Summary
This study investigated the effect of the bark extract of Bathysa cuspidata on paraquat (PQ)-induced extra-pulmonary acute lung injury (ALI) and mortality in rats. ALI was induced with a single dose of PQ (30 mg/kg, i.p.), and animals were treated with B. cuspidata extract (200 and 400 mg/kg). Analyses were conducted of survival, cell migration, lung oedema, malondialdehyde, proteins carbonyls, catalase, superoxide dismutase, histopathology and the stereology of lung tissue. Rats exposed to PQ and treated with 200 and 400 mg of the extract presented lower mortality (20% and 30%), compared with PQ alone group (50%). Furthermore, lung oedema, septal thickening, alveolar collapse, haemorrhage, cell migration, malondialdehyde and proteins carbonyl levels decreased, and catalase and superoxide dismutase activity were maintained. These results show that the bark extract of B. cuspidata reduced PQ-induced extra-pulmonary ALI and mortality in rats and suggest that these effects may be associated with the inhibition of oxidative damage.
Keywords: acute lung injury, Bathysa cuspidata, oxidative stress, paraquat
The treatment of human diseases using medicinal plants and their derivatives is an ancient practice that is currently gaining popularity worldwide (Farnsworth 1994; Park et al. 2010). At the moment, there is considerable interest in natural plant products, particularly those used in traditional medicine. These products may be used in the development of new drugs and applications, which would include lung diseases induced by toxic agents (Lo et al. 2005; Park et al. 2010). This type of investigation has been shown to represent an effective strategy for selecting medicinal plants for the development of new drugs, as around 80% of the world population uses products derived from plants in their basic healthcare (Farnsworth 1994; Kaur et al. 2005).
Paraquat (1, 1′-dimethyl-4, 4′-bipyridinium dichloride) (PQ) is a quaternary nitrogen herbicide, which is used worldwide because of its high efficacy and the low residue of the product left in crops (Sittipunt 2005; Chang et al. 2009). Its high toxicity to humans and animals when absorbed through ingestion, skin contact or inhalation has been extensively demonstrated (Dasta 1978; Suntres 2002; Marrs & Adjei 2003). This herbicide accumulates mainly in the lungs through the highly developed polyamine uptake system, leading to the generation of a superoxide radical (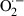 ) through an oxygen- and NADPH-dependent redox cycle, resulting in acute oxidative stress-related insults (Tsukamoto et al. 2002; Marrs & Adjei 2003; Wang et al. 2006; Chang et al. 2009). PQ-induced extra-pulmonary acute lung injury (ALI) results in alveolar epithelial cell (type I and II pneumocytes) and Clara cell disruption, impairment of surfactant production, haemorrhage, oedema, hypoxaemia and infiltration of inflammatory cells into the interstitial and alveoli spaces (Rocco et al. 2001, 2003; Park et al. 2010; Novaes et al. 2012). The principal cause of death in cases of PQ toxicity is respiratory failure associated with diffuse damage to the alveolar epithelium owing to oxidation of lipids and membrane proteins, which culminates in partial or total loss of cell and lung function (Mustafa et al. 2002; Tsukamoto et al. 2002; Rocco et al. 2004). In view of its reproducibility and simplicity of administration, this herbicide has been widely used as an experimental model in studies on ALI (Rocco et al. 2001, 2003; Chang et al. 2009; Novaes et al. 2012). Moreover, it constitutes an inexpensive model in which effects are rapid (Silva & Saldiva 1998; Rocco et al. 2003), thus ensuring its frequent use when investigating the effects of vegetable extracts on lung disorders (Kim et al. 2006; Park et al. 2010).
) through an oxygen- and NADPH-dependent redox cycle, resulting in acute oxidative stress-related insults (Tsukamoto et al. 2002; Marrs & Adjei 2003; Wang et al. 2006; Chang et al. 2009). PQ-induced extra-pulmonary acute lung injury (ALI) results in alveolar epithelial cell (type I and II pneumocytes) and Clara cell disruption, impairment of surfactant production, haemorrhage, oedema, hypoxaemia and infiltration of inflammatory cells into the interstitial and alveoli spaces (Rocco et al. 2001, 2003; Park et al. 2010; Novaes et al. 2012). The principal cause of death in cases of PQ toxicity is respiratory failure associated with diffuse damage to the alveolar epithelium owing to oxidation of lipids and membrane proteins, which culminates in partial or total loss of cell and lung function (Mustafa et al. 2002; Tsukamoto et al. 2002; Rocco et al. 2004). In view of its reproducibility and simplicity of administration, this herbicide has been widely used as an experimental model in studies on ALI (Rocco et al. 2001, 2003; Chang et al. 2009; Novaes et al. 2012). Moreover, it constitutes an inexpensive model in which effects are rapid (Silva & Saldiva 1998; Rocco et al. 2003), thus ensuring its frequent use when investigating the effects of vegetable extracts on lung disorders (Kim et al. 2006; Park et al. 2010).
