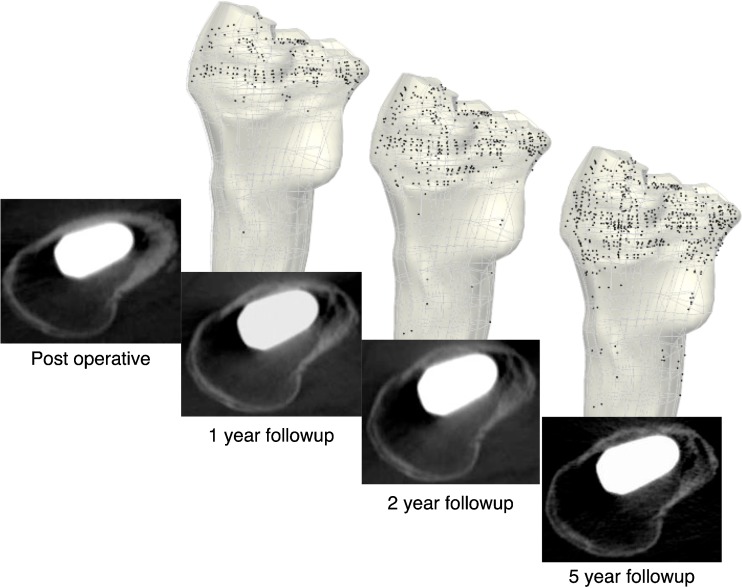
Our three dimensional model was able to reveal fine details in BD changes over time. We identified three regions within the proximal femur showing substantial BD loss. The regions are the posteromedial region, the lesser trochanter region and the calcar region (Fig. 4). The average percentage density loss in the proximal region was 20% [10]. However, our FE model was able to show the BD loss pattern in more detail, and the amount of average bone loss in the three regions were 37% (SD 19%) for posteromedial, 45% (SD 22%) for lesser trochanter and 42% (SD 13%) for calcar regions, indicating that these were the major regions of bone loss within the proximal region. Our model revealed that cortical bone initially became porous in the greater trochanter, but this phenomenon progressed to the cortex of the lesser trochanter and the posterior aspect of the metaphysis (Fig. 5). The diaphyseal area did not experience major change in bone density for either cortical or cancellous bone (Fig. 5).
Fig. 4.
The quantitative CT (qCT) / finite element (FE) modelling results from one patient shows progressive density loss in the metaphyseal region. The density loss was calculated by subtracting the density values at each follow-up from baseline values. The similar pattern was seen in all patients
Fig. 5.
Increase in cortical bone porosity over the five-year period observed in one patient (71-year-old male). The black dots in the finite element (FE) models represent areas that transformed from cortical to porous cortical bone. These images show a pronounced porosity increase in the calcar and posterior metaphyseal regions, which is also shown in the patient’s CT scans. All patients showed similar increase in porosity
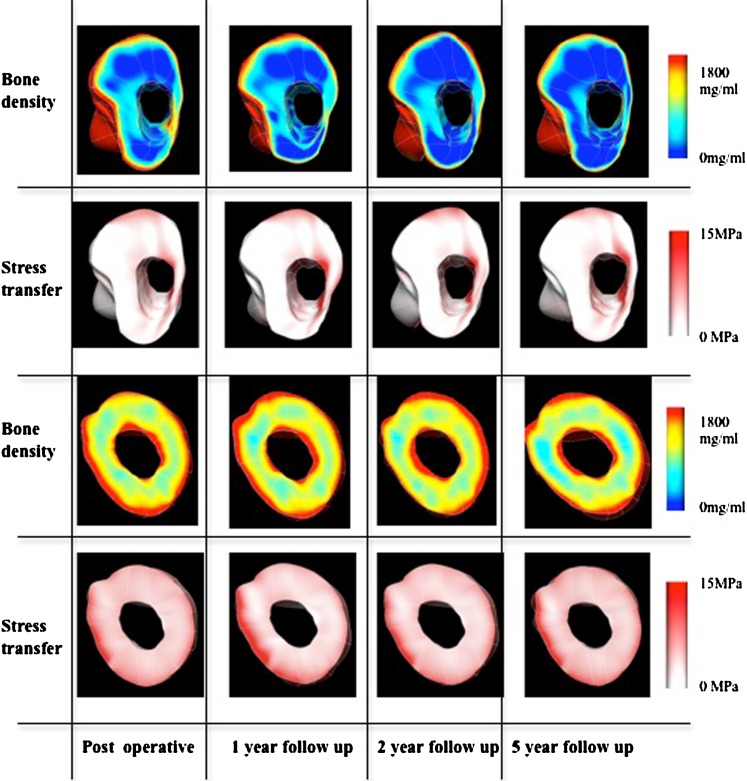
Patterns in BD loss and stress transfer were highly correlated both qualitatively and quantitatively. Von Mises stress transfer patterns show that the anterior and medial side of the proximal region did not incur significant stress transfer after insertion of the implant and the situation did not change over time as the region remained largely unloaded over the five-year period. The posterior side of the proximal region at first experienced some stress transfer right after the surgery but gradually became unloaded as time progressed. The diaphyseal zone was loaded over the five-year period and the stress pattern change was minimal (Fig. 6).
