Summary
Previous studies by our research group using a model of insulin resistance induced by dexamethasone (DEX) showed that in the rat ventral prostate there was epithelial and smooth muscle cell atrophy and there were also alterations in fibroblasts. Proteins of the insulin signalling pathway are known to be very important for cell proliferation and development. Thus, we investigated the insulin signalling pathway and epithelial proliferation in the rat ventral prostate in this model and correlated the findings with expression of glucocorticoid (GR) and androgen (AR) receptors. Insulin resistance was induced in adult male Wistar rats by injection of DEX (1 mg/kg, ip for 5 consecutive days), whereas control (CTL) rats received saline. DEX treatment resulted in a significant decrease in body weight, but not in prostate weight. Reductions in insulin receptor 1 (IRS-1) (CTL 1.11 ± 0.06; DEX 0.85 ± 0.03), IRS-2 (CTL 0.95 ± 0.05; DEX 0.49 ± 0.04), AKT (CTL 0.98 ± 0.03; DEX 0.78 ± 0.02), mammalian target of rapamycin (mTOR; CTL 0.65 ± 0.08; DEX 0.22 ± 0.05), GR (CTL 1.30 ± 0.09; DEX 0.57 ± 0.10) and AR (CTL 1.83 ± 0.16; DEX 0.55 ± 0.08) protein levels were observed in the prostate of DEX-treated rats. The expression of the IRα-subunit, phosphoinositide 3-kinase, p-AKT, p70S6K, extracellular signal-regulated kinase (ERK) and p-ERK was not altered. The frequency of AR-positive cells in the epithelium of the prostate decreased in the glucocorticoid-treated group, and the intensity of the reaction for this receptor in the cell nuclei was lower in this group. Furthermore, the treatment with DEX reduced the frequency of proliferating cell nuclear antigen-positive (PCNA) cells 30-fold. This study suggests that the reduction in the insulin signalling pathway proteins IRS-1/IRS-2/AKT/mTOR in the prostate of DEX-treated rats may be associated with the morphological alterations observed previously.
Keywords: cell proliferation, dexamethasone, insulin resistance, insulin signalling, ventral prostate
Insulin resistance is a disorder in which target cells fail to respond to normal levels of circulating insulin. Recently, it was demonstrated that the administration of dexamethasone (DEX), a synthetic glucocorticoid, for five consecutive days in adult rats induces hyperinsulinaemia and insulin resistance in a dose-dependent fashion (Rafacho et al. 2008).
Clinical and experimental studies demonstrate the deleterious effects of diabetes on the genital system, associated mainly with erectile dysfunction and infertility (Jackson & Hutson 1984; Scarano et al. 2006). Experimentally induced diabetes impairs spermatogenesis, which can be accompanied by decrease in the weight of reproductive organs, such as the prostate, and a reduction in serum testosterone levels (Seethalakshmi et al. 1987; Saito et al. 1996). Prostate atrophy, induced by diabetes, was accompanied by lower cell proliferation and higher apoptotic rates in acinar epithelium (Arcolino et al. 2010). Androgens are probably the main trophic factor in the ventral prostate acinar epithelium, being fundamental for normal cellular proliferation and differentiation and also for malignant transformation (Cunha et al. 1992; Marker et al. 2003; Yan & Brown 2008; Yuan & Balk 2009). While mounting evidence has implicated testosterone withdrawal and androgen receptor (AR) signalling in the prostate modifications induced by diabetes, the understanding of the cellular mechanisms underlying these alterations is incomplete, and their putative interactions with impaired insulin actions are unknown (Cagnon et al. 2000). Furthermore, most experimental studies concerning the response of the prostate to diabetes are based on experimental protocols of type 1 diabetes and knowledge of the effect of the more common type 2 diabetes and insulin resistance on this gland are still incipient (Ribeiro et al. 2008; Vikram et al. 2010a). In previous studies of the rat ventral prostate DEX treatment resulted in epithelial and smooth muscle cell atrophy and alterations in fibroblasts. Mitochondrial changes have also been detected in the ventral prostate smooth muscle cells, indicating possible deleterious effects of glucocorticoid (Ribeiro et al. 2008).
Insulin exerts important metabolic and cellular mitogenic effects mediated through the insulin receptor (IR) that is present in virtually all vertebrate tissues (Kahn 1985). IR undergoes rapid autophosphorylation and subsequently phosphorylates intracellular protein substrates, including IRS-1 and IRS-2 (Cheatham & Kahn 1995). Phosphorylated IRS acts as a protein scaffold that activates the phosphoinositide 3-kinase (PI3K)/AKT pathway (Yenush & White 1997), which has a pivotal role in the regulation of various biological processes, including apoptosis, proliferation, differentiation and intermediary metabolism (Downward 1998; Chen et al. 2001). AKT phosphorylates many proteins with essential physiological roles, including mammalian target of rapamycin (mTOR), which phosphorylates the p70S6K, resulting in augmented protein synthesis. The mTOR pathway is a key regulator of cell growth and proliferation, and increasing evidence suggests that its dysregulation is associated with human diseases, including cancer and diabetes (Ueno et al. 2005; Sabatini 2006). Besides PI3K activation, a contribution of mitogen-activated protein kinase pathway, especially extracellular signal-regulated kinase (ERK) activation, to proliferation, protection against cell death and gene expression has been observed although its significance is not completely understood (Briaud et al. 2003; Lawrence et al. 2005).
