Abstract
Purpose
Pentoxifylline (PTX) is a derivative of methylxanthine and is used in peripheral vascular and cerebrovascular diseases for its effect on the regulation of blood circulation. We investigated whether PTX could be beneficial for femoral head osteonecrosis associated with steroid through these effects.
Methods
Sixty mature Leghorn type chickens were chosen and divided into three groups. The 25 chickens in group A were given a weekly dose of 3 mg/kg/week methylprednisolone acetate intramuscularly. Four chickens in group B died after the first drug injection and were excluded from the study. Therefore, the remaining 21 chickens in group B were additionally given 25 mg/kg/day pentoxifylline intramuscularly, along with the steroid medication as given in group A. The ten chickens in group C were not given any injections, as they were accepted as the control group. After the sacrifice of the animals at week 14, both femoral heads were taken from each animal. The animals which died along the course of the study also underwent pathological examination but were not a part of the statistical analysis.
Results
In this study, steroid induced femoral head osteonecrosis has been experimentally observed in chickens after high doses of corticosteroid therapy. The chickens were given pentoxifylline in order to prevent the effects of steroid on bones and bone marrow. The results showed that chickens are suitable osteonecrosis models, and that steroid causes adipogenesis and necrosis in the bone marrow and the death of the subchondral bone.
Conclusions
The results of this study hint at the assumption that PTX may have a positive benefit on ONFH. PTX seems to minimise the effects of the steroid and reduce the incidence of ONFH.
Introduction
The incidence of osteonecrosis of the femoral head (ONFH) has been on the rise in recent years due to the widespread use of steroid medication for immunosuppression, especially in transplant patients and in the treatment of rheumatic diseases [1–3]. However, the pathogenesis of steroid-induced ONFH has not yet been identified. Furthermore, protective and therapeutic methods have not yet proven successful either [4, 5]. Many researchers consider that systemic or local alterations of lipid metabolism may have an important role in steroid-induced ONFH. It was suggested that occlusion of arteries mainly located in the subchondral area by fat embolism or compression of sinusoids by accumulation of bone marrow fat stores and consequent arterial obstruction or lipid accumulation within osteocytes were responsible for ONFH [6–14]. Prevention of ONFH with antilipidemic drugs in test animals further supported this theory [14–20]. Recently, a direct adverse effect of steroids on osteoblastogenesis resulting in apoptosis of osteoblasts and osteocytes was shown in both experimental and human studies [5, 21–23]. PTX is a derivative of methylxanthine and is used in cerebral and peripheral vascular diseases for its effect on the regulation of blood circulation. It increases the microcirculation and tissue oxygenation by decreasing the viscosity of the blood and increasing the erythrocyte flexibility [24, 25]. In bone, pentoxifylline reduces osteoclast activation, increases the osteoblast differentiation and enhances bone morphogenetic protein-2(BMP-2)-induced new bone formation [26–30]. Previous studies have shown that PTX may be useful for treatment of prosthetic loosening, bisphosphonate-associated osteonecrosis and radiotherapy related chondrocyte apoptosis [26, 31, 32]. We postulated that PTX might also be beneficial for the prevention of steroid-induced osteonecrosis of the femoral head.
Material and methods
Steroid and pentoxifylline medication on the animals
This study was carried out in the animal laboratory of the Veterinary Faculty of Istanbul University. Institutional review board approval was obtained prior to the study. An animal osteonecrosis model described by Cui et al. [15] was used. Sixty male mature Leghorn type chickens were chosen and randomly divided into three groups. All animals in each group were fed the same standard diet adding two units of oksitetracycline (Geosol powder, Vetas, Turkey) prophylactically. All the animals in each group were weighed prior to the study and their medication doses were determined according to their weights. The 25 chickens in group A were injected with a weekly dose of 3 mg/kg/week methylprednisolone acetate (Depo-medrol 40 mg flacon, Eczacibasi, Turkey). The 25 chickens in group B were also given 25 mg/kg/day pentoxifylline (Trentilin 100 mg ampule, Santa Farma, Turkey) intramuscularly. Four chickens in group B died after the first drug injection and were excluded from the study. Therefore, the remaining 21 chickens in group B were given 25 mg/kg/day pentoxifylline intramuscularly, together with the steroid medication as given in group A. No treatment was given to the ten animals of the control group (group C). Animals were sacrificed with an overdose of i.v. pentobarbital sodium injection in the 14th week.