Fig. 6.
Correlation between density change and stress transfer in the metaphyseal and diaphyseal regions. Bone density decrease is remarkable in the posterior aspect of the metaphysis (dark blue area). The von Mises stress transfer from the finite element (FE) simulation shows that the amount of stress shielding in the metaphyseal area increases over time. There are unremarkable changes in the diaphyseal areas
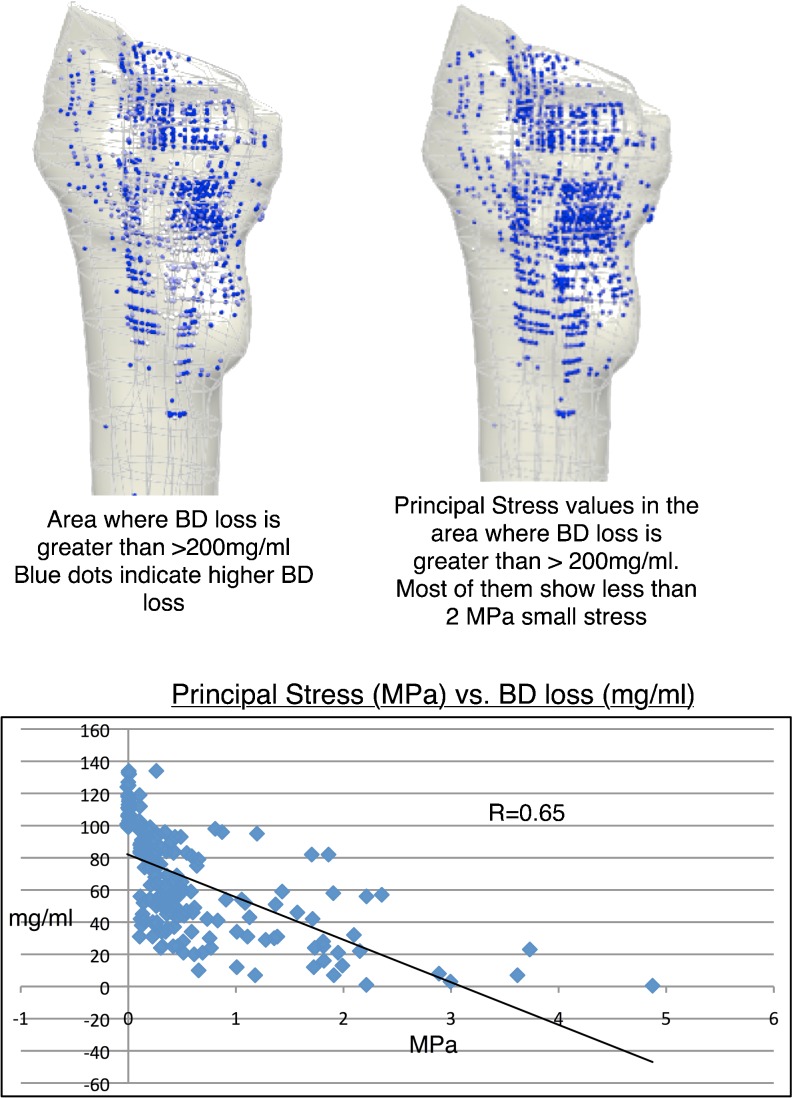
We further analysed the correlation between BD loss pattern at five-year follow-up and initial principal stress pattern (at post-op) using data from three patients and found out that there is a strong correlation (R = 0.65) between initial principal stress transfer pattern and BD loss pattern. Specifically, regions showing greatest BD loss were characterized by small magnitude of initial principal stresses. The initial stresses in the proximal region at post-op were very low initially and this was the area where loss of cancellous bone was the greatest. In particular, the posterior region of the metaphysis was more unloaded than the anterior region and subsequently BD loss was greatest on this side. On the other hand, the diaphyseal zone experienced a high magnitude of stress transfer and maintained its bone density over time (Fig. 7).