Bathysa cuspidata (A. St. Hil.) Hook f. belongs to the Rubiaceae family. It is a plant popularly known in South America as ‘quina-do-mato’, and its bark is used in popular medicine for the prevention and treatment of stomach, liver and lung disorders, as well as an anti-inflammatory and healing agent (Novaes et al. 2012). In addition, in toxicity tests in vitro, the ethanolic extracts of the leaves and bark of this species showed no mutagenic and genotoxic effects in the Ames test, using TA98 and TA100 strains of Salmonella typhimurium, and plasmid (DNA plasmid pUC 18) cleavage test respectively (Nunes 2008). Considering that lung lesions from exposure to PQ occur through the induction of oxidative stress and activation of inflammatory response, compounds with antioxidant and anti-inflammatory action may potentially affect ALI and mortality from this condition (Suntres 2002; Kim et al. 2006). To clarify its relevance, its efficacy and possible mechanism of action, the objective of this study was to investigate the effect of the ethanolic bark extract of B. cuspidata on PQ-induced extra-pulmonary ALI and mortality in Wistar rats.
Materials and methods
Chemicals
Paraquat (Composition: 1,1′-dimetil-4,4′-bipridilio dicloreto íon [200 g/l] and inerts ingredients [876 g/l]) was purchased from the manufacturer Syngenta (Basel, Switzerland). Thiobarbituric acid, hydrogen peroxide, sodium phosphate, formaldehyde, glutaraldehyde, trypan Blue, comassie blue, haematoxylin and phloxine were purchased from Sigma Chemical Co. (St Louis, MO, USA).
Preparation and chemical analysis of Bathysa cuspidata extract
Samples of B. cuspidata were collected in a biome of Brazilian Atlantic forest in the state of Minas Gerais, Brazil (20°43′00.0′′S and 42°29′10.8′′W, 1200 m above sea level) and adequately documented in the herbarium of the Federal University of Viçosa under registration VIC 21559. Stem bark samples were separated, dried for 48 h at room temperature in a darkened, well-ventilated room, pulverized in a knife mill and stored. The powdered, air-dried stem bark of B. cuspidata (500 g) was extracted exhaustively by percolation with ethanol 95%. The extract was concentrated under vacuum at 50 °C using a rotary evaporator and then lyophilized until complete removal of the solvent, yielding an ethanolic extract (139 g). Phytochemical analysis of the extract was performed on chromatography plates coated with silica gel GF 254® (Merck, Darmstadt, Germany) using different mobile phases and detection reagents in accordance with the protocol described by Wagner and Bladts (1996). The preparation of B. cuspidata was analysed for the presence of phytochemicals by qualitative and quantitative chemical analysis previously described (Wagner & Bladts 1996).The vehicle Dimethyl sulfoxide (DMSO) was selected to dilute the B. cuspidata extract used in the treatment of animals.
Animals and their care
Three-month-old male Wistar rats were obtained from the main animal laboratory of the Federal University of Viçosa (Brazil) and kept in individual steel cages under controlled temperature conditions (21 ± 2 °C) with relative air humidity of 60–70% and 14 h of light daily (5 am–7 pm). The animals had free access to rat chow and water. All experiments were conducted in accordance with the internationally accepted laboratory animal use and care stated in the guide lines and rules of the ethical committee, Federal University of Viçosa, Brazil (approval protocol 064/2010).
Model of PQ-induced extra-pulmonary ALI and treatment groups
The animal model of ALI was prepared as previously reported (Wang et al. 2006). Briefly, PQ was dissolved in 0.5 ml of 0.9% NaCl saline solution (10 mg/ml) and injected intraperitoneally (i.p.) in a single dose of 30 mg/kg of body weight. The ethanolic extract of the stem bark of B. cuspidata (BCE) was resuspended in 700 μl of vehicle (VE) DMSO (w/v) and administered to the animals by gavage. Treatment with this extract at the doses of 200 mg/kg (BCE 200) and 400 mg/kg of body weight (BCE 400) began 2 days before the application of PQ and continued to be administered until 2 days after application of the herbicide. The same protocol used for the groups receiving the extract was followed in the control groups, which received BCE 400, DMSO, paraquat or saline 0.9% (700 μl) alone. Seventy rats were randomized into seven study groups with 10 animals in each group. 1) SAL (control): saline solution (NaCl 0.9%); 2) PQ (chemical control): Paraquat; 3) PQ/VE: Paraquat + DMSO; 4) VE (vehicle control): DMSO; 5) BCE 400 (extract control): BCE 400 mg/kg; 6) PQ/BCE 200: Paraquat + BCE 200 mg/kg; 7) PQ/BCE 400: Paraquat + BCE 400 mg/kg. As the doses of 200 and 400 mg/kg of the extract were effective in reducing oxidative markers in a pretest, these doses were selected for the study.