Much is known about the glucose metabolism and the insulin signalling pathway in insulin-responsive tissues such as muscle, fat and liver (Saad et al. 1992; Páez-Espinosa et al. 1998). However, there are few studies that investigate the role of this pathway in prostate gland function, and the downstream signalling pathways in this gland are have not been explored previously. The current study sought to associate the morphological alterations observed in DEX treated, insulin-resistant rats with expression of key proteins of the insulin pathway especially those that are involved in cellular proliferation, such as IRS-2/PI3K/AKT/mTOR/p70S6K. We observed significant reduction in IRS-1, IRS-2, AKT, mTOR, AR and glucocorticoid receptor (GR) protein expression, indicating possible participation of at least some of the insulin cascade proteins in the atrophy of epithelial and smooth muscle cells of ventral prostate that occurs in this model of insulin resistance.
Materials and methods
Materials
Dexamethasone-phosphate (Decadron) was from Aché (Campinas, SP, Brazil). SDS-PAGE and Western blotting were performed using Bio-Rad systems (Hercules, CA, USA). All chemicals used were from Bio-Rad and from Sigma (St. Louis, MO, USA). Anti-IRα-subunit (sc-710; 1:1500), anti-IRS-1 (sc-560; 1:700), anti-AKT (sc-8312; 1:1000), anti-phospho-AKT Ser473 (sc-7985-R; 1:500), anti-p70S6K (sc-8418; 1:1000), anti-phospho-ERK (sc-7383; 1:500), anti-AR (sc-816; 1:500), anti-GRα/β-subunit (sc-1004; 1:500) and anti-proliferating cell nuclear antigen (PCNA) (sc-56) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-mTOR (7C10; 1:500) and anti-IRS-2 (4502; 1:500) were from Cell Signaling Technology (Beverly, MA, USA). Anti-PI3K, subunit p85, (06-195; 1:2000) and anti-ERK (06-182; 1:1000) were from Upstate (Lake Placid, NY, USA). Anti-β-actin (ab8227; 1:10000) was from Abcam (Cambridge, MA, USA). Glucose levels were measured using a glucometer (One-Touch, Johnson & Johnson, CA, USA). Hormonal measurements, except for insulin, were made by ELISA (Human do Brasil, MG, Brazil), according to the instructions of the manufacturer. Insulin was measured by RIA utilizing guinea pig anti-rat insulin antibody and rat insulin as standard (Scott et al. 1981). All measurements were performed in 10-12 h fasted animals.
Animals and dexamethasone treatment
Adult male Wistar rats (320–420 g) obtained from the UNESP-São Paulo State University Animal Care Unit, Campus of Botucatu, were kept at 22 ± 2 °C on a 12 h light/dark cycle. The rats had free access to food and water. The experimental group (DEX) received daily injections of dexamethasone-phosphate (1 mg/kg body weight, dissolved in saline, intraperitoneally for 5 consecutive days), whereas control (CTL) rats received saline (1 ml/kg body weight intraperitoneally for 5 consecutive days). Rats were weighed daily. Following treatment, animals of both groups were anaesthetized by CO2 inhalation and killed by decapitation. The ventral prostates were removed and weighed. We used the ventral prostate because this lobe responds to androgens in a more similar way to the human prostate than the other lobes (Prins 1992). Experiments with animals were approved by the institutional UNESP Committee for Ethics in Animal Experimentation and conform to the Guide for the Care and Use of Laboratory Animals.
Protein extraction and immunoblotting
Fragments of ventral prostate from six animals of each group were homogenized for 15 s at the maximum speed in ice-cold cell lysis buffer (Cell Signaling). The samples were centrifuged for 15 min at 4 °C and 12,000 g, and the protein concentration in the supernatant was determined using the reducing agent compatible and detergent compatible assay (RCDC), according to the manufacturer’s instructions (Bio-Rad). For electrophoresis, aliquots of 100 μg of protein were separated on a 10% denaturing polyacrylamide gel. Electrotransfer of proteins from the gel to nitrocellulose membrane was performed for 90 min at 120 V (constant) in a Bio-Rad miniature transfer apparatus (Mini-Protean, Hercules, CA, USA.) with 0.02% SDS and 20% methanol. After blocking at room temperature for 2 h in Tris buffer salt tween (TBST) plus 5% dry skimmed milk, membranes containing ventral prostate cell lysates were washed in TBST and incubated overnight with the appropriate primary antibodies at the dilutions recommended by the manufacturers in TBST plus 3% dry skimmed milk. After washing in TBST, membranes were incubated with the appropriate secondary antibody in TBST plus 1% dried skimmed milk. Antibody binding was detected by enhanced SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA), as described by the manufacturer. Blots were scanned, and the densitometry of protein bands was determined by pixels intensity using Scion Image software (Scion Corporation, Frederick, MD, USA). The protein content values were corrected by expression of β-actin (used as internal control) and are expressed as optical arbitrary units.