Fig. 7.
Correlation between the initial principal stress at post-op vs. bone density (BD) loss over five years. Good correlation (r = 0.65) is shown
Discussion
This study has demonstrated that qCT and FE modalities, when used together, improve the ability to monitor changes in bone architecture and assess bone quality after THA. Combination of the two modalities with reference to cortical (dense and porous) and cancellous bone allows correlation of bone architectural changes with their respective stress patterns over time.
There were a number of limitations for this study. We restricted the number of patients to five out of a total of 31 due to time and labour constraints. Therefore caution is required in interpreting the results. However, due to the relatively small variations we had in our results are an encouraging sign that our results are likely to reflect the general trend in the dataset. We also note that similar studies in this field of research have utilized small sample sizes [17]. For all five-year follow-up patient datasets, the contra-lateral (non-operated) side was not available for assessment. Information on the non-operated side would have been useful for comparison of bone remodeling changes. Thus the influence of natural physiological aging of the femoral bone could not be assessed within this study.
Technical limitations included our inability to resolve fine detail with CT below 1 mm. The qCT protocol included a 5-mm scan feed in the metaphyseal region and 1 cm in the diaphyseal region. A spiral mode of CT imaging would have allowed a better resolution of bone remodeling changes enabling better density change descriptions and the ability to assess mechanics at a smaller scale.
For our models we assigned cancellous properties to porous cortical bone regions. This might compromise the validity of the mechanical calculations on a fine scale. However, to the best of our knowledge there is no porous cortical bone material data available in the literature.
Outcomes were related to the design of the stem used for these patients. A different stem design would have distributed load differently and we expect that this would have influenced the remodeling response. However, our study was focused on evaluation of a method and not on specific remodeling responses for any one design. We would anticipate that our combined methods would be equally sensitive to identifying other patterns of remodeling for other stems.
Our contribution has been to apply qCT to three-dimensional FE models for biomechanical assessment. This is an advancement on other studies that have used imaging modalities such as DEXA [15, 16, 22, 23], which cannot distinguish between cortical and cancellous bone. Kerner et al. [16] used DEXA combined with FE to test the bone remodelling algorithm previously published by Huiskes et al. [24]. They utilized autopsy specimens of five elderly patients who had uncemented stem in-situ for 17–84 months. The contralateral non-operated femur was used for control. The predicted pattern matched well with the DEXA results. Lengsfeld et al. [17] used conventional CT in conjunction with FE models for simulation of the strain response to remodelled bone in THA. CT imaging was retrospective and bone density analysis was not calibrated with respect to a hydroxyapatite standard. They found significant reduction of strain energy density values in all regions of interest for 11 femora at 12 years after cemented THA. Their analysis averaged data across Gruen zones, thus it was not suitable for 3D assessment and did not account for porous cortical changes .
The cases that we have used for our analysis are a subset of a previous five-year follow-up qCT osteodensitometry study [10]. The study showed changes of BD suggestive of proximal femur diaphysis load transfer with osteointegration and moderate metaphyseal stress-shielding. In addition the present study identified a gradual increase in porosity in the stress-shielded posterior cortex of the proximal femur. We also showed that there is a strong correlation between initial principal stress pattern and BD loss pattern. It poses an interesting hypothesis to explore as this quantitative relationship can be further refined into a new and efficient algorithm which can predict the general pattern of BD loss after THA. All these findings were possible because we used high order elements for accurate mesh generation and Gauss points for fine sampling of qCT osteodensitometry data, which enabled us to efficiently generate patient-specific imaging in-vivo.