Survival analysis and euthanasia
The animals were monitored every 12 h from the onset of treatment, and mortality was recorded up to 24 h after the final treatment (sixth day). Mortality was investigated and reported for each initial group of 10 animals. To ensure the required number of animals for analysis (10 from each group), the animals that died were replaced by other animals submitted to the same treatment given in the group to which they were allocated. The animals were anesthetized with ketamine (10 mg/kg of body weight, i.p.) and xylazine (2 mg/kg of body weight, i.p.). Laparotomy was performed, and the abdominal aorta and vena cava were sectioned, resulting in a massive haemorrhage that quickly killed the animals.
Cell migration and lung oedema index
Cell migration was analysed in the bronchoalveolar lavage fluid (BALF) of five animals from each group. Following euthanasia, the lungs were removed en bloc and the BALF was collected using a polyethylene cannula (PE 205, Clay Adams, NJ, USA) inserted into the trachea. BAL was carried out by injecting and withdrawing three aliquots of 2 ml of phosphate-buffered saline (0.15 M). The samples of BALF were centrifuged (5 min at 300 g) for total and differential cell counts (Henderson 2005). Total leucocytes were counted in a Neubauer chamber (LaborOptik, Friedrichsdorf, Germany). Trypan blue (0.1%) dye exclusion was used to determine 95% cell viability. For differential cell counts, 100 μl of BALF were smeared on glass slides, then dried and stained using rapid panoptic staining (Laborclin, Paraná, Brazil). Two hundred cells per animal were classified as neutrophils, macrophages or lymphocytes and expressed as a percentage of recovered cells (Valença et al. 2008).
Lung oedema was assessed using a gravimetric method to determine the water content of the lung by calculating the wet/dry weight ratio of lung tissues. The lung was dissected from non-lung tissue and weighed before (wet weight 0) and after (wet weight 1) the bronchoalveolar lavage. Dry weight was determined after the lung was dried at 60 °C for 96 h, and the oedema index ([wet weight 1 − wet weight 0] − wet weight 1/dry weight) was calculated (Lo et al. 2005).
Biochemical analysis
For the biochemical study, the right lungs were removed from five animals per group and used for assessing oxidative markers in this organ. For analysis of tissue malondialdehyde (MDA), an end product of lipid peroxidation, an aliquot of frozen lung (100 mg) was homogenized in PBS, the homogenate was reacted with thiobarbituric acid and the formation of thiobarbituric acid-reactive substance was monitored at 535 nm as described previously (Gutteridge & Halliwel 1990). Catalase activity was evaluated according to the method described by Aebi (1984) by measuring the rate of decomposition of hydrogen peroxide (H2O2), and superoxide dismutase activity was estimated by a method based on the production of H2O2 by xanthine oxidase and reduction of nitroblue tetrazolium (Sarban et al. 2005). Protein carbonyl content was measured using the 2,4-dinitrophenylhydrazine (DNPH) procedure (Sohal et al. 1993). Total protein levels in lung tissue were measured using the Bradford method (Bradford 1976).
Lung histopathology and stereology
Under anaesthesia, the trachea of five animals from each group was exposed and occluded with suture line after the expiration. After laparotomy, the left main bronchus was occluded, and the left lung was removed en bloc and fixed (Lacerda et al. 2009) in Karnovsky’s solution for 24 h. Lung fragments were obtained through the orientator method as isotropic sections are necessary for the stereological study (Mandarim-de-Lacerda 2003). These fragments were dehydrated in ethanol and embedded in glycol-methacrylate (Historesin, Leica, Germany). Sections 4 μm thick were cut with a Multicut 2045® rotary microtome (Reichert-Jung, Germany), stained with haematoxylin and phloxine and mounted using Entellan® mounting medium (Merck, Frankfurter, Germany). To avoid repeated analysis of the same histological area, sections were evaluated in semi-series, using 1 in every 20 sections. The slides were visualized, and the images captured using a light microscope (Olympus BX-60®, Tokyo, Japan) connected to a digital camera (Olympus QColor-3®).
Fifty random lung fields from each group were analysed with 20× objective lens over a total lung area of 4.37 × 106 μm2. The histopathological parameters analysed consisted of the presence of haemorrhage, thickening of the alveolar septum, obstruction and alveolar collapse. For the stereological analysis, a test system of 300 points and 24 cycloid arcs was used in a standard test area of 73 × 103 μm2 (Mandarim-de-Lacerda 2003). The stereological parameter of volume density (Vv) was estimated by point counting for alveolar septum [septum], alveolar space [space] and cell [cell] using the following formula: Vv = PP [structure]/PT, where PP is the number of points that hit the structure and PT is the total test points. In addition, the relationship between the number of intersections of the alveolar septum with the cycloid arcs (I) and the arc length (LT) was used to calculate the surface density (Sv) of the alveolar epithelium (epi) according to the formula: Sv = 2 × I/LT (Mandarim-de-Lacerda 2003). All stereological analyses were performed using the Image Pro-Plus 4.5® image analysis software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
The data were expressed as means ± standard deviations (SD). The coefficient of variation (CV) was calculated to investigate the homogeneity in the distribution of animal weights. The survival data were submitted to the log-rank (Mantel–Cox) test to investigate the effect of the extract on the animal’s mortality. Biochemical and morphological data were submitted to the one-way anova test, followed by Tukey’s test for multiple comparisons. All the tests were performed using the GraphPad Prism 5.01® statistical software program (GraphPad Software, Inc, San Diego, CA, USA), and statistical significance was established at P < 0.05.
