Abstract
Purpose
The use of inappropriate cementation techniques has been suggested as an adverse factor for the long-term survival of hip-resurfacing arthroplasty. Inadequate initial fixation, thermal osteonecrosis and interface biological reactions are possible causes of failure. We analysed morphological changes associated with the cementation technique in a large collection of retrieved femoral components.
Methods
One hundred and fifty femoral components (mean time to failure of 8.3 months ± 11.0) obtained at revision surgery were analysed morphometrically and histopathologically. Cement mantle and penetration were quantified in six different regions of interest. Histopathological analysis of the bone–cement interface was performed on undecalcified processed bone tissue.
Results
The vast majority of the cases differed substantially from laboratory-based cement-penetration depth recommendations. Fifty-nine cases had a fibrous membrane at the cement–bone interface. This membrane was significantly thicker in cases with osteonecrosis compared to cases viable bone.
Conclusions
Our results demonstrate that most failures were cemented inappropriately. We suggest that poor cementation was an important adverse factor; however, the cause of the failures was obviously multifactorial. The thickness of the fibrous membrane at the cement–bone interface differed significantly between cases with osteonecrosis and specimens with viable bone tissue.
Introduction
Hip-resurfacing arthroplasty (HRA) has experienced a revival over the last decade. In 2009 in the United Kingdom, 33% of male patients under the age of 55 years who required primary hip replacement underwent a resurfacing procedure [1]. Femoral-head or -neck fractures [2, 3] and aseptic loosening of the femoral component [3, 4] are the most common early failure mechanisms of HRA. In later follow-up sessions, adverse soft-tissue reactions to metal wear seem to play an important role in prosthesis failures in a subset of patients. In particular, metallosis [5], pseudotumours [6], excessive intraosseous lymphocyte infiltration [7], proliferative desquamative synovitis [7, 8] and so-called aseptic lymphocytic-vasculitis-associated lesions (ALVAL) [9, 10] have been reported in cases revised for unexplained groin pain.
The success of cemented HRA is presumably based upon establishing solid bonds between implant and bone using cement. However, different cementing strategies apply to different implant designs [11]. Whereas some prosthetic designs allow a cement mantle of about 1 mm by providing a greater clearance between implant and bone, such as the Durom (Zimmer Orthopaedics, Warsaw, IN, USA), ASR (DePuy Orthopaedics, Warsaw, IN, USA) and ReCap (Biomet Orthopaedics, Warsaw, IN, USA), other designs such as the BHR (Smith & Nephew PLC, London, UK) or Cormet (Corin Medical, Cirencester, UK) have a tighter femoral component fit and allow minimal or no cement mantle [12–14].
Although many factors influence cement penetration into the bone remnant (cement amount, type, viscosity; timing and application type; quality of femoral bone remnant tissue; preparation of femoral remnant using jet lavage or drilling additional fixation holes), it is not possible to prove cement penetration in vivo, and recommendations regarding cement penetration are scarce. Several experimental studies recommend cement penetration into the bone of about 3–5 mm [15, 16] to achieve optimal fixation and prevent harmful polymerisation temperatures [17], to achieve successful long-term fixation. Several studies [11, 18, 19] emphasise the importance of proper cementation. However, investigations into the clinical realisation of the recommended cementation technique for HRA, including quantitative cement data, are rare [13]. The primary objective of this study was to quantify the cement mantle and penetration depth for each failed prosthesis and to analyse morphological changes associated with cement status.
Materials and methods
Patient demographics
In this international multicentre study, 150 femoral components of patients who had undergone revision surgery between 2004 and 2007 [64 (50.4%) men (mean age 56.4 years ± 9.2) and 63 (49.6%) women (mean age 55.5 years ± 10.4)] were analysed. Following revision surgery, all specimens were fixed in 4% buffered formalin solution and sent to our laboratory. Most specimens were early failures (mean time to failure averaged 8.3 months ± 11.0). Data on the reason for revision surgery were available in 125 cases: 90 (72.0%) failed due to atraumatic periprosthetic fracture, nine (7.2%) were revised for acetabular component loosening and 26 (20.8%) for pain or luxation of the femoral component (Table 1). Valid data on cement type were available for 56 of 150 retrievals. Correlation between patient age and time of implantation was close to zero (adjusted R² = 0.027; p = 0.038).
Table 1.