Histological preparation and grading
Both femoral heads were taken from every animal and cut along the coronal plane. Bone samples were fixed in 40% formalin, decalcified in 10% formic acid and put into paraffin. Five-micron serial cross-sections were cut by microtome and samples were stained with haematoxylin and eosin. The pathological changes in the femoral heads were classified into four stages according to the method described by Matsui et. al. [33] and modified by Cui et. al. [15] (Table 1). Two investigators experienced in bone pathology, blinded to treatment protocols of the groups, assessed the slides individually.
Table 1.
The pathological classification of osteonecrosis
| Grade | Histological changes |
|---|---|
| 0 | Normal histology |
| 1 | Proliferation of the fat cells and bone marrow oedema |
| 2 | Bone marrow necrosis without trabecular necrosis, cytolysis, karyorrhexis, karyolysis in the bone cells and bone marrow |
| 3 | Additional to the changes in grade 2, subchondral bone death, resorption and new / live bone formation surrounding the dead trabeculae |
Statistical analysis
Statistical analysis was done on GraphPad Prisma V.3 program for Windows (Graphpad Software, San Diego, CA). Besides average and standard deviation, the data was evaluated using the Kruskal-Wallis test in the intergroup comparisons, Wilcoxon signed rank test for percent change from zero, and chi-square test for equal proportions in the qualitative comparisons. Results were evaluated in the p < 0.05 level of significance.
Results
The body weight of the chickens which were given steroid injections (groups A and B) decreased from their initial weight. (20.22 ± 4.98% and 18.77 ± 7.06 %, respectively). The decrease in the control group was only 0.46 ± 1.51%. The decrease in body weight of the chickens in groups A and B was significantly higher from that of the control group (P < 0.05).
During the 14 weeks, there were 12 (48%) deaths in group A, and there were 11 (52%) deaths in group B. The animals which died also underwent pathological examination, but were not included in the final statistical analysis. We did not see any visible change in any femurs or femoral heads in the macroscopic examination. All samples were seen to have intact joint cartilage. No deformity or collapse was observed.
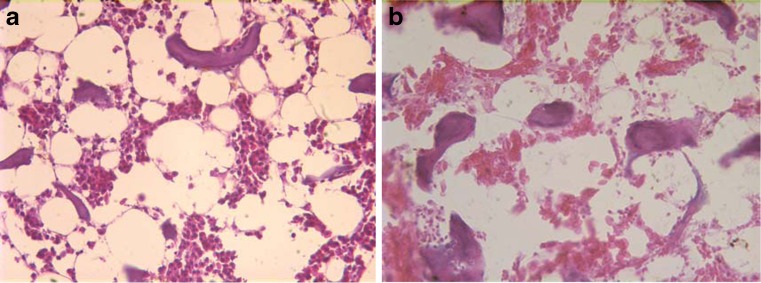
In the frontal cross-sectional preparations prepared from animals that died in the third week from group A, there was a pale and yellowish appearance seen macroscopically. In the microscopic examination, we determined remarkable fat cell proliferation in the bone marrow taken from all of the animals which died in group A in the third week. Despite the increase in both the number and size of the fat cells, and bone marrow oedema in all of the femoral heads in group A, group B experiments showed no pathological change at week three. In the femoral head cross-section examination of the chickens that died in the fifth week from group A, we found decreased haematopoesis and focal bone marrow necrosis in six out of the eight femoral heads (grade 2 osteonecrosis). We also determined no pathological change in the group B cross-section examinations at week 5 (two femoral heads) (Fig. 1).