Immunohistochemical reactions
Prostate fragments were fixed by immersion in metacarn and embedded in paraffin. Histological sections were submitted to antigen retrieval in citrate buffer, pH 6.0, for 20 min and treated for a further 30 min in 3% H2O2 in methanol to block endogenous peroxidases. Sections were treated for 15 min with a background sniper blocker to eliminate non-specific binding (Biocare Medical, Concord, CA, USA). Sequentially, slides were incubated with the following primary antibodies, diluted 1:100 in 1% BSA: rabbit anti-AR (overnight, at 4 °C) and mouse anti-PCNA (1 h, at 37 °C). AR detection was performed with biotinilated secondary anti-rabbit antibody, for 45 min, followed by the ABC Staining System (sc-2018, Santa Cruz Biotechnology), according to the manufacturer’s instructions. Anti-PCNA was detected by Polymer conjugated to peroxidase (Novolink Polymer; Novocastra, Norwell, MA, USA), for 45 min. The reactions were revealed with diaminobenzidine (0.03% in TBS), and sections were stained with haematoxylin. Negative controls were obtained by omission of the primary antibody. Slides were observed using a Zeiss-Jenamed light microscope (Jena, Germany) coupled with a camera and a semi-automatic image analysis system (Image-Pro Plus Media Cybernetics version 4.5 for Windows software, Bethesda, MD, USA).
Quantitative and statistical analysis
The quantification of PCNA- and AR-positive cells in the acinar epithelium of the ventral prostate was performed using 20 microscopic randomly selected fields at 200×. Three rats were used, resulting in 60 fields per group. The frequency of immunolabelled cells was calculated as the number of positive nuclei in the epithelial cells divided by the total number of epithelial cell nuclei counted, expressed as a percentage. Statistical comparisons between data from the DEX and CTL groups were performed using the unpaired Student’s t-test. Values of P < 0.05 were considered statistically significant, and results are expressed as means ± SE.
Results
Biometric parameters and hormonal measurements
Table 1 shows the mean values of body weight and prostate weight of the control and DEX-treated groups. The body weight of DEX animals was reduced significantly by 20% when compared with the controls (P < 0.05, n = 10). The absolute prostate weight did not differ significantly between groups. Relative prostate weight shows an increase (not significant) of approximately 19% in DEX group.
Table 1.
Mean values of body, ventral prostate and relative weight of control (CTL) and dexamethasone-treated rats (DEX)
| Initial body weight (g) | Final body weight (g) | Prostate weight (mg) | Relative weight | |
|---|---|---|---|---|
| CTL | 383.0 ± 9.3 | 397.0 ± 8.1 | 527.1 ± 6.6 | 1.33 ± 0.03 |
| DEX | 372.5 ± 15.9 | 332.5 ± 17.2* | 515.9 ± 13.1 | 1.58 ± 0.08 |
Data are mean ± SEM.
Significantly different vs. control, P < 0.05, n = 10.
As expected, fast glucose (CTL 99.20 ± 2.50 vs. DEX 118.70 ± 15.60, P < 0.05, N = 10) and insulin (CTL 2.40 ± 0.27 vs. DEX 15.30 ± 1.80, P < 0.05, N = 10) increased significantly in DEX group.
Glucocorticoids are known to exert effects on plasmatic levels of a variety of other hormones. However, the treatment with DEX did not produce significant alterations in luteinizing hormone, follicle stimulating hormone, testosterone and estradiol levels (Table 2, n = 10).
Table 2.
Blood glucose (mg/dl) and serum hormones (ng/ml) in control (CTL) and dexamethasone-treated rats (DEX)
| CTL | DEX | |
|---|---|---|
| Glucose | 99.20 ± 2.50 | 118.70 ± 15.60* |
| Insulin | 2.40 ± 0.27 | 15.30 ± 1.80* |
| Estradiol | 40.21 ± 1.13 | 37.41 ± 0.78 |
| Follicle stimulating hormone | 6.86 ± 0.10 | 6.32 ± 0.04 |
| Luteinizing hormone | 0.38 ± 0.01 | 0.33 ± 0.009 |
| Testosterone | 145.00 ± 0.01 | 135.00 ± 0.09 |
Data are mean ± SEM.
Significantly different vs. control, P < 0.05, n = 10.
Insulin signalling in the prostate of insulin-resistant rats
Western blotting experiments were performed to determine possible alterations in the insulin signalling pathway in the prostate of insulin-resistant rats. IR α subunit, protein expression was similar in the prostate of control and DEX-treated rats (CTL 0.90 ± 0.15 vs. DEX 0.89 ± 0.1, Figure 1a). IRS-1 (CTL 1.11 ± 0.06 vs. DEX 0.85 ± 0.03, P < 0.05, Figure 1b) and IRS-2 (CTL 0.95 ± 0.05; DEX 0.49 ± 0.04, P < 0.05, Figure 1c) protein expression were significantly reduced in the DEX group. PI3K protein expression, p85 regulatory subunit, was not different between groups (CTL 0.69 ± 0.08 vs. DEX 0.54 ± 0.03, Figure 1d).