Characterisation of the study group according to cement status, time to failure, patient age and indication for revision
| No. | Cement mantle (mm) | Cement penetration (mm) | Time to failure (months) | Age (years) | Indication for revision (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 3 | Zone 1 | Zone 2 | Zone 3 | Fracture | Acetabular | Other | ||||
| Implant type | ||||||||||||
| Biomet: ReCap | 7 | 1.7 ± 0.6 | 1.4 ± 0.2 | 1.0 ± 0.3 | 3.2 ± 1.5 | 8.0 ± 3.8 | 4.8 ± 2.5 | 3.6 ± 4.5 | 62.4 ± 5.5 | 6 (85%) | 0 (0%) | 1 (15%) |
| Corin: Cormet | 15 | 2.7 ± 2.3 | 2.0 ± 2.0 | 1.0 ± 0.9 | 1.5 ± 2.7 | 2.4 ± 3.7 | 1.6 ± 2.1 | 35.1 ± 17.3 | 52.3 ± 11.3 | 2 (17%) | 1 (8%) | 9 (75%) |
| DePuy: ASR | 110 | 3.0 ± 2.1 | 1.6 ± 1.6 | 0.8 ± 0.8 | 2.8 ± 3.1 | 3.0 ± 3.7 | 2.0 ± 2.4 | 5.7 ± 6.5 | 56.4 ± 9.6 | 73 (80%) | 5 (5%) | 14 (15%) |
| S&N: BHR | 7 | 2.3 ± 1.5 | 2.2 ± 1.6 | 0.4 ± 0.5 | 3.5 ± 3.4 | 5.8 ± 4.7 | 4.7 ± 3.8 | 18.3 ± 10.8 | 46.7 ± 13.5 | 3 (50%) | 2 (25%) | 2 (33%) |
| Zimmer: Durom | 11 | 1.8 ± 2.2 | 2.2 ± 0.9 | 1.5 ± 0.6 | 4.0 ± 3.2 | 2.4 ± 2.2 | 2.0 ± 1.3 | 6.7 ± 5.1 | 55.9 ± 4.0 | 6 (75%) | 1 (17%) | 0 (0%) |
| Pooled | 150 | 2.7 ± 2.1 | 1.7 ± 1.5 | 0.9 ± 0.8 | 2.9 ± 3.0 | 3.3 ± 3.8 | 2.2 ± 2.5 | 8.3 ± 11.0 | 55.9 ± 4.0 | 90 (72%) | 9 (7.2%) | 26 (21%) |
| Sex | ||||||||||||
| Females | 64 | 3.0 ± 2.0 | 1.7 ± 1.5 | 0.9 ± 0.9 | 2.1 ± 2.6 | 2.6 ± 3.2 | 2.1 ± 2.6 | 7.2 ± 10.0 | 56.4 ± 9.2 | 48 (79%) | 4 (7%) | 9 (14%) |
| Males | 63 | 2.8 ± 2.3 | 1.6 ± 1.7 | 0.8 ± 0.7 | 3.5 ± 3.1 | 4.1 ± 4.4 | 2.4 ± 2.8 | 9.6 ± 12.0 | 55.5 ± 10.4 | 34 (67%) | 5 (10%) | 12 (23%) |
| Cement type | ||||||||||||
| Low Visc | 19 | 2.8 ± 1.8 | 2.1 ± 2.3 | 0.7 ± 0.8 | 3.7 ± 3.2 | 3.9 ± 4.6 | 2.9 ± 3.8 | 9.6 ± 14.8 | 55.6 ± 7.2 | 11 (64%) | 3 (18%) | 3 (18%) |
| Medium Visc | 9 | 2.3 ± 1.2 | 1.9 ± 1.9 | 1.2 ± 1.3 | 2.5 ± 2.2 | 2.7 ± 2.9 | 2.4 ± 3.5 | 5.1 ± 3.4 | 54.7 ± 5.1 | 8 (89%) | 0 (0%) | 1 (11%) |
| High Visc | 28 | 3.2 ± 2.3 | 1.5 ± 1.4 | 0.8 ± 0.6 | 2.1 ± 2. | 3.6 ± 4.5 | 2.3 ± 2.7 | 4.4 ± 4.9 | 55.2 ± 12.2 | 22 (92%) | 0 (0%) | 2 (8%) |
| Implant Size | ||||||||||||
| Small | 33 | 2.5 ± 2.0 | 1.4 ± 1.3 | 0.8 ± 0.7 | 3.0 ± 2.8 | 3.7 ± 4.2 | 2.2 ± 2.3 | 9.7 ± 9.8 | 55.5 ± 10.8 | 21 (78%) | 1 (4%) | 5 (18%) |
| Medium | 116 | 2.8 ± 2.1 | 1.7 ± 1.6 | 0.9 ± 0.8 | 2.8 ± 3.1 | 3.2 ± 3.7 | 2.3 ± 2.5 | 7.9 ± 11.3 | 56.0 ± 9.4 | 68 (70%) | 8 (8%) | 21 (22%) |
| Large | 1 | 2.8 ± 0.0 | 3.3 ± 0.0 | 2.0 ± 0.0 | 1.0 ± 0.0 | 0.5 ± 0.0 | 0.0 ± 0.0 | 4.4 ± 4.9 | 55.2 ± 12.2 | 1 (100%) | 0 (0%) | 0 (0%) |
visc viscosity
Material
Five different implant types were investigated: ReCap, Cormet, ASR, BHR and Durom.
Undecalcified preparation, grinding and histology
The standard preparation technique is described in detail elsewhere [20]. Briefly, a 4-mm slice of the femoral head was sectioned in the coronal plane, leaving the implant bone composite and the cemented interface intact. For further preparation, the specimens were subjected to an undecalcified infiltration process [7, 20–22] to preserve the cement status [21]. The specimens were ground and stained with toluidine blue [21] (Fig. 1). Ground specimens with in situ cement and metal components were analysed morphologically and microscopically. Up to four additional histological sections – depending on the amount of available bone tissue under the femoral component – were prepared in the anteroposterior plane. The undecalcified samples were plastic embedded, cut into 5-μm sections, and stained using the von Kossa technique, toluidine blue and the Goldner trichrome.