Fig. 1.
a In the third week of steroid medication, there is significant fat cell proliferation in the bone marrow. An increase in the size and number of fat cells, and bone marrow oedema is observed in group A (haematoxylin + eosin ×400). b Focal necrosis areas and decreased heamatopoesis in the bone marrow in week 5 resulting from steroid medication in group A (grade 2 osteonecrosis) (haematoxylin + eosin ×400)
In the examination of the dead chickens in the eighth week from group A, we found wide bone marrow necrosis and decreased haematopoesis in two out of four femoral heads of two animals, and widespread bone marrow oedema and fat cell proliferation in the other two femoral heads. Only one of the eight femoral heads showed grade 1 osteonecrosis in group B. In all of the six femoral heads we examined from group A at week 11, we found grade 1 and 2 osteonecrosis according to the grading system. Four of the femoral heads were grade 2, and two were grade 1 osteonecrosis. In the examinations of group B in week 11, we found no pathological change in two out of six femoral heads (grade 0), and four of them were seen to have bone marrow oedema and an increase in the number of fat cells (grade 1). In the examination of 22 femoral heads from group B in weeks 3, 5, 8, and 11, we determined a significant decrease in the number and size of the fat cells compared to group A in adipogenesis, and only 11 of the group B femoral heads (50%) developed bone oedema at week 11 (Fig. 2).
Fig. 2.
a In the steroid medicated group A, necrosis is seen in the bone marrow cells in week 8 (grade 2 osteonecrosis) (haematoxylin + eosin ×400). b Steroid and pentoxifylline medicated group B experiments which showed no pathological changes in week 11 (grade 0) (haematoxylin + eosin ×100)
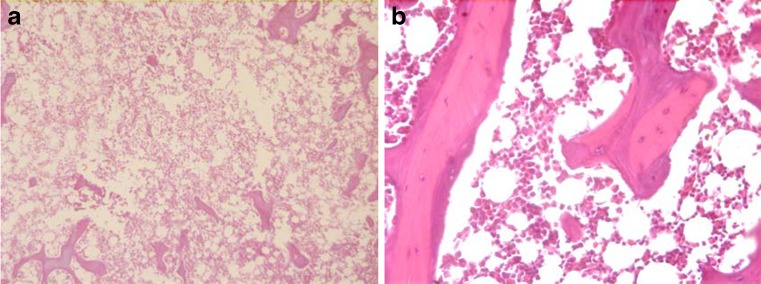
In the macroscopic examination, no change in any femur or femoral head was found. In group A at week 14, grade 2 or 3 osteonecrosis was found in 24 out of 26 (16 were grade 2 and eight were grade 3) femoral heads. The specimens with grade 3 necrosis, which showed widespread bone marrow necrosis, did not have subchondral bone trabeculae and showed that the former trabecules were being replaced by new bone trabeculae which were thinner in formation and fewer in number. In group B in week 14, we found no pathological change in 13 out of the 20 femoral heads (grade 0), while seven of them were seen to have bone marrow oedema and an increase in the number of fat cells (grade 1) (Fig. 3). The difference in the pathological grading between group A and group B was statistically significant (p < 0.05). The specimens of the control group did not show any pathological change at week 14. Histological results are summarised in Table 2.
Fig. 3.
a In group A, trabecular bone is shown to be disappearing in week 14 (haematoxylin + eosin ×100). b Group B experiments show no pathological change in week 14 (grade 0) (haematoxylin + eosin ×40)
Table 2.
Pathological grading of the femoral heads at week 14
| Groups | Number of femoral heads | Percentage | |
|---|---|---|---|
| Steroid (group A) | Grade 1 | 2 | 8% |
| Grade 2 | 16 | 62% | |
| Grade 3 | 8 | 31% | |
| Steroid + PTX (group B) | Grade 0 | 13 | 65% |
| Grade 1 | 7 | 35% | |
| P value | χ² = 39,67 | ||
| p < 0.0001 | |||
Discussion
Studies have been performed on humans for ONFH using various drug and treatment methods [34–39]. There have also been various steroid-induced ONFH experiments performed on animal models [7–20, 40–44]. In most of these studies, the experiment was done with quadruped animals, whose hip biomechanics are significantly different from that of human’s. Cui et al. [15] used chickens as bipedal test animal and developed ONFH successfully. The authors further showed that the Lovastatine, a lipid-decreasing drug, may decrease the rate of ONFH. Therefore, we used the system of Cui et al. [15] in our study.