Figure 1.
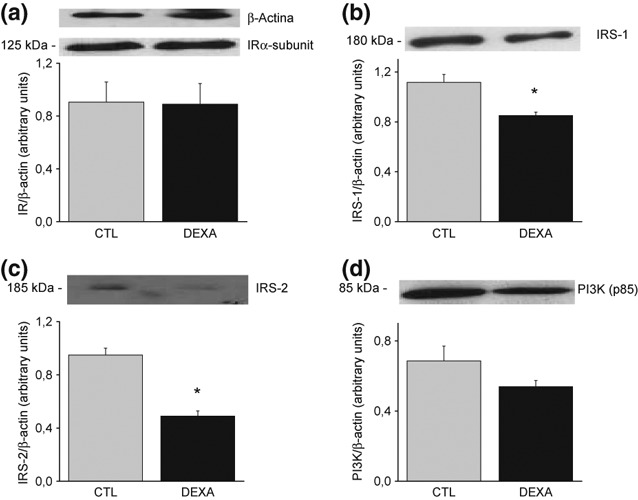
Protein expression of insulin receptor α-subunit (a); insulin receptor substrate (IRS)-1 (b); IRS-2 (c) and phosphoinositide 3-kinase (d) in the ventral prostate of rats treated with saline (CTL) and dexamethasone (DEX). Data are reported as means ± SE. *P < 0.05 compared to control (Student’s t-test), n = 6.
AKT protein levels were reduced significantly in the DEX group (CTL 0.98 ± 0.03 vs. DEX 0.78 ± 0.04, P < 0.02, Figure 2a), but no difference in the p-AKT expression was observed (CTL 0.61 ± 0.05 vs. DEX 0.52 ± 0.09, Figure 2b). mTOR protein content was decreased significantly in the ventral prostate of DEX animals (CTL 0.65 ± 0.08 vs. DEX 0.22 ± 0.07, P < 0.05, Figure 2c). p70S6K protein expression was not significantly different between groups (CTL 0.89 ± 0.09 vs. DEX 0.72 ± 0.12, Figure 2d).
Figure 2.
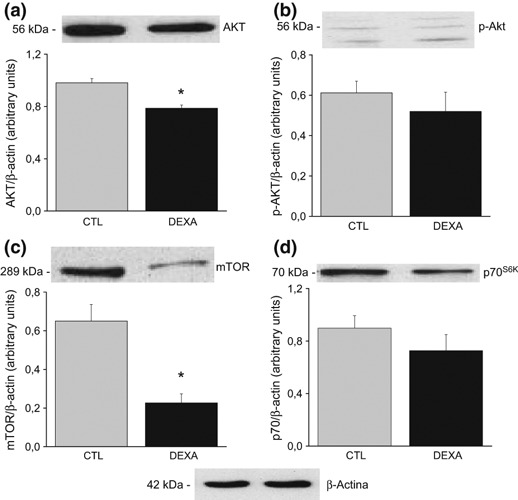
Protein expression of AKT (a); p-AKT (b); mammalian target of rapamycin (c) and p70S6K (d) in the ventral prostate of rats treated with saline (CTL) and dexamethasone (DEX). Data are reported as means ± SE. *P < 0.05 compared to control (Student’s t-test), n = 6.
In both groups, the ERK protein expression was similar (0.91 ± 0.03 for CTL and 0.92 ± 0.03 for DEX animals, Figure 3a). The levels of p-ERK were not different in DEX rats compared to control (CTL 0.59 ± 0.08 vs. DEX 0.86 ± 0.09, Figure 3b).
Figure 3.
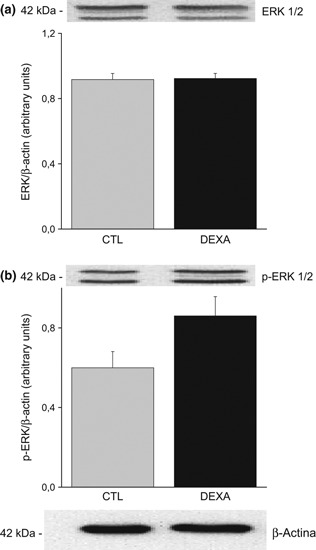
Protein expression of extracellular signal-regulated kinase (ERK) (a) and p-ERK (b) in the ventral prostate of rats treated with saline (CTL) and dexamethasone (DEX). Data are reported as means ± SE, P > 0.05 (Student’s t-test), n = 6.
Treatment with dexamethasone decreased GR and AR expression
A significant reduction in GR (GRα/β-subunit) protein expression (CTL 1.30 ± 0.09 vs. DEX 0.57 ± 0.10, P < 0.05, Figure 4a) and AR (CTL 1.83 ± 0.16 vs. DEX 0.55 ± 0.18, P < 0.08, Figure 4b) protein expression were observed in the prostate of DEX animals.