Results
Chemical composition of Bathysa cuspidata
The phytochemical analysis of the stem bark of the B. cuspidata ethanolic extract showed positive results for alkaloids, coumarins, flavonoids, tannins and triterpenes. The quantitative analysis indicated the content of total phenolic compounds (58.7 mg/g), proanthocyanidins (39.7 mg/g) and total flavonoids (3.4 mg/g).
Animal’s weight and mortality
The initial (364 ± 37 g; CV = 0.10) and final (361 ± 40 g; CV = 0.11) weight of the animals used in this study was similar in both groups, without significant differences. As shown in Figure 1, the administration of PQ resulted in the death of the Wistar rats. The mortality rate was higher in the PQ and PQ/VE groups compared with the other groups (50%). Moreover, both concentrations of the ethanol extract of B. cuspidata used in this study (200 and 400 mg/kg) reduced significantly the mortality of the rats exposed to PQ (P < 0.05), the best results being found in the PQ/BCE 400 group (mortality of 20%). The administration of the extract or vehicle alone did not result in the death of these animals.
Figure 1.
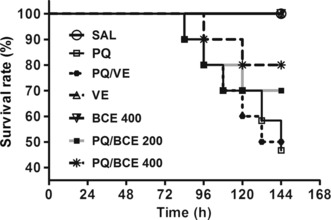
Effects of treatment with bark extract of Bathysa cuspidata (BCE 200 and 400 mg/kg, wt.) on survival of Wistar rats with extra-pulmonary acute lung injury induced by a single dose of paraquat (PQ, 30 mg/kg, wt, i.p). SAL, saline; PQ, paraquat; VE, vehicle (DMSO). There was significant reduction in mortality in the groups intoxicated and treated with both doses of the extract at the end of the experiment (P < 0.01), Log-rank (Mantel–Cox) test.
Cell migration and lung oedema
Analysis of the BALF showed increased cell migration in the lungs of animals exposed to PQ (Table 1). The total number of leucocytes in the BALF of the animals in the PQ and PQ/VE groups was significantly higher compared with that of the animals in the other groups (P < 0.05) except in relation to the PQ/BCE 200 group. There was a significant reduction in cell migration in the PQ/BCE 400 group compared with the PQ and PQ/VE groups (P < 0.05). Moreover, no statistically significant differences were found in the total number of leucocytes in the PQ/BCE 400 group compared with the SAL, VE and BCE 400 groups. With respect to differential cell count, the number of neutrophils was significantly higher in the PQ and PQ/VE groups compared with all the other groups (P < 0.05) except the PQ/BCE 200 group. In addition, the percentage of neutrophils in the PQ/BCE 400 group was significantly higher than in the SAL, VE and BCE 400 groups (P < 0.05). The amount of alveolar macrophages was significantly lower in the PQ and PQ/VE groups (P < 0.05) compared with all the other groups except for the PQ/BCE 200 group. Furthermore, the percentage of macrophages in the PQ/BCE 400 group was significantly lower in relation to the SAL, VE and BCE 400 groups (P < 0.05). There were no significant differences in the number of lymphocytes between the PQ, PQ/VE, PQ/BCE 200 and PQ/BCE 400 groups. Moreover, the percentage of lymphocytes in these groups was significantly lower in relation to the SAL, VE and BCE 400 groups (P < 0.05). There was no statistically significant difference in the number of total leucocytes, neutrophils, alveolar macrophages and lymphocytes between the PQ/BCE 200 and PQ/BCE 400 groups.
Table 1.