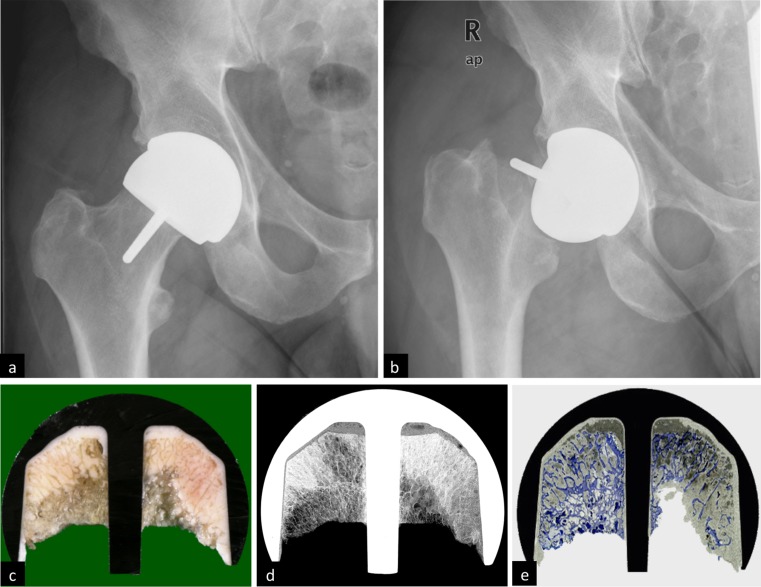
Fig. 1.
a Postoperative anteroposterior radiograph after hip-resurfacing arthroplasty (HRA) and b subsequent failure 2 months later. c For failure analysis, all specimens were dissected with a diamond-coated saw, d subjected to contact X-rays and e processed to give 300-μm-thick specimens for full appreciation of bone structure and cement beneath the metal surface
We specifically focused on a histopathological analysis of the cement–bone interface with regard to morphological changes in the interface membrane. We quantified the occurrence and width of the fibrous interface membrane. Osteonecrosis (ON) was microscopically defined by the presence of trabeculae without stainable osteocytes, disorganised bone marrow and bordering fibrosis [23].
Quantitative cement data analysis
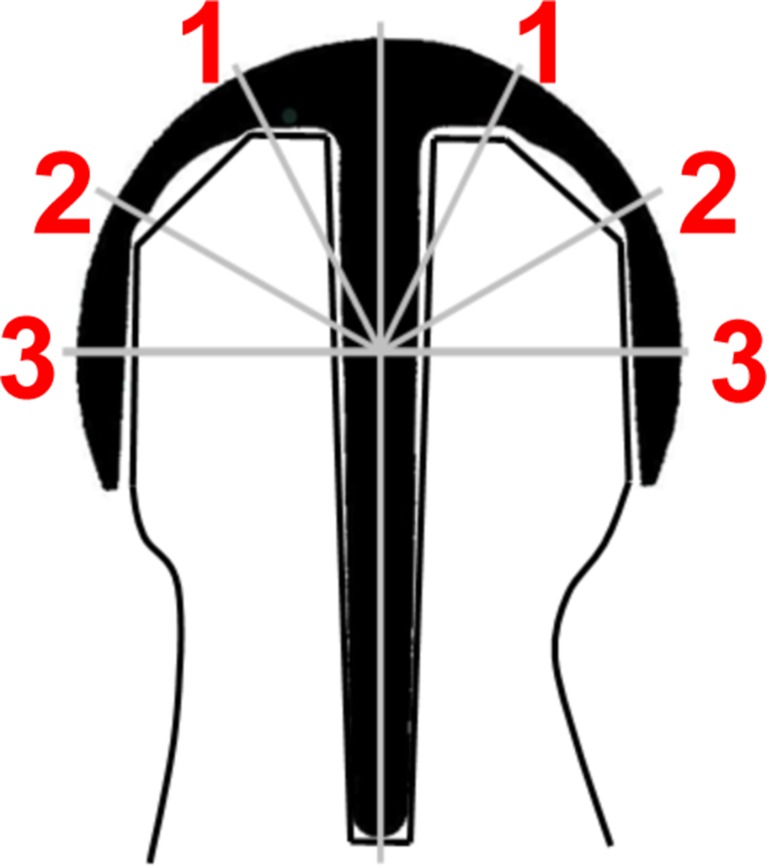
The cement mantle was defined as the cement layer between the resurfacing component and the border of the cancellous bone. Cement penetration was defined as the interdigitating cement material between the surface of the reamed bone and deeper bone tissue. Cement thickness was determined directly from the ground specimens using a measuring eyepiece (Carl Zeiss AG, Germany, accuracy 0.1 mm). Cement measurements were taken from the dome of the cap (zone 1), the intermediate (zone 2), and the radial (zone 3) region on each side of the stem (Fig. 2) [18]. Regions of interest (ROIs) with cement-filled cysts or cement pegs were excluded from the analysis. Previous studies show that the depth of cement penetration seems to be independent of implant design [24]. As there are no predefined product-specific values, we further analysed cement penetration with respect to laboratory recommendations based on experimental data, stating that cement penetration should ideally measure 3–5 mm [15, 16, 18, 25]. Histopathological analysis of the femoral remnant was also performed in each case.
Fig. 2.
Different regions of interest (1–3) used to analyse cement-mantle thickness and penetration
Statistical analysis
Statistical analysis was conducted using SPSS 17.0 (SPSS Inc. 2008, Chicago, IL, USA). Mean values ± standard deviations (SD) are reported for each implant type. Student’s t tests and analysis of variance (ANOVA) were used to assess univariate comparisons of mantle thickness and penetration; chi-square tests were performed to test univariate relations between categorical variables. ANOVA was conducted to determine the relations between femoral component size, patient age and gender, implant time in situ, cement and implant type and cement mantle thickness and depth of penetration. All statistical tests were two sided, with a significance level of 5%. Linear regression analysis was used to assess the relative effect of age on implant survival until revision.