Figure 4.
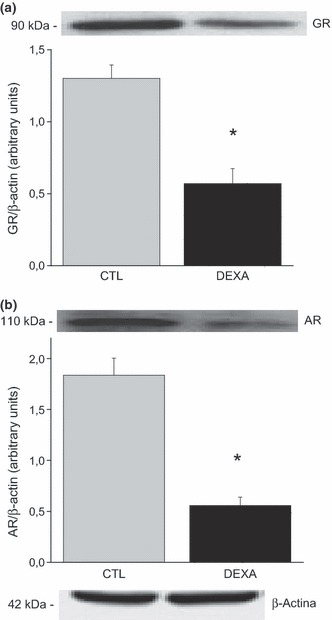
Protein expression of GR (a) and AR (b) in the ventral prostate of rats treated with saline (CTL) and dexamethasone (DEX). Data are reported as means ± SE. *P < 0.05 compared to control (Student’s t-test), n = 6.
Immunohistochemical analysis
Immunohistochemical methods were used to detect PCNA (Figure 5) and AR (Figure 6) positive cells. The frequency of PCNA-positive cells in the acinar epithelium of the prostate decreased 30-fold in the dexamethasone-treated group, when compared with controls (CTL 5.40 ± 0.64%vs. DEX 0.18 ± 0.05%; P < 0.001; Figure 7a). Although treatment with DEX caused only a small reduction in the frequency of AR-positive cells in the rat prostate, the intensity of staining in each nucleus decreased sharply, in particular in the intermediate gland region (CTL 97.71 ± 1.09%vs. DEX 88.64 ± 1.82%; P < 0.05; Figure 7b).
Figure 5.
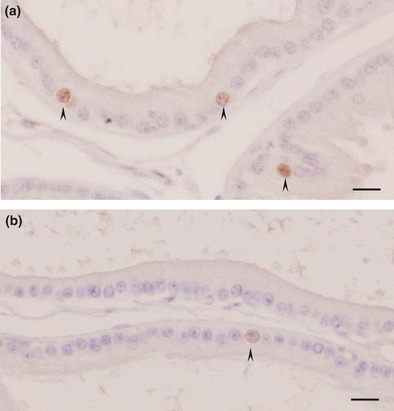
Proliferating cell nuclear antigen immunocytochemistry of the intermediate region of the ventral prostate of control rats (a) or rats treated with dexamethasone (b). Proliferating cell nuclear antigen-positive cells are indicated by arrowheads. Note that proliferation in the prostate epithelium decreases significantly in the dexamethasone group (b). Scale bar: 20 μm.
Figure 6.
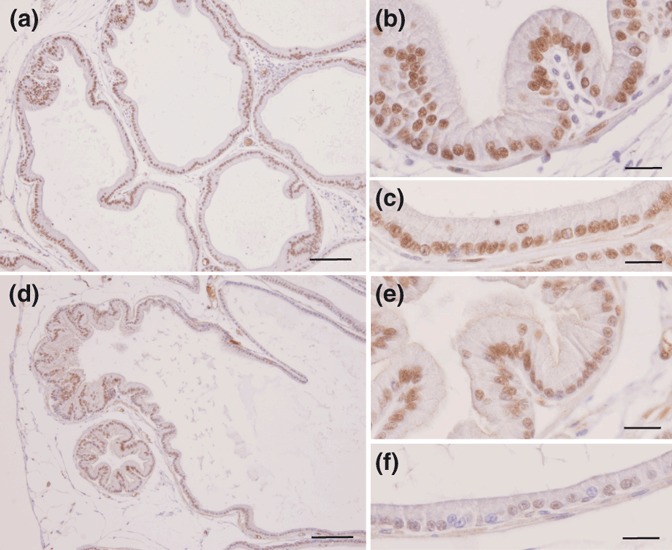
Immunocytochemistry for AR in the ventral prostate of control rats (a–c) or rats treated with DEX (d–f). Distal region (a, b, d and e) and intermediate region of the acini (c and f). Note the reduction in the staining of the AR in the prostate epithelium of the DEX-treated group and the decreasing intensity of nuclear staining for this receptor after treatment with DEX. Scale bars = 100 μm for a and d; 20 μm for b, c, e and f.
Figure 7.
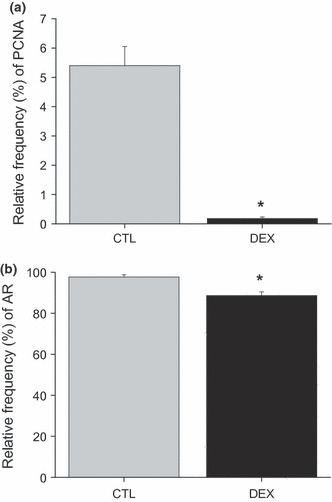
Relative frequency (%) of immunoreactions for PCNA-positive cells (a) and AR-positive cells (b). Data are reported as means ± SE. *P < 0.05 compared to control (Student’s t-test).