Effects of treatment with bark extract of Bathysa cuspidata on the total and differential cell count in brochoalveolar lavage of Wistar rats with extra-pulmonary acute lung injury induced by paraquat
| Groups | Total (×106/ml) | Neutrophils (%) | Macrophages (%) | Lymphocytes (%) |
|---|---|---|---|---|
| SAL | 0.91 ± 0.56a | 3.26 ± 1.62a | 82.87 ± 7.25a | 13.87 ± 1.13a |
| PQ | 4.02 ± 1.15b | 27.11 ± 6.24b | 65.01 ± 9.77b | 7.88 ± 2.27b |
| PQ/VE | 3.97 ± 1.37b | 25.18 ± 8.09b | 66.51 ± 11.12b | 8.31 ± 2.45b |
| VE | 1.12 ± 0.46a | 3.15 ± 1.07a | 82.16 ± 6.29a | 14.69 ± 1.33a |
| BCE 400 | 1.07 ± 0.35a | 2.94 ± 1.03a | 83.01 ± 5.42a | 14.05 ± 1.18a |
| PQ/BCE 200 | 2.86 ± 1.61b,c | 19.47 ± 9.86b,c | 70.35 ± 8.11b,c | 10.18 ± 2.07b |
| PQ/BCE 400 | 1.85 ± 1.29a,c | 15.13 ± 7.85c | 73.91 ± 8.49c | 10.96 ± 1.97b |
Acute lung injury was induced by a single dose of paraquat (PQ, 30 mg/kg, wt, i.p), and the animals were treated with extract of Bathysa cuspidata (BCE 200 and 400 mg/kg, wt.) diluted in vehicle DMSO (VE). Control animals received saline (SAL), paraquat (PQ), vehicle DMSO (VE) or BCE 400 mg/kg alone. The data are expressed as means ± SD. Different letters in columns indicate statistical difference between the groups (P < 0.05), and groups that have some common letter do not differ statistically, anova followed by Tukey’s test.
The oedema index was significantly higher in the PQ and PQ/VE groups in relation to the other groups (P < 0.05). In the PQ/BCE 200 group, the oedema index was significantly lower compared with the PQ and PQ/VE group, but significantly higher compared with the SAL, VE, BCE 400 and PQ/BCE 400 groups (P < 0.05). There were no statistically significant differences between any of the other groups (Figure 2).
Figure 2.
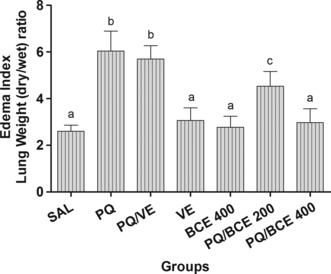
Effects of treatment with bark extract of Bathysa cuspidata (BCE 200 and 400 mg/kg, wt.) on index of lung edema determined by calculating the wet/dry weight ratio of lung tissue of Wistar rats with extrapulmonary acute lung injury induced by a single dose of paraquat (PQ, 30 mg/kg, wt, i.p). SAL, saline; PQ, paraquat; VE, vehicle (DMSO). The data are expressed as means ± SD. a,b,c Different letters in columns indicate statistical difference between the groups (P < 0.05) and groups that have some common letter do not differ statistically, anova followed by Tukey’s test.
Oxidative stress markers and antioxidant enzymes
According to the biochemical analysis, protein carbonyls and MDA levels were significantly higher in the lung tissue from animals in the PQ and PQ/VE groups compared with the other groups (P < 0.05), with the exception of the PQ/BCE 200 group. In addition, protein carbonyls and MDA levels were significantly higher in the PQ/BCE 200 group compared with the SAL, VE and BCE 400 (P < 0.05) groups; however, there was no statistically significant difference with respect to the PQ/BCE 400 group (Figure 3).
Figure 3.
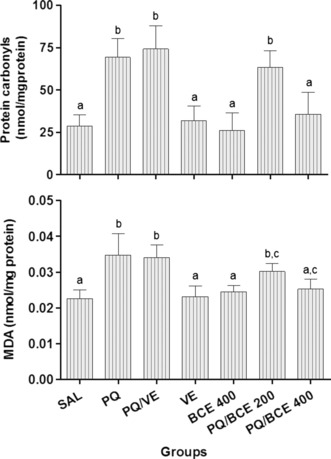
Effects of treatment with bark extract of Bathysa cuspidata (BCE 200 and 400 mg/kg, wt.) on protein carbonyls and malondialdehyde (MDA) levels in lung tissue of Wistar rats with extra-pulmonary acute lung injury induced by a single dose of paraquat (PQ, 30 mg/kg, wt, i.p). SAL, saline; PQ, paraquat; VE, vehicle (DMSO). The data are expressed as means ± SD. a,b,c Different letters in columns indicate statistical difference between the groups (P < 0.05) and groups that have some common letter do not differ statistically, anova followed by Tukey’s test.
Catalase activity in lung tissue was significantly lower in the PQ and PQ/VE groups compared with the other groups (P < 0.05), and no statistically significant differences were found between any of the other groups. In addition, superoxide dismutase activity was significantly lower in the groups PQ, PQ/VE and PQ/BCE200 in relation to the other groups (P < 0.05), which showed no statistical difference between them (Figure 4).
Figure 4.
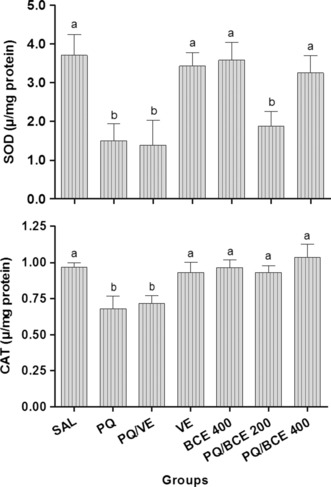
Effects of treatment with bark extract of Bathysa cuspidata (BCE 200 and 400 mg/kg, wt.) on superoxide dismutase (SOD) and catalase (CAT) activity in lung tissue of Wistar rats with extra-pulmonary acute lung injury induced by a single dose of paraquat (PQ, 30 mg/kg, wt, i.p). SAL, saline; PQ, paraquat; VE, vehicle (DMSO). The data are expressed as means ± SD. a,b,c Different letters in columns indicate statistical difference between the groups (P < 0.05) and groups that have some common letter do not differ statistically, anova followed by Tukey’s test.