Results
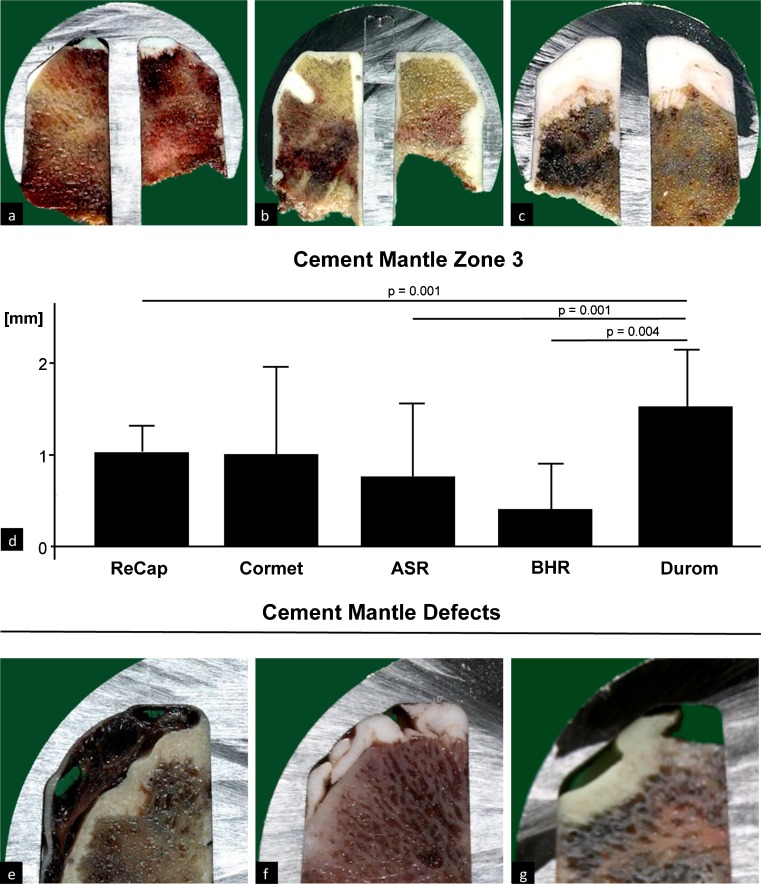
Cement mantle analysis revealed no substantial differences regarding prosthetic designs between zones 1 and 2 (Table 1). In zone 3, the cement mantle was substantially greater in Durom, at 1.5 mm ± 0.6, compared with ReCap (1.0 mm ± 0.3, p = 0.001), ASR (0.8 mm ± 0.8, p = 0.001) and BHR (0.4 mm ± 0.5, p = 0.004) (Fig. 3d) (Table 1). However, no differences between Durom and Cormet (1.0 mm ± 0.9) could be found. Overall, the mantle was thickest in zone 1 (2.72 mm ± 2.07), followed by zone 2 (1.66 mm ± 1.52) and zone 3 (0.86 mm ± 0.78). Eleven cases showed major cement-mantle defects, most likely caused intraoperatively by bleeding (Fig. 3e), folded cement layers (Fig. 3f) or trapped air (Fig. 3g).
Fig. 3.
Heterogeneity in cement-mantle thickness. Examples of a a rather thin, b regular and c excessively thick cement mantle. d Cement-mantle analysis for zone 3 revealed substantial differences regarding prosthesis design: it was thicker in Durom than in BHR, ASR and ReCap. Examples of cement/mantle defects: e improperly seated cemented femoral component and osseous remnant, with bleeding into the empty area, f irregularly folded cement mantle, g trapped air mixed into the cement mantle
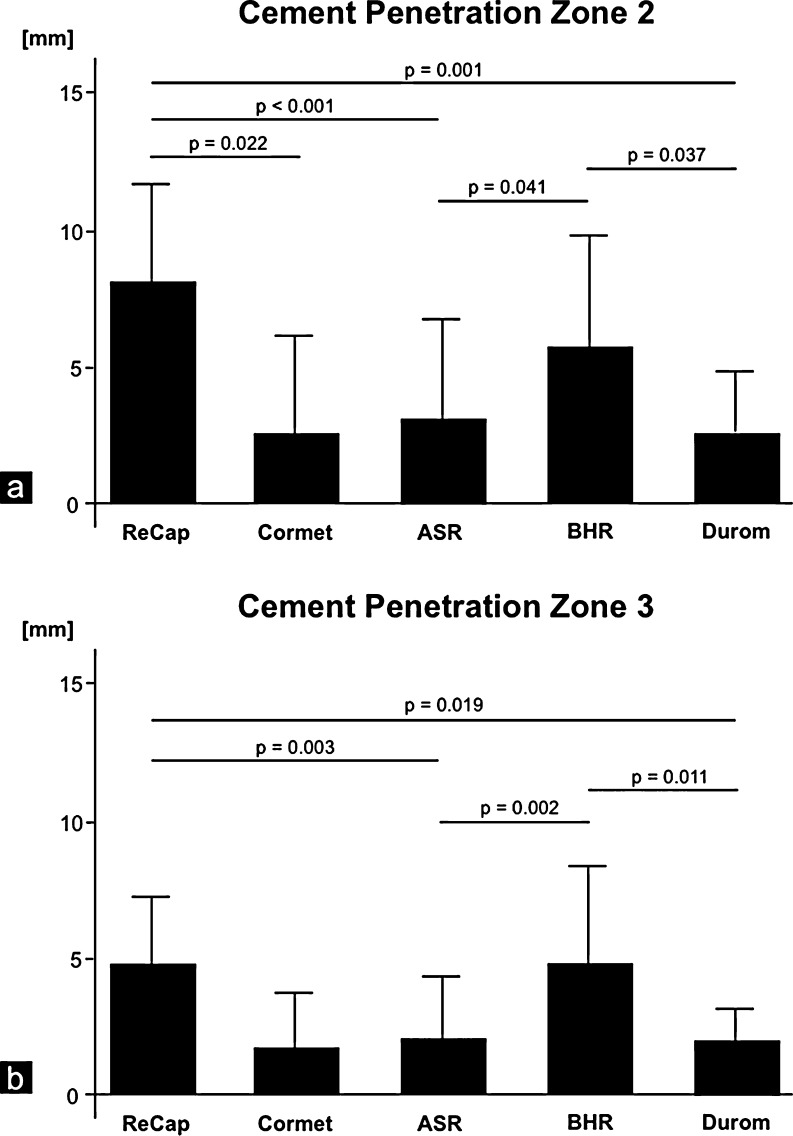
Implant design also had a substantial influence on cement penetration. Although 567 (65.1%) of 871 analysed ROIs showed cement penetration <3 mm, 164 (18.8%) ROI displayed a penetration depth >5 mm (Fig. 4). The deepest cement penetration values were recorded in zone 2 (3.27 mm ± 3.78). No significant differences were found in zone 1. ReCap (8.0 mm ± 3.8) showed greater cement penetration