Discussion
The effects of glucocorticoids in vivo include both impaired insulin-dependent glucose uptake in the periphery and enhanced gluconeogenesis in the liver (Rizza et al. 1982; Rooney et al. 1994). Peripheral insulin resistance is established as demonstrated by the high levels of glucose and fast serum insulin measured in dexamethasone-treated rats (Rafacho et al. 2009).
A previous investigation reported that glucocorticoid treatment led to epithelial and smooth muscle cell atrophy in the rat ventral prostate and structural alterations in fibroblasts and mitochondria (Ribeiro et al. 2008). A novel set of experiments based on this model of hyperinsulinaemia now examines the insulin signalling pathway in this gland. As previously observed (Rafacho et al. 2008), DEX treatment significantly reduced body weight. DEX rats also showed hyperglycaemia and hyperinsulinemia, indicating the establishment of insulin resistance. Prostate weight did not change in DEX rats and, in consequence, relative prostate weight increases approximately 21%, indicating different degrees of tissue sensitivity to glucocorticoids. However, besides the absence of effect on prostate weight, the effect of DEX treatment upon the morphology of the gland was remarkable. Our results also show that, after treatment with DEX, there was no change in IR levels. There was a significant reduction in the expression of IRS-1 and IRS-2 in the rat ventral prostate. This is in agreement with other studies, such as in the muscle of DEx-treated rats (Saad et al. 1993; Rojas et al. 2003). Additionally, a significant decrease in AKT content was observed in the prostate of insulin-resistant rats. The decrease in the IR/IRS/PI3K/AKT pathway could also be demonstrated in the liver and muscle of hyperinsulinaemic rats (Ueno et al. 2005) and in adipocyte cells incubated with DEX (Burén et al. 2002).
A major downstream target of AKT is mTOR, which has key roles in cell survival, growth, protein synthesis, cellular metabolism and angiogenesis (Garcia & Danielpour 2008). Our findings showed that, after treatment with DEX, there was drastic reduction in mTOR expression in the ventral prostate. The downregulation in AKT/mTOR signalling in the prostate explains the reduction in cell proliferation detected in the gland in this model of insulin resistance. The mTOR signalling pathway is associated with the regulation of protein synthesis, cell size and proliferation. In vascular smooth muscle cells (VSMC), rapamycin, an mTOR pathway inhibitor, induces AKT activation and increases VSMC expression of contractile proteins, used as differentiation markers (Rzucidlo et al. 2007). This reduction in mTOR expression may be involved in the smooth muscle atrophy shown previously in this model (Ribeiro et al. 2008). Current estimates suggest that PI3K/AKT/mTOR signalling is upregulated in 30–50% of prostate cancers. Multiple inhibitors of this pathway have been developed and are being assessed, with attention focusing on mTOR inhibition (Morgan et al. 2009). In prostate cancer cells, mTOR and p70S6K are downstream of PI3K and AKT in regulating G1 cell cycle progression. PI3K transmits the mitogenic signal through AKT, mTOR to p70S6K, suggesting that the PI3K/AKT/mTOR/p70S6K signalling pathway could serve as a target for therapeutic intervention in prostate cancer (Gao et al. 2003). Additional in vivo studies with lower doses of DEx may provide new information about an anti-proliferative effects on prostate cells and its potential use in the treatment of proliferative prostate disorders.
The cell growth effect of insulin is also mediated by the MEK/ERK pathway that acts together with PI3K/AKT as regulators of insulin sensitivity. Changes in the balance between these two pathways can alter the responsiveness to insulin as well as cell growth. Differential regulation of these two pathways has been demonstrated in many tissues and cells, like cardiac myocyte (Carvalheira et al. 2003), VSMC (Jiang et al. 1999; Lightell et al. 2011), 3T3-L1 cells (Yu et al. 2011) among others. The treatment with DEX used in this study had no effect on the expression of ERK and pERK. This finding indicates that prostate responsiveness to insulin is altered, because the balance between the pathways is impaired, probably because the hyperinsulinemia imposed by compensatory response of pancreatic B cells to the insulin resistance caused by DEX treatment. The impairment of PI3K/AKT together with no alteration of MEK/ERK is in accordance with our previous observations of epithelial and smooth muscle cell atrophy (Ribeiro et al. 2008).
Besides the reduction in the insulin signalling pathway IRS-1/IRS-2/AKT/mTOR proteins, we also noticed a significant decrease in the expression of GRs and ARs in the prostate of rats treated with DEX. Glucocorticoids can inhibit androgen signalling: in cell culture studies that GR inhibits AR transcriptional activity (Chen et al. 1997). A study in the prostate cancer cell lines, DU145 and PC-3, showed a dose-dependent decreased level of GR after treatment with DEX, where this reduction was more evident in PC-3 cells than in DU145 cells (Nishimura et al. 2001). With regard to the AR, a recent study demonstrated that the inhibition of the PI3K pathway activates AR signalling. Despite the increase in AR signalling, which has proliferative effects, PI3K pathway inhibition has anti-proliferative effects, suggesting that the PI3K pathway is dominant over AR signalling in prostate cancer cells (Kaarbo et al. 2010).