In spite of osteogenic and haematopoetic cell death and subchondral trabecular bone necrosis that developed in the course of our study, a collapse in the femoral head did not occur. Short duration of steroid exposure, the decrease of the load applied on the femoral head related to limited physical activity associated with the catabolic effects of high-dose steroid leading to significant cachexia, may explain the decrease in the rate of subchondral microfracture and collapse formation.
PTX is a phosphodiesterase (PDE) inhibitor. PDE is an enzyme coded by different genes that is composed of nine tissue-specific isoenzymes (PDE1-9). The first action of PDE is to reduce the intracellular cAMP and/or cGMP levels [45]. Therefore, the inhibition of PDE results in the intracellular deposition of cyclic nucleotides and the increase in the cAMP and/or cGMP levels. PTX, which is a general inhibitor of those enzymes, used for many years to increase the cerebral and peripheral circulation, and has recently been used for its TNF-production decreasing effect for the treatment of septic shock [46]. There is very little information on the effects of PDE inhibitors on bone metabolism or osteoblastic function. Earlier studies showed that the osteoblastic effect of PTX occurred by its inhibition of the tumour necrosis factor α (TNF-α) production, which is a cytokine that inhibits bone formation [46, 47]. TNF-α inhibits fracture healing, bone formation related to BMP, and BMP-dependent alkaline phosphatase activity in osteoblastic cells. PDE inhibitors increase intracellular cAMP levels, which in turn obstructs TNF-α gene transcription [27, 48–50]. This is thought to be mediated by a transcriptional factor release inhibiting TNF gene expression [45]. Thus, bone formation can continue its normal process. Schwarz et al. [26] also showed that PTX blocks the TNF-α response and bone marrow related macrophages by the help of interleukin-18 and IFN-γ inhibition.
PTX and other PDE inhibitors activate osteoblast differentiation by increasing the intracellular cAMP levels by triggering protein kinase [51]. Rawadi et al. [28] report that PTX induced the osteoblastic markers, osteocalcin and Osf2/Cbfa1 in mesenchymal cells. They also report that this mechanism occurs independently from cAMP-dependent protein, kinase A (PKA). In the same study, it is suggested that the main mechanism occurs with the help of mitogen-activated protein kinase.
In our study, we found that PTX decreased histological evidence of steroid-induced ONFH. As the steroid induces the differentiation of osteoprogenitor cells to adipocytes and inhibits the osteoblasts, we thought that PTX might block the steroid‘s effect on changing osteoprogenitor cells to fat cells by increasing differentiation of mesenchymal cells to osteoblasts and promoting new bone formation. Moreover, when it is considered that steroids cause an intraosseous compartment syndrome after fat cell proliferation and a significant fat cell deposit resulting in sinusoidal compression, venous stasis and arterial obstruction and ischaemia, we suspect that PTX, with its microcirculation regulative effect, minimises these side effects. However, as it is a short-term study, the long-term effects of this treatment and questions such as whether it will prevent collapse formation on femoral head and whether the timing of PTX administration is important, remain unanswered. The possible side effects of PTX in long-term use along with steroid medication should be investigated in future studies. Since our study was based only on pathological criteria, further in vitro studies, including histomorphological and imaging methods, are necessary to support positive effects of PTX on the decrease of steroid-induced ONFH.