in zone 2 than did ASR (3.0 mm ± 3.7, p < 0.001), Cormet (2.4 mm ± 3.7, p = 0.022) or Durom (2.4 mm ± 2.2, p = 0.001). In addition, differences were found between BHR (5.8 mm ± 4.7) and Durom (p = 0.037) and ASR (p = 0.041) (Fig. 5a) (Table 1). For zone 3, ReCap (4.8 mm ± 2.5) and BHR (4.7 mm ± 3.8) showed greater cement penetration in comparison to ASR (2.0 mm ± 2.4; ReCap p = 0.003; BHR p = 0.002) and Durom (2.0 mm ± 1.3 ReCap p = 0.019; BHR p = 0.011) (Fig. 5b) (Table 1). The deepest cement penetration values were recorded in zone 2 (3.27 mm ± 3.78). A cement penetration depth of 3–5 mm for all ROI, as suggested by numerous experimental studies, was only found in one case (0.68%).
Fig. 4.
Cement penetration: a–c Examples demonstrate the variety from none, d–f to normal or g–i deep cement penetration. At a higher resolution, the specimens revealed how the cement penetrates the cancellous bone and how trabecular bone is trapped within the cement
Fig. 5.
a Cement penetration in zone 2 was greatest in ReCap. b In zone 3, however, cement penetration was lowest in ASR and Durom compared with BHR and ReCap
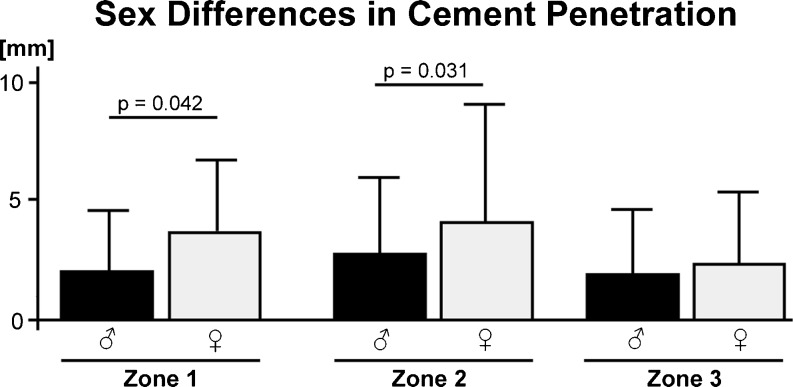
Multivariate analysis confirmed significant independent differences in penetration between male and female patients. In specimens from women, cement penetration was significantly deeper than in men in zone 1 (mean penetration 3.53 mm ± 3.12 compared with 2.15 mm ± 2.60 in men; p = 0.042) and zone 2 (4.07 mm ± 4.43 compared with 2.56 mm ± 3.21 in men; p = 0.031). Zone 3 showed no significant differences (p = 0.879) (Fig. 6). No relationship was found between cement viscosity and depth of penetration (p = 0.947).
Fig. 6.