Histopathological and stereological analysis
The six images in Figure 5 show that the histological organization of the lung tissue samples in the SAL, VE and BCE 400 groups was normal, with thin-lined alveolar septa and no obstruction of the alveolar space. Samples from the PQ and PQ/VE groups showed intense areas of haemorrhage with obvious septal thickening, obstruction and alveolar collapse. The images from the PQ/BCE 200 group show the presence of septal thickening with a reduction in the alveolar space and no signs of haemorrhage or alveolar collapse. The histological patterns of the samples from the PQ/BCE 400 group were similar to those of the control groups (SAL, VE and BCE 400); however, a slight thickening of the alveolar septum was observed.
Figure 5.
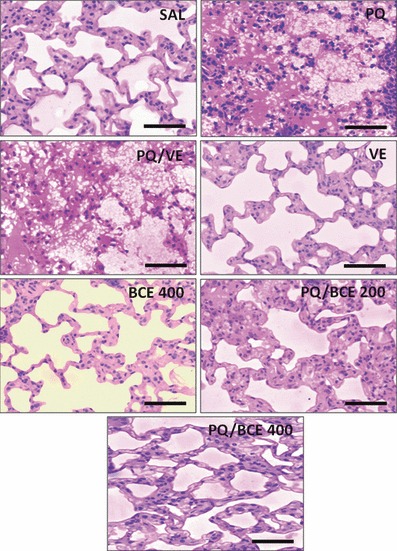
Representative photomicrographs of histological section of the lung of Wistar rats stained with hematoxylin and phloxine and observed under light microscope (bar = 150 μm). Extra-pulmonary acute lung injury was induced by a single dose of paraquat (PQ, 30 mg/kg, wt, i.p.) and the animals were treated with extract of Bathysa cuspidata (BCE 200 and 400 mg/kg, wt.) diluted in vehicle DMSO (VE). Control animals received saline (SAL), paraquat (PQ); vehicle DMSO (VE) or BCE 400 mg/kg alone.
Stereology (Table 2) confirmed the histopathological abnormalities and showed that the proportion of alveolar septum (Vv[septum]) in the lung area was significantly higher in the PQ, PQ/VE and PQ/BCE 200 groups compared with the other groups (P < 0.05). In addition, the volume density of alveolar spaces and the surface density of the lung epithelium were significantly lower in the PQ, PQ/VE and PQ/BCE 200 groups. Moreover, the volume density of cells (Vv[cell]) in lung parenchyma was significantly higher in the PQ and PQ/VE groups in relation to the others groups (P < 0.05). There were no significant differences in all stereological parameters between SAL, VE, BCE 400 and PQ/BCE 400 groups.
Table 2.
Effects of treatment with bark extract of Bathysa cuspidata on lung stereological parameters of Wistar rats with extra-pulmonary acute lung injury induced by paraquat
| Groups | Vv [septum] (%) | Vv [space] (%) | Vv [cell] (%) | Sv [epi] (mm2/mm3) |
|---|---|---|---|---|
| SAL | 53.59 ± 3.98a | 46.41 ± 3.99a | 6.23 ± 0.75a | 19.55 ± 3.34a |
| PQ | 74.55 ± 6.40b | 25.45 ± 6.38b | 10.33 ± 2.90b | 9.96 ± 2.73b |
| PQ/VE | 72.26 ± 7.23b | 27.74 ± 7.08b | 9.52 ± 2.47b | 11.25 ± 3.79b |
| VE | 56.01 ± 4.11a | 43.99 ± 3.72a | 6.48 ± 0.81a | 17.25 ± 2.47a |
| BCE 400 | 54.45 ± 5.53a | 45.55 ± 5.41a | 5.98 ± 0.96a | 17.92 ± 1.91a |
| PQ/BCE 200 | 63.14 ± 10.61b | 36.86 ± 10.52b | 6.85 ± 1.63a | 14.16 ± 2.10b |
| PQ/BCE 400 | 55.51 ± 2.12a | 44.49 ± 7.05a | 5.22 ± 1.98a | 18.72 ± 2.17a |
Acute lung injury was induced by a single dose of paraquat (PQ, 30 mg/kg, wt, i.p.), and the animals were treated with extract of Bathysa cuspidata (BCE 200 and 400 mg/kg, wt.) diluted in vehicle DMSO (VE). Control animals received saline (SAL), paraquat (PQ), vehicle DMSO (VE) or BCE 400 mg/kg alone. Vv, volume density; Sv, surface density; septum, alveolar septum; space, alveolar space; epi, epithelium. The data are expressed as means ± SD. Different letters in columns indicate statistical difference between the groups (P < 0.05), and groups that have some common letter do not differ statistically, anova followed by Tukey’s test.