Acknowledgments
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Lieberman JR, Roth KM, Elsissy P, et al. Symptomatic osteonecrosis of the hip and knee after cardiac transplantation. J Arthroplasty. 2008;23(1):90–96. doi: 10.1016/j.arth.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Shibatani M, Fujioka M, Arai Y, et al. Degree of corticosteroid treatment within the first 2 months of renal transplantation has a strong influence on the incidence of osteonecrosis of the femoral head. Acta Orthop. 2008;79(5):631–636. doi: 10.1080/17453670810016641. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa K, Tada Y, Koarada S, et al. Very early development of steroid-associated osteonecrosis of femoral head in systemic lupus erythematosus: prospective study by MRI. Lupus. 2005;14(5):385–390. doi: 10.1191/0961203305lu2103oa. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein RS (2011) Glucocorticoid-induced osteonecrosis. Endocrine Dec 15. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 5.Kerachian MA, Séguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114(3–5):121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher DE. The role of fat embolism in the etiology of corticosteroid-induced avascular necrosis: clinical and experimental results. Clin Orthop Relat Res. 1978;130:68–80. [PubMed] [Google Scholar]
- 7.Jones LC, Hungerford DS. Models of ischemic necrosis of bone. In: Arlet J, Ficat RP, Hungerford DS, editors. Bone circulation. Baltimore: Williams and Wilkins; 1984. pp. 30–34. [Google Scholar]
- 8.Kawai K, Maruno H, Hirohata K. Fat necrosis of osteocytes as a causative factor in idiopathic osteonecrosis of the femoral head in man. Trans Orthop Res Soc. 1983;8:263. [PubMed] [Google Scholar]
- 9.Wang GJ, Sweet DE, Reger SI, et al. Fatcell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg. 1977;59A:729–735. [PubMed] [Google Scholar]
- 10.Wang GJ. Pathogenesis of steroid-induced avascular necrosis and its response to lipid clearing agent. In: Hirohata K, Mizuno K, Matsubara T, editors. Trends in research and treatment of joint diseases. Tokyo: Springer-Verlag; 1992. pp. 59–71. [Google Scholar]
- 11.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Wang GJ, Dughman SS, Reger SI. Femoral head blood flow in long- term steroid treatment (study of rabbit model) In: Arlet J, Ficat RP, Hungerford DS, editors. Bone circulation. Baltimore: Williams and Wilkins; 1984. pp. 35–37. [Google Scholar]
- 13.Pengde K, Fuxing P, Bin S, et al. Lovastatin inhibits adipogenesis and prevents osteonecrosis in steroid-treated rabbits. Joint Bone Spine. 2008;75(6):696–701. doi: 10.1016/j.jbspin.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Kabata T, Kubo T, Matsumoto T, et al. Onset of steroid-induced osteonecrosis in rabbits and its relationship to hyperlipaemia and increased free fatty acids. Rheumatology. 2005;44(10):1233–1237. doi: 10.1093/rheumatology/keh721. [DOI] [PubMed] [Google Scholar]
- 15.Cui Q, Wang GJ, Su CC, et al. The Otto Aufranc award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;344:8–19. doi: 10.1097/00003086-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients with receiving steroids. Clin Orthop Relat Res. 2001;386:173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Wang GJ, Moga DB, Richemer WG, et al. Cortisone induced bone changes and its response to lipid clearing agents. Clin Orthop Relat Res. 1978;130:81–85. [PubMed] [Google Scholar]
- 18.Kang P, Gao H, Pei F, et al. Effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Int J Exp Pathol. 2010;91(3):235–243. doi: 10.1111/j.1365-2613.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motomura G, Yamamoto T, Miyanishi K, et al. Risk factors for developing osteonecrosis after prophylaxis in steroid-treated rabbits. J Rheumatol. 2008;35(12):2391–2394. doi: 10.3899/jrheum.080416. [DOI] [PubMed] [Google Scholar]