Analysis for sex-specific differences showed significantly higher cement penetration in women for zones 1 and 2 but not in zone 3
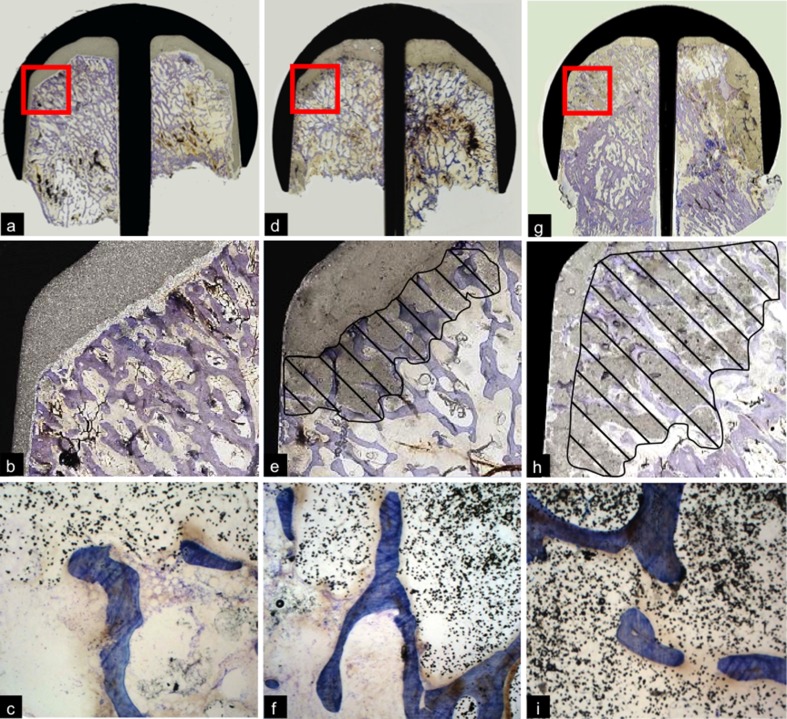
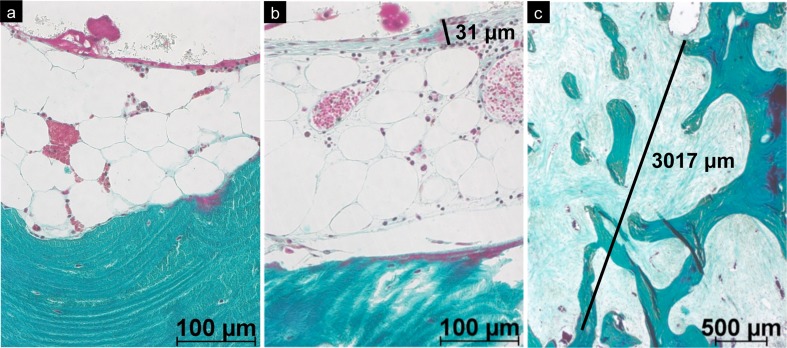
A fibrous interface membrane was microscopically detected in 59 (39.3%) of 150 cases. Interestingly, the thickness of the fibrous membrane varied substantially and was correlated with other histopathological findings, particularly with the presence of osteonecrosis (ON) (Fig. 7). The fibrous membrane was thicker in specimens with ON (mean thickness of fibrous membrane 1.01 mm ± 3.26) than in hips with viable bone remnants (mean thickness of fibrous membrane 0.31 mm ± 1.13; p = 0.021, Kruskal–Wallis test). Furthermore, fibrous membrane thickness increased in cases with a morphologically defined mode of loosening of the femoral component [4] in the following order: cement–implant debonding (mean thickness of fibrous membrane 0.1 mm ± 0.14), loosening of the bone–cement interface (mean thickness of fibrous membrane 0.49 mm ± 1.05), collapsed ON (mean thickness of fibrous membrane 0.88 mm ± 1.05) and pseudoarthrosis (mean thickness of fibrous membrane 1.18 mm ± 1.32, p = 0.256, Kruskal–Wallis test). Statistical analysis of the latter comparison found no significant differences, but it should be noted that two groups contained fewer than five cases each.
Fig. 7.
Bone–cement interface in retrieved hip-resurfacing arthroplasty (HRA) specimens: a Specimen with viable bone and absent fibrous interface membrane. A discrete layer of macrophages was visible on the surface of the cement (plastic embedding; stain: Goldner trichrome, original magnification ×200). b Thin fibrous membrane on the bone–cement interface (plastic embedding; stain: Goldner trichrome, original magnification ×200). c Thick fibrous membrane (mid) at the border of advanced osteonecrosis (above; plastic embedding; stain Goldner trichrome, original magnification ×25)
Discussion
Experimental studies [16, 18, 25] have demonstrated the importance of proper cementation, particularly against the background of the different cementing philosophies being applied to the different implant designs [11]. However, clinical retrieval analyses of present-generation HRAs, demonstrating the cementation technique and associated structural changes beneath the femoral component, are rare [13]. Our study demonstrated substantial variability in the cement mantle thickness in failed HRA. Some cases had regions with a cement mantle over ten millimetres; there were also cases in which the same prosthesis design had no cement mantle at all. Furthermore, we observed the presence of cement mantles of up to several millimetres in designs in which the manufacturer intended practically no cement mantle, which is in accordance with the results of Campbell et al. [24]. In zone 3, the cement mantle in the Durom prosthesis was substantially greater than that found in other designs (ReCap, ASR and BHR), which might be explained by the greater gap between the implant and the edge provided by the implant. However, we observed no substantial differences in cement mantle regarding specific design features of zones 1 and 2. An adequate cement mantle is considered a basic requirement for mechanical stability [18]; however, an excessive mantle might cause incorrect positioning, high local intraosseous temperatures [26] or femoral-neck lengthening – notably associated with altered bending moments [2] and the possibility of uncovered reamed bone.
Our specimens also showed a wide range of cement penetration depths. Due to the lack of specific manufacturers’ specifications, we evaluated the penetration depths according to laboratory-based recommendations of three to five millimetres. With respect to these recommendations only one case complied with all ROIs. Almost 20% of the analysed ROIs presented cement penetration depths over five millimetres. Excessive cement penetration was found in particular in zone 2 of the ReCap prostheses (average penetration 8.0 ± 3.8 mm). In agreement with Campbell et al., who considered values over five millimetres to be excessive [13], large cement masses have been demonstrated to reach temperatures of up to 68°C [26]. This could result in thermally induced ON [19, 27] or reduced osseointegration [28]. Even though large masses of cement were observed in our specimens, the characteristic features of direct thermal injury to the viable bone, or ON, were not identifiable. This might reflect the longer time period (several months) between implantation surgery and revision [23]. In this study, more than half of all periprosthetic fractures were classified as postnecrotic fractures [2]. Despite the absence of evidence of thermal damage to bone remnants in retrieval studies, other potentially adverse effects of inappropriate surgical or cementing techniques were suggested: arterial damage [29] and cardiopulmonary complications [26, 30].