Discussion
The present study investigated the effect of bark extract of B. cuspidata on PQ-induced extra-pulmonary ALI and mortality in rats. The experimental model used in this study was previously validated as a model of ALI (Rocco et al. 2001, 2003; Wang et al. 2006). There is evidence that the metabolic processes, immuno-inflammatory features and histological lung damage in acute PQ intoxication are similar in humans and rats (Dasta 1978; Rocco et al. 2001, 2003, 2004). Moreover, the dose of PQ used in the present study (30 mg/kg of body weight. i.p.) may be classified as moderate exposure to PQ (60% of the LD50) in rats (LD50 = 50 mg/kg) and high exposure in humans considering the human LD50 of 35 mg/kg (Nwabisi & Nwanze 1997; Sittipunt 2005). This dose was sufficient to induce an inflammatory response with increased cell migration and to elevate lipid ant protein oxidation, reduce the catalase and superoxide dismutase activity of lung tissue and result in lung injury, as shown by the lung oedema, haemorrhage, alveolar obstruction and collapse found in the PQ and PQ/VE groups.
The down-regulation of the oxidative and inflammatory processes represents an important mechanism for reducing PQ-induced ALI, which has been extensively investigated in an attempt to find alternative approaches to reduce the mortality rate related to intoxication with this herbicide (Dasta 1978; Lock & Wilks 2001; Suntres 2002; Wang et al. 2006). In the present study, the mortality rate of animals in the PQ and PQ/VE groups was 50%, while treatment of the PQ-intoxicated rats with 200 and 400 mg/kg of B. cuspidata extract reduced the mortality of the animals to 30% and 20% respectively. In addition, both doses of B. cuspidata extract reduced the lung oedema in PQ-intoxicated animals, with the best result being observed in the PQ/VE 400 group. These findings indicate that the reduction in mortality is associated with attenuation of the lung damage, suggesting a possible protective dose-dependent effect of B. cuspidata extract.
Morphologically, PQ-induced ALI is characterized by the infiltration of inflammatory cells, the death of lung epithelial cells, alveolitis and pulmonary oedema (Schoenberger et al. 1984; Tsukamoto et al. 2002; Kim et al. 2006). This occurs because of inhibition of the neural regulation of fluid reabsorption in the pulmonary interstitium. Inhibition of this regulation may cause pulmonary haemorrhage and interstitial oedema (Satomi et al. 2004; Kim et al. 2006). These events lead to alveolar obliteration and a reduction in lung volume and complacency that culminate in hypoxaemia and respiratory failure, which constitute the main cause of death in humans and animals following acute intoxication with PQ (Rocco et al. 2001, 2003; Suntres 2002).
This study confirms that treatment with bark extract of B. cuspidata reduces lipid and protein oxidation and prevents a reduction in catalase and superoxide dismutase activity. These findings were evident in the PQ/BCE 400 group. The cytotoxic effects of PQ have been attributed to the generation of superoxide radicals after the reduction of PQ by intracellular oxidases (Day & Crapo 1996; Tsukamoto et al. 2002). This process is amplified by the generation of highly reactive compounds such as malondialdehyde (MDA), lipid peroxyl radicals (LOO-) and alkoxy radicals (RO-) that propagate the oxidative damage resulting in marked lung injury (Dasta 1978; Nwabisi & Nwanze 1997; Mustafa et al. 2002). Previous studies have shown that compounds with antioxidant activity are capable of down-regulating the oxidation of lipids and cell proteins and the inflammatory process, reducing functional and morphological lung damage (Day & Crapo 1996; Suntres 2002; Kim et al. 2006; Chang et al. 2009; Park et al. 2010). As the preliminary phytochemical analysis of B. cuspidata extract indicated the presence of antioxidant compounds such as alkaloids, coumarins, flavonoids, tannins and triterpenes, the potential benefits of this extract in reducing the lung lipid peroxidation resulting from PQ poisoning were confirmed.