- 20.Nishida K, Yamamoto T, Motomura G, et al. Pitavastatin may reduce risk of steroid-induced osteonecrosis in rabbits: a preliminary histological study. Clin Orthop Relat Res. 2008;466(5):1054–1058. doi: 10.1007/s11999-008-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calder JD, Buttery L, Revell PA, et al. Apoptosis—a significant cause of bone cell death in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2004;86(8):1209–1213. doi: 10.1302/0301-620X.86B8.14834. [DOI] [PubMed] [Google Scholar]
- 22.Calder JD, Buttery L, Revell PA, et al. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85(8):2907–2912. doi: 10.1210/jc.85.8.2907. [DOI] [PubMed] [Google Scholar]
- 23.Zalavras C, Shah S, Birnbaum MJ, et al. Role of apoptosis in glucocorticoid-induced osteoporosis and osteonecrosis. Crit Rev Eukaryot Gene Expr. 2003;13(2–4):221–235. [PubMed] [Google Scholar]
- 24.Ward A, Clissold SP. Pentoxifylline: a review of its pharmacodynamic and pharmacokinetic properties and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Xu YJ, Mengi SA, et al. Therapeutic potentials of pentoxifylline for treatment of cardiovascular diseases. Exp Clin Cardiol. 2004;9(2):103–111. [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz EM, Benz EB, Lu AP, et al. Quantitative small-animal surrogate to evaluate drug efficacy in preventing wear debris-induced osteolysis. J Orthop Res. 2000;18(6):849–855. doi: 10.1002/jor.1100180602. [DOI] [PubMed] [Google Scholar]
- 27.Horiuchi H, Saito N, Kinoshita T, et al. Enhancement of bone morphogenetic protein-2-induced new bone formation in mice by the phosphodiesterase inhibitor pentoxifylline. Bone. 2001;28(3):290–294. doi: 10.1016/S8756-3282(00)00450-6. [DOI] [PubMed] [Google Scholar]
- 28.Rawadi G, Ferrer C, Spinella-Jaegle S, et al. 1-(5-oxohexyl)-3,7-dimethylxanthine, a phosphodiesterase inhibitor, activates MAPK cascades and promotes osteoblast differentiation by a mechanism independent of PKA activation (pentoxifylline promotes osteoblast differentiation) Endocrinology. 2001;142(11):4673–4682. doi: 10.1210/en.142.11.4673. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita T, Ebara S, Kamimura M, et al. Phosphodiesterase inhibitors, Pentoxifylline and Rolipam, increase bone mass by promoting bone formation in normal mice. Bone. 2000;27:811–817. doi: 10.1016/S8756-3282(00)00395-1. [DOI] [PubMed] [Google Scholar]
- 30.Horiuchi H, Saito N, Kinoshita T, et al. Enhancement of recombinant human bone morphogenetic protein-2 (rhBMP-2)-induced new bone formation by concurrent treatment with parathyroid hormone and a phosphodiesterase inhibitor, pentoxifylline. J Bone Miner Metab. 2004;22(4):329–334. doi: 10.1007/s00774-003-0490-y. [DOI] [PubMed] [Google Scholar]
- 31.Pateder DB, Sheu TJ, O'Keefe RJ, et al. Role of pentoxifylline in preventing radiation damage to epiphyseal growth plate chondrocytes. Radiat Res. 2002;157(1):6. doi: 10.1667/0033-7587(2002)157[0062:ROPIPR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Epstein MS, Wicknick FW, Epstein JB, et al. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(5):593–596. doi: 10.1016/j.tripleo.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 33.Matsui M, Saito S, Ohzono K, et al. Experimental steroid-induced osteonecrosis in adult rabbits with hypersensitivity vasculitis. Clin Orthop Relat Res. 1992;277:61–72. [PubMed] [Google Scholar]
- 34.Gao YS, Zhang CQ. Cytotherapy of osteonecrosis of the femoral head: a mini review. Int Orthop. 2010;34(6):779–782. doi: 10.1007/s00264-010-1009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jäger M, Zilkens C, Bittersohl B, et al. Efficiency of iloprost treatment for osseous malperfusion. Int Orthop. 2011;35(5):761–765. doi: 10.1007/s00264-010-0998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jäger M, Zilkens C, Westhoff B, et al. Efficiency of iloprost treatment for chemotherapy-associated osteonecrosis after childhood cancer. Anticancer Res. 2009;29(8):3433–3440. [PubMed] [Google Scholar]