Obviously, the thickness of the cement mantle, and the depth of cement penetration, is influenced by the cementing technique used. Reasons for high penetration depths include the use of jet lavage, especially at a high pressure in osteopenic bone, which itself is another influencing parameter [31]. Cement viscosity has also been regarded as a very important factor [32, 33]. However, we were not able to prove a significant influence of cement viscosity on the depth of penetration in vivo. We are aware of the limited validity of our data, as - despite the international study design and the participation of 68 surgical departments from 18 countries worldwide - detailed information on cement viscosity was only available in one third of the study cases.
Concerning the development of fibrous membranes: Major biomechanical stresses seem to be absorbed by the implant itself; mechanical factors that possibly lead to repetitive trauma to the interface bone seem to play a subordinate role in the development of fibrous membranes in well-fixed femoral components. In fact, we did not detect a fibrous membrane in many of cases with a well-fixed femoral component, which is consistent with findings reported by Morberg et al. [34]. The cases with typical bone–cement loosening [4], however, demonstrated a continuous fibrous membrane over the entire surface of the remnant bone tissue.
We recognise the fact that there are several limitations to this study. First, this morphological and morphometric investigation was performed in the setting of a retrospective, international multisurgeon study over several years. Therefore, specific information, such as detailed descriptions of surgical and cementation techniques or the duration of implantation surgery, was not available in all cases. Furthermore, we had no cadaver specimens and were not able to include an adequate control group of functioning HRAs. Our results, however, showed considerable variability in cementation characteristics in hips retrieved from resurfacing arthroplasty. Furthermore, the vast majority of specimens showed a cement mantle and penetration thickness that substantially differed from the values recommended in recent experimental studies. As we were unable to identify a specific mode of failure associated with the cementing techniques, the cause of these failures was probably multifactorial. We also demonstrated substantial differences in the thickness of the interface fibrous membrane in cases with distinct failure modes.
Acknowledgments
We thank all cooperating orthopaedic surgeons who provided us with cases. The authors further thank Dr. Christine zu Eulenburg for conducting statistical analyses. The Hamburg Retrieval Study on Hip Resurfacing was supported by Biomet Orthopaedics (Warsaw, IN, USA), Corin Medical (Cirencester, UK), DePuy Orthopaedics International (Leeds, UK), Smith and Nephew (London, UK), and Zimmer (Warsaw).
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Matthias Krause and Stefan Breer contributed equally to this work and share first authorship
References
- 1.National Joint Registry for England and Wales. 7th Annual Report. National Joint Registry Steering Committee.
- 2.Zustin J, Krause M, Breer S, Hahn M, Domarus C, Ruther W, Sauter G, Morlock MM, Amling M. Morphologic analysis of periprosthetic fractures after hip resurfacing arthroplasty. J Bone Joint Surg Am. 2010;92:404–410. doi: 10.2106/JBJS.H.01113. [DOI] [PubMed] [Google Scholar]
- 3.Amstutz HC, Duff MJ. Eleven years of experience with metal-on-metal hybrid hip resurfacing: a review of 1000 conserve plus. J Arthroplasty. 2008;23:36–43. doi: 10.1016/j.arth.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Zustin J, Hahn M, Morlock MM, Ruther W, Amling M, Sauter G. Femoral component loosening after hip resurfacing arthroplasty. Skeletal Radiol. 2010;39:747–756. doi: 10.1007/s00256-009-0862-z. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs JJ, Urban RM, Hallab NJ, Skipor AK, Fischer A, Wimmer MA. Metal-on-metal bearing surfaces. J Am Acad Orthop Surg. 2009;17:69–76. doi: 10.5435/00124635-200902000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 7.Zustin J, Amling M, Krause M, Breer S, Hahn M, Morlock MM, Ruther W, Sauter G. Intraosseous lymphocytic infiltrates after hip resurfacing arthroplasty : a histopathological study on 181 retrieved femoral remnants. Virchows Arch. 2009;454:581–588. doi: 10.1007/s00428-009-0745-7. [DOI] [PubMed] [Google Scholar]
- 8.Burkandt A, Katzer A, Thaler K, Baehr V, Friedrich RE, Ruther W, Amling M, Zustin J. Proliferation of the synovial lining cell layer in suggested metal hypersensitivity. In Vivo. 2011;25:679–686. [PubMed] [Google Scholar]
- 9.Reito A, Puolakka T, Pajamaki J. Birmingham hip resurfacing: five to eight year results. Int Orthop. 2011;35:1119–1124. doi: 10.1007/s00264-010-1066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 11.Beaule PE, Matar WY, Poitras P, Smit K, May O. 2008 Otto Aufranc Award: component design and technique affect cement penetration in hip resurfacing. Clin Orthop Relat Res. 2009;467:84–93. doi: 10.1007/s11999-008-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimmin A, Beaule PE, Campbell P. Metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am. 2008;90:637–654. doi: 10.2106/JBJS.G.01012. [DOI] [PubMed] [Google Scholar]