Glucocorticoids act either directly and indirectly in cells. Nishimura et al. (2001) showed that DU145 cells treated with DEX accumulated in G1 phase, favouring for an inhibition of cell proliferation. Also, they found an accumulation of NF-κB that was accompanied by increase in IκBα protein levels and decrease in secreted the IL-6 levels, which suggest that DEX anti-proliferative effects may be mediated by the IL-6 pathway. Because the DEX dose used in this study is high, this possibility cannot be disounted. However, we observed a decrease in GR protein expression, indicating a possible down regulation of the receptor. More experiments are necessary to clarify this point. Also, hormones that participate in the physiological regulation of the prostate showed normal concentration in plasma, which lead us to believe that the alterations observed in the morphology and in protein expression in this model of DEX treatment may be mainly a result of the imposed insulin resistance status. Very recently, Vieira et al. (2011) showed that DEX treatment (resulting in hyperglycaemia) increased the activity of insulin-degrading enzyme and prevented, at least in part, the atrophy of the ventral prostate caused by castration, a model that causes profound modifications in hormone levels and prostate physiology.
We have demonstrated here, for the first time, a reduction in the insulin signalling pathway proteins IRS-1/IRS-2/AKT/mTOR in the ventral prostate of DEX-treated rats that may be implicated in modifying of the gland morphology.
Acknowledgments
This work was supported by grants from the Foundation for Research Support of São Paulo (FAPESP). We thank Mr. Luis Roberto Faleiros Jr. and Ms. Samantha Yuri Maeda for technical support.
References
- Arcolino FO, Ribeiro DL, Gobbo MG, Taboga SR, Góes RM. Proliferation and apoptotic rates and increased frequency of p63-positive cells in the prostate acinar epithelium of alloxan-induced diabetic rats. Int. J. Exp. Pathol. 2010;91:144–154. doi: 10.1111/j.1365-2613.2009.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briaud I, Lingohr MK, Dickson LM, Wrede CE, Rhodes CJ. Differential activation mechanisms of Erk-1/2 and p70(S6K) by glucose in pancreatic beta-cells. Diabetes. 2003;52:974–983. doi: 10.2337/diabetes.52.4.974. [DOI] [PubMed] [Google Scholar]
- Burén J, Liu H, Jensen J, Eriksson JW. Dexamethasone impairs insulin signalling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase B in primary cultured rat adipocytes. J. Endocrinol. 2002;146:419–429. doi: 10.1530/eje.0.1460419. [DOI] [PubMed] [Google Scholar]
- Cagnon VHA, Camargo AM, Rosa RM, Fabiani CR, Padovani CR, Martinez FE. Ultrastructural study of the ventral lobe of the prostate of mice with streptozotocin induced diabetes (C57bl/6j) Tissue Cell. 2000;32:275–283. doi: 10.1054/tice.2000.0123. [DOI] [PubMed] [Google Scholar]
- Carvalheira JBC, Calegari VC, Zecchin HG, et al. The cross-talk between angiotensin and insulin differentially affects phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-mediated signaling in rat heart: implications for insulin resistance. Endocrinology. 2003;144(12):5604–5614. doi: 10.1210/en.2003-0788. [DOI] [PubMed] [Google Scholar]
- Cheatham B, Kahn CR. Insulin action and the insulin signaling network. Endocr. Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, Yu G, Liu W, Pearce D. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J. Biol. Chem. 1997;272:14087–14092. doi: 10.1074/jbc.272.22.14087. [DOI] [PubMed] [Google Scholar]
- Chen R, Kim O, Yang J, et al. Regulation of Akt/PKB activation by tyrosine phosphorylation. J. Biol. Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Alarid ET, Turner T, Donjacour AA, Boutin EL, Foster BA. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J. Androl. 1992;13:465–475. [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Gao N, Zhang Z, Jiang B, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem. Biophy. Res. Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- Garcia JA, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol. Cancer Ther. 2008;7:1347–1354. doi: 10.1158/1535-7163.MCT-07-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson FL, Hutson JC. Altered responses to androgens in diabetic male rats. Diabetes. 1984;33:819–824. doi: 10.2337/diab.33.9.819. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Lin Y-W, Clemont A, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarbo M, Mikkelsen OL, Malerod L, et al. PI3K-AKT-mTOR pathway is dominant over androgen receptor signaling in prostate cancer cells. Cell Oncol. 2010;32:11–27. doi: 10.3233/CLO-2009-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn CR. The molecular mechanism of insulin action. Ann. Rev. Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J. Biol. Chem. 2005;280:26751–26759. doi: 10.1074/jbc.M503158200. [DOI] [PubMed] [Google Scholar]