Draenert [25] used histomorphology in specimens retrieved from patients with uncemented THR and observed the same phenomenon of increased cortical porosity. Maloney et al. [26] assessed femoral bone remodelling in 24-specimen femora using plain radiographs and histomorphology. They found remarkable bone resorption in the proximal regions around both cemented and uncemented stems. Interestingly, the posterior cortex showed the maximum bone loss [26].
In summary, we have shown that qCT in conjunction with FE analysis enables the production of accurate patient-specific meshes on which to model femoral components and the effect these components have on bone remodelling. In particular, we found a strong correlation between initial stress pattern at post-op and BD loss pattern at five-year follow-up. Therefore this technology can prove to be useful in predicting bone remodeling and the quality of implant fixation with different design and/or biomaterials.
At present, the method of choice in THA patient management is simple 2D radiographs. The radiographs’ availability, low cost and ease of use make it a very tough act to beat. However, as the design and material of implants develop, the indicators of implant failure become more and more subtle, making it harder for clinicians to use just radiographs to detect early sign of implant failure. Therefore a tool that allows more detailed examination of the health of the hip joint and progression of surgery is required and a tool that combines qCT and FE is one of the most promising candidates. At present the technologies for combining these two methods are already available. But the challenge is to develop a user-friendly tool that clinicians can use in their daily practices. Considering the enormous research effort being put towards the goal, we expect this technology to be introduced in a clinical environment during this decade. In the future, this tool could be used for pre-clinical validation of new implants before their widespread introduction in clinical practice.
Footnotes
One or more of the authors have received funding from the Wishbone Trust New Zealand and Stevenson Charitable Trust (RPP).
References
- 1.Bobyn JD, Engh CA, Glassman AH (1987) Histologic analysis of a retrieved microporous-coated femoral prosthesis. A seven-year case report. Clin Orthop Relat Res (224):303 [PubMed]
- 2.Engh CA, Bobyn JD, Glassman AH, Porous-coated hip replacement The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1997;69(1):45. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs CR, Simo JC, Beaupre GS, Carter DR. Adaptive bone remodeling incorporating simultaneous density and anisotropy considerations. J Biomech. 1997;30(6):603. doi: 10.1016/S0021-9290(96)00189-3. [DOI] [PubMed] [Google Scholar]
- 4.Oh I, Carlson CE, Tomford WW, Harris WH. Improved fixation of the femoral component after total hip replacement using a methacrylate intramedullary plug. J Bone Joint Surg Am. 1978;60(5):608. [PubMed] [Google Scholar]
- 5.Rüegsegger P, Koller B, Müller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int. 1996;58(1):24. doi: 10.1007/BF02509542. [DOI] [PubMed] [Google Scholar]
- 6.Cody DD, Gross GJ, Hou FJ, Spencer HJ, Goldstein SA, Fyhrie DP. Femoral strength is better predicted by finite element models than QCT and DXA. J Biomech. 1999;32(10):1013. doi: 10.1016/S0021-9290(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 7.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33(4):744. doi: 10.1016/S8756-3282(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 8.Pandit S, Graydon A, Bradley L, Walker C, Munro J, Pitto RP. CT-osteodensitometry in modern uncemented taper-design stem with hydroxyapatite coating. Australia and New Zealand. J Surg. 2006;76:778. [Google Scholar]
- 9.Pitto RP, Bhargava A, Pandit S, Walker C, Munro JT. Quantitative CT-assisted osteodensitometry of femoral adaptive bone remodelling after uncemented total hip arthroplasty. Int Orthop. 2008;32(5):589. doi: 10.1007/s00264-007-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitto RP, Hayward A, Walker C, Shim VB (2009) Femoral bone density changes after total hip arthroplasty with uncemented taper-design stem. Int Orthop 34(6):783–787 [DOI] [PMC free article] [PubMed]