- 37.Petje G, Radler C, Aigner N, et al. Pharmacological management of aseptic osteonecrosis in children. Expert Opin Pharmacother. 2004;5(7):1455–1462. doi: 10.1517/14656566.5.7.1455. [DOI] [PubMed] [Google Scholar]
- 38.Yoshioka T, Mishima H, Akaogi H, et al. Concentrated autologous bone marrow aspirate transplantation treatment for corticosteroid-induced osteonecrosis of the femoral head in systemic lupus erythematosus. Int Orthop. 2011;35(6):823–829. doi: 10.1007/s00264-010-1048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangji V, Hauzeur JP (2005) Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am 87 Suppl 1(Pt 1):106–112 [DOI] [PubMed]
- 40.Motomura G, Yamamoto T, Irisa T, et al. Dose effects of corticosteroids on the development of osteonecrosis in rabbits. J Rheumatol. 2008;35(12):2395–2399. doi: 10.3899/jrheum.080324. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Yang S, Duan D, et al. Experimental osteonecrosis induced by a combination of low-dose lipopolysaccharide and high-dose methylprednisolone in rabbits. Joint Bone Spine. 2008;75(5):573–578. doi: 10.1016/j.jbspin.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Kuribayashi M, Fujioka M, Takahashi KA, et al. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 2010;81(1):154–160. doi: 10.3109/17453671003628772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Han R, Geng C, et al. A new osteonecrosis animal model of the femoral head induced by microwave heating and repaired with tissue engineered bone. Int Orthop. 2009;33(2):573–580. doi: 10.1007/s00264-008-0672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang TT, Lu B, Yue B, et al. Treatment of osteonecrosis of the femoral head with hBMP-2-gene-modified tissue-engineered bone in goats. J Bone Joint Surg Br. 2007;89(1):127–129. doi: 10.1302/0301-620X.89B1.18350. [DOI] [PubMed] [Google Scholar]
- 45.Sounes J, Griffin M, Malslen C, et al. Evidence that cyclic AMP phosphodiesterase inhibitors suppress TNF α generation from human monocytes by interaction with a low-affinity phosphodiesterase 4 conformer. Br J Pharmacol. 1996;118:649–658. doi: 10.1111/j.1476-5381.1996.tb15450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badger AM, Olivera DL, Eser KM. Beneficial effects of the phosphodiesterase inhibitors BRL 61063, pentoxifylline, and rolipram in a murine model of endotoxin shock. Circ Shock. 1994;44:188–195. [PubMed] [Google Scholar]
- 47.Sensky PL, Prise VE, Ward AE, et al. The effects of pentoxifylline on the relative perfusion of tumours growing in three sites in the mouse. Br J Cancer. 1993;68(6):1110–1114. doi: 10.1038/bjc.1993.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimato J, Takaoka K, Yoshikawa H, et al. Preservation of ectopically induced bone in the mouse by estradiol. Bone. 1991;12:249–255. doi: 10.1016/8756-3282(91)90071-P. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu T, Mehdi R, Yoshimura Y, et al. Sequential expression of bone morphogenetic protein, tumor necrosis factor, and their receptors in bone-forming reaction after mouse femoral marrow ablation. Bone. 1991;23:127–133. doi: 10.1016/S8756-3282(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhou FH, Foster BK, Zhou XF, et al. TNF-alpha mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J Bone Miner Res. 2006;21(7):1075–1088. doi: 10.1359/jbmr.060410. [DOI] [PubMed] [Google Scholar]
- 51.Boguslawski G, Hale LV, Yu XP, et al. Activation of osteocalcin transcription involves interaction of protein kinase A and protein kinase C dependent pathways. J Biol Chem. 2000;275:999–1000. doi: 10.1074/jbc.275.2.999. [DOI] [PubMed] [Google Scholar]