- Lightell DJ, Moss SC, Woods TC. Loss of canonical insulin signaling accelerates vascular smooth muscle cell proliferation and migration through changes in p27Kip1 regulation. Endocrinology. 2011;152(2):651–658. doi: 10.1210/en.2010-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal cellular and molecular control of ventral prostate development. Dev. Biol. 2003;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr. Cancer Drug Targets. 2009;9:237–249. doi: 10.2174/156800909787580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Nonomura N, Satoh E, et al. Potential mechanism for the effects of dexamethasone on growth of androgen-independent prostate cancer. J. Natl Cancer Inst. 2001;93:1739–1746. doi: 10.1093/jnci/93.22.1739. [DOI] [PubMed] [Google Scholar]
- Páez-Espinosa V, Carvalho CRO, Alvarez-Rojas F, et al. Insulin induces tyrosine phosphorylation of Shc and stimulates Shc/Grb2 association in insulin-sensitive tissues of the intact rat. Endocrine. 1998;8:193–200. doi: 10.1385/ENDO:8:2:193. [DOI] [PubMed] [Google Scholar]
- Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- Rafacho A, Giozzet VAG, Boschero AC, Bosqueiro JR. Functional alterations in endocrine pancreas of rats with different degrees of dexamethasone-induced insulin resistance. Pancreas. 2008;36:284–293. doi: 10.1097/MPA.0b013e31815ba826. [DOI] [PubMed] [Google Scholar]
- Rafacho A, Cestari TM, Taboga SR, Boschero AC, Bosqueiro JR. High doses of dexamethasone induces increased β-cell proliferation in pancreatic rat islets. Am. J. Physiol. Endocrinol. Metab. 2009;296:681–689. doi: 10.1152/ajpendo.90931.2008. [DOI] [PubMed] [Google Scholar]
- Ribeiro DL, Rafacho A, Bosqueiro JR, Taboga SR, Góes RM. Cellular changes in the ventral prostate stroma of glucocorticoid treated rats. Cell Tissue Res. 2008;332:499–508. doi: 10.1007/s00441-008-0581-0. [DOI] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ, Gerich J. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. J. Clin. Endocrinol. Metab. 1982;54:131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Rojas FA, Hirata AE, Saad MJA. Regulation of insulin receptor substrate-2 tyrosine phosphorylation in animal models of insulin resistance. Endocrine. 2003;21:115–122. doi: 10.1385/ENDO:21:2:115. [DOI] [PubMed] [Google Scholar]
- Rooney DP, Neely RDG, Cullen C, Ennis CN, Sheridan B, Atkinson AB. The effect of cortisol on glucose/glucose-6-phosphate cycle activity and insulin action. J. Clin. Endocrinol. Metab. 1994;77:1180–1183. doi: 10.1210/jcem.77.5.8077310. [DOI] [PubMed] [Google Scholar]
- Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J. Vasc. Surg. 2007;45:25A–32A. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Saad MJ, Araki E, Miralpeix M, Rothenberg PL, White MF, Kahn CR. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J. Clin. Invest. 1992;90:1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad MJA, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J. Clin. Invest. 1993;92:2065–2072. doi: 10.1172/JCI116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Saito M, Nishi K, Fukumoto Y, Weiss RM, Latifpour J. Effect of experimental diabetes on rat prostate endothelin receptors. Eur. J. Pharmacol. 1996;310:197–200. doi: 10.1016/0014-2999(96)00422-0. [DOI] [PubMed] [Google Scholar]
- Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int. J. Androl. 2006;29:482–488. doi: 10.1111/j.1365-2605.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- Scott AM, Atwater I, Rojas E. A method for the simultaneous measurement of insulin release and B cell membrane potential in single mouse islets of Langerhans. Diabetologia. 1981;21:470–475. doi: 10.1007/BF00257788. [DOI] [PubMed] [Google Scholar]
- Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin-induced diabetes on the neuroendocrine-male reproductive tract axis of adult rat. J. Urol. 1987;138:190–194. doi: 10.1016/s0022-5347(17)43042-4. [DOI] [PubMed] [Google Scholar]
- Ueno M, Carvalheira JBC, Tambascia RC, et al. Regulation of insulin signalling by hyperinsulinaemia: role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia. 2005;48:506–518. doi: 10.1007/s00125-004-1662-6. [DOI] [PubMed] [Google Scholar]
- Vieira JSBC, Saraiva KLA, Barbosa MCL, et al. Effect of dexamethasone and testosterone treatment on the regulation of insulin-degrading enzyme and cellular changes in ventral prostate after castration. Int. J. Exp. Pathol. 2011;92:272–280. doi: 10.1111/j.1365-2613.2011.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikram A, Jena GB, Ramarao P. Increased cell proliferation and contractility of prostate in insulin resistant rats: linking hyperinsulinemia with benign prostate hyperplasia. Prostate. 2010a;70:79–89. doi: 10.1002/pros.21041. [DOI] [PubMed] [Google Scholar]
- Yan J, Brown TR. Cell proliferation and expression of cell cycle regulatory proteins that control the g1/s transition are age dependent and lobe specific in the brown Norway rat model of ventral prostate hyperplasia. Endocrinology. 2008;149:193–207. doi: 10.1210/en.2007-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenush L, White MF. The IRS-signalling system during insulin and cytokine action. BioEssays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]
- Yu X, Shen N, Zhang M-L, et al. Egr-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice. EMBO J. 2011;30(18):3754–3765. doi: 10.1038/emboj.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol. Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