- 13.Campbell P, Takamura K, Lundergan W, Esposito C, Amstutz HC. Cement technique changes improved hip resurfacing longevity - implant retrieval findings. Bull NYU Hosp Jt Dis. 2009;67:146–153. [PubMed] [Google Scholar]
- 14.Heisel C, Kleinhans JA, Menge M, Kretzer JP. Ten different hip resurfacing systems: biomechanical analysis of design and material properties. Int Orthop. 2009;33:939–943. doi: 10.1007/s00264-008-0607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krause WR, Krug W, Miller J (1982) Strength of the cement-bone interface. Clin Orthop Relat Res 290–299 [PubMed]
- 16.Bitsch RG, Heisel C, Silva M, Schmalzried TP. Femoral cementing technique for hip resurfacing arthroplasty. J Orthop Res. 2007;25:423–431. doi: 10.1002/jor.20311. [DOI] [PubMed] [Google Scholar]
- 17.Askew MJ, Steege JW, Lewis JL, Ranieri JR, Wixson RL. Effect of cement pressure and bone strength on polymethylmethacrylate fixation. J Orthop Res. 1984;1:412–420. doi: 10.1002/jor.1100010410. [DOI] [PubMed] [Google Scholar]
- 18.Chandler M, Kowalski RS, Watkins ND, Briscoe A, New AM. Cementing techniques in hip resurfacing. Proc Inst Mech Eng H. 2006;220:321–331. doi: 10.1243/09544119JEIM113. [DOI] [PubMed] [Google Scholar]
- 19.Little JP, Gray HA, Murray DW, Beard DJ, Gill HS. Thermal effects of cement mantle thickness for hip resurfacing. J Arthroplasty. 2008;23:454–458. doi: 10.1016/j.arth.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Morlock MM, Bishop N, Ruther W, Delling G, Hahn M. Biomechanical, morphological, and histological analysis of early failures in hip resurfacing arthroplasty. Proc Inst Mech Eng H. 2006;220:333–344. doi: 10.1243/095441105X69015. [DOI] [PubMed] [Google Scholar]
- 21.Hahn M, Vogel M, Delling G. Undecalcified preparation of bone tissue: report of technical experience and development of new methods. Virchows Arch A Pathol Anat Histopathol. 1991;418:1–7. doi: 10.1007/BF01600238. [DOI] [PubMed] [Google Scholar]
- 22.Morlock MM, Bishop N, Stahmer F, Zustin J, Sauter G, Hahn M, Krause M, Ruther W, Amling M. Reasons for failure of hip resurfacing implants. A failure analysis based on 250 revision specimens. Orthopade. 2008;37:695–703. doi: 10.1007/s00132-008-1298-1. [DOI] [PubMed] [Google Scholar]
- 23.Zustin J, Sauter G, Morlock MM, Ruther W, Amling M. Association of osteonecrosis and failure of hip resurfacing arthroplasty. Clin Orthop Relat Res. 2010;468:756–761. doi: 10.1007/s11999-009-0979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell P, Beaule PE, Ebramzadeh E, LeDuff M, Smet K, Lu Z, Amstutz HC. The John Charnley Award: a study of implant failure in metal-on-metal surface arthroplasties. Clin Orthop Relat Res. 2006;453:35–46. doi: 10.1097/01.blo.0000238777.34939.82. [DOI] [PubMed] [Google Scholar]
- 25.Beaule PE, Campbell P, Lu Z, Leunig-Ganz K, Beck M, Leunig M, Ganz R. Vascularity of the arthritic femoral head and hip resurfacing. J Bone Joint Surg Am. 2006;88(Suppl 4):85–96. doi: 10.2106/JBJS.F.00592. [DOI] [PubMed] [Google Scholar]
- 26.Gill HS, Campbell PA, Murray DW, Smet KA. Reduction of the potential for thermal damage during hip resurfacing. J Bone Joint Surg Br. 2007;89:16–20. doi: 10.1302/0301-620X.89B1.18369. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh PH, Tai CL, Liaw JW, Chang YH. Thermal damage potential during hip resurfacing in osteonecrosis of the femoral head: an experimental study. J Orthop Res. 2008;26:1206–1209. doi: 10.1002/jor.20639. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson RA, Albrektsson T. The effect of heat on bone regeneration: an experimental study in the rabbit using the bone growth chamber. J Oral Maxillofac Surg. 1984;42:705–711. doi: 10.1016/0278-2391(84)90417-8. [DOI] [PubMed] [Google Scholar]
- 29.Aust JC, Bredenberg CE, Murray DG. Mechanisms of arterial injuries associated with total hip replacement. Arch Surg. 1981;116:345–349. doi: 10.1001/archsurg.1981.01380150063017. [DOI] [PubMed] [Google Scholar]
- 30.Andersen KH, Nielsen JM. Heat of polymerization of bone cement can induce cardiac arrest. Ugeskr Laeger. 1998;160:4905–4906. [PubMed] [Google Scholar]
- 31.Bitsch RG, Jager S, Lurssen M, Loidolt T, Schmalzried TP, Clarius M. Influence of bone density on the cement fixation of femoral hip resurfacing components. J Orthop Res. 2010;28:986–991. doi: 10.1002/jor.21094. [DOI] [PubMed] [Google Scholar]
- 32.Bitsch RG, Loidolt T, Heisel C, Schmalzried TP. Cementing techniques for hip resurfacing arthroplasty: in vitro study of pressure and temperature. J Arthroplasty. 2011;26:144–151. doi: 10.1016/j.arth.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Bitsch RG, Jager S, Lurssen M, Loidolt T, Schmalzried TP, Weiss S (2011) The influence of cementing technique in hip resurfacing arthroplasty on the initial stability of the femoral component. Int Orthop [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 34.Morberg P, Albrektsson T, Reigstad A, Rokkum M, Lindgren U, Svensson O, Romanus B. A qualitative and quantitative study of retrieved femoral heads in three different types of resurface hip arthroplasties. J Arthroplasty. 1993;8:617–624. doi: 10.1016/0883-5403(93)90009-S. [DOI] [PubMed] [Google Scholar]