- 11.Bradley CP, Pullan AJ, Hunter PJ. Geometric modeling of the human torso using cubic hermite elements. Ann Biomed Eng. 1997;25(1):96. doi: 10.1007/BF02738542. [DOI] [PubMed] [Google Scholar]
- 12.Shim VB, Pitto RP, Streicher RM, Hunter PJ, Anderson IA (2008) Development and validation of patient-specific finite element models of the hemipelvis generated from a sparse CT data set. J Biomech Eng 130(5) [DOI] [PubMed]
- 13.Fernandez JW, Mithraratne P, Thrupp SF, Tawhai MH, Hunter PJ. Anatomically based geometric modelling of the musculo-skeletal system and other organs. Biomech Model Mechanobiol. 2004;2(3):139. doi: 10.1007/s10237-003-0036-1. [DOI] [PubMed] [Google Scholar]
- 14.Keller TS. Predicting the compressive mechanical behavior of bone. J Biomech. 1994;27(9):1159. doi: 10.1016/0021-9290(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 15.Herrera A, Panisello JJ, Ibarz E, Cegonino J, Puertolas JA, Gracia L. Long-term study of bone remodelling after femoral stem: a comparison between dexa and finite element simulation. J Biomech. 2007;40(16):3615. doi: 10.1016/j.jbiomech.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Herrera A, Panisello JJ, Ibarz E, Cegonino J, Puertolas JA, Gracia L. Comparison between DEXA and finite element studies in the long-term bone remodeling of an anatomical femoral stem. J Biomech Eng. 2009;131(4):041013. doi: 10.1115/1.3072888. [DOI] [PubMed] [Google Scholar]
- 17.Lengsfeld M, Burchard R, Gunther D, Pressel T, Schmitt J, Leppek R, Griss P. Femoral strain changes after total hip arthroplasty—patient-specific finite element analyses 12 years after operation. Med Eng Phys. 2005;27(8):649. doi: 10.1016/j.medengphy.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Shim V, Bohme J, Vaitl P, Klima S, Josten C, Anderson I. Finite element analysis of acetabular fractures—development and validation with a synthetic pelvis. J Biomech. 2010;43(8):1635. doi: 10.1016/j.jbiomech.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Stolk J, Verdonschot N, Huiskes R. Hip-joint and abductor-muscle forces adequately represent in vivo loading of a cemented total hip reconstruction. J Biomech. 2001;34(7):917. doi: 10.1016/S0021-9290(00)00225-6. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN. Hip contact forces and gain patterns from routine activities. J Biomech. 2001;34:859–871. doi: 10.1016/S0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 21.Shirazi-Adl A, Dammak M, Paiement G. Experimental determination of friction characteristics at the trabecular bone/porous-coated metal interface in cementless implants. J Biomed Mater Res. 1993;27(2):167. doi: 10.1002/jbm.820270205. [DOI] [PubMed] [Google Scholar]
- 22.Kerner J, Huiskes R, Lenthe GH, Weinans H, Rietbergen B, Engh CA, Amis AA. Correlation between pre-operative periprosthetic bone density and post-operative bone loss in THA can be explained by strain-adaptive remodelling. J Biomech. 1999;32(7):695. doi: 10.1016/S0021-9290(99)00041-X. [DOI] [PubMed] [Google Scholar]
- 23.Turner AWL, Gillies RM, Sekel R, Morris P, Bruce W, Walsh WR. Computational bone remodelling simulations and comparisons with DEXA results. J Orthop Res. 2005;23(4):705. doi: 10.1016/j.orthres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Huiskes R, Weinans H, Grootenboer HJ, Dalstra M, Fudala B, Slooff TJ. Adaptive bone-remodeling theory applied to prosthetic-design analysis. J Biomech. 1987;20(11–12):1135. doi: 10.1016/0021-9290(87)90030-3. [DOI] [PubMed] [Google Scholar]
- 25.Draenert K. Forschung und Fortbildung in der Chirurgie des Bewegungsapparates 2. Munich: Kastner & Callwey; 1988. [Google Scholar]
- 26.Maloney WJ, Sychterz C, Bragdon C, McGovern T, Jasty M, Engh CA, Harris WH (1996) The Otto Aufranc Award. Skeletal response to well fixed femoral components inserted with and without cement. Clin Orthop Relat Res (333):15 [PubMed]