There is evidence of a relationship between markers of oxidative damage and histological lung injury (Fukushima et al. 2002; Suntres 2002; Kim et al. 2006; Park et al. 2010). The data on MDA in addition to the histopathological and stereological analysis observed in the present study corroborate this information. Besides reducing the levels of MDA and protein carbonyls, both doses of B. cuspidata extract (200 and 400 mg/kg) attenuated the morphological lesions of the lung parenchyma, the best results being found in the PQ/BCE 400 group. In this group, lung oedema was found to be reduced, there was a slight thickness of the alveolar septum and a greater area of free alveolar surface compared with the PQ and PQ/VE groups. In addition, modulation of the inflammatory process was found in BALF through the reduction of cell migration and in tissue through lower cellularity in lung parenchyma (Vv[cell]). In PQ intoxication, the destruction of type I and II epithelial cells leads to the release of cytokines and growth factors that induce the migration of inflammatory cells, mainly, neutrophils (Schoenberger et al. 1984; Lock & Wilks 2001). As a result of the infiltration and activation of macrophages, neutrophils and lymphocytes in lung tissue, there is an increased formation of oxidizing agents such as nitric oxide (NO), superoxide radicals (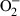 ) and hydroxyl radicals (OH−) (Dasta 1978; Lock & Wilks 2001; Fukushima et al. 2002; Mustafa et al. 2002). These agents are known to amplify oxidative damage and lung dysfunction (Schoenberger et al. 1984; Fukushima et al. 2002; Suntres 2002). Thus, the present study admits the hypothesis that B. cuspidata extract acts through two mechanisms, one direct and other indirect, to reduce damage resulting from lipid protein oxidation. The first is caused by the direct neutralization of oxidizing agents produced by a cytotoxic effect of PQ on pneumocytes and resident leucocytes, while the second is attributed to a reduction in inflammatory cell migration and secondary oxidative lung injury. In this context, the phytochemicals indentified in the extract could have a radical scavenger activity able to act together with CAT and SOD in the antioxidant defence process, reducing the depletion of these enzymes and the pneumotoxicity induced by paraquat. In fact, the bark extract of B. cuspidata showed high content of total phenolic compounds, substances with recognized antioxidant activity found in many plant extracts (Rice-Evans et al. 1997; Kähkönen et al. 1999; Fukumoto & Mazza 2000).
) and hydroxyl radicals (OH−) (Dasta 1978; Lock & Wilks 2001; Fukushima et al. 2002; Mustafa et al. 2002). These agents are known to amplify oxidative damage and lung dysfunction (Schoenberger et al. 1984; Fukushima et al. 2002; Suntres 2002). Thus, the present study admits the hypothesis that B. cuspidata extract acts through two mechanisms, one direct and other indirect, to reduce damage resulting from lipid protein oxidation. The first is caused by the direct neutralization of oxidizing agents produced by a cytotoxic effect of PQ on pneumocytes and resident leucocytes, while the second is attributed to a reduction in inflammatory cell migration and secondary oxidative lung injury. In this context, the phytochemicals indentified in the extract could have a radical scavenger activity able to act together with CAT and SOD in the antioxidant defence process, reducing the depletion of these enzymes and the pneumotoxicity induced by paraquat. In fact, the bark extract of B. cuspidata showed high content of total phenolic compounds, substances with recognized antioxidant activity found in many plant extracts (Rice-Evans et al. 1997; Kähkönen et al. 1999; Fukumoto & Mazza 2000).
The present study demonstrated that the bark extract of B. cuspidata reduced the PQ-induced extra-pulmonary ALI and the mortality in Wistar rats. The results indicated a possible dose-dependent effect of B. cuspidata extract, which can mainly be associated with its inhibitory activities on lipid and protein oxidation, maintenance of catalase and superoxide dismutase activity and reduction of morphological damage to the lung parenchyma. Further pharmacological evaluations are essential to elucidate the detailed mechanism of action of this extract, which might relate to a high potential for the prevention and treatment of extra-pulmonary ALI.
Author contributions
All listed authors meet ICMJE authorship criteria, and nobody who qualifies for authorship has been excluded. Authors contributed to research design, acquisition, analysis and interpretation of data, drafting the manuscript or revising it critically, and approval of the submitted and final versions.
Acknowledgments
The authors received no external funding for this study.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang X, Shao C, Wu Q, Wu Q, Huang M, Zhou Z. Pyrrolidine dithiocarbamate attenuates paraquat-induced lung injury in rats. J. Biomed. Biotechnol. 2009;61948:7. doi: 10.1155/2009/619487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasta JF. Paraquat poisoning: a review. Am. J. Hosp. Pharm. 1978;35:1368–1372. [PubMed] [Google Scholar]
- Day BJ, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced lung injury in vivo. Toxicol. Appl. Pharmacol. 1996;140:94–100. doi: 10.1006/taap.1996.0201. [DOI] [PubMed] [Google Scholar]
- Farnsworth NR. Ethnopharmacology and drug development. Ciba Found. Symp. 1994;185:42–51. doi: 10.1002/9780470514634.ch4. [DOI] [PubMed] [Google Scholar]
- Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food. Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Tanaka K, Lim H, Moriyama M. Mechanism of cytotoxicity of paraquat. Envrion. Health Prev. Med. 2002;7:89–94. doi: 10.1265/ehpm.2002.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge JMC, Halliwel B. The measurement and mechanism of lipid peroxidation in physiological systems. Trends Biochem. Sci. 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Henderson RF. Use of bronchoalveolar lavage to detect respiratory tract toxicity of inhaled material. Exp. Toxicol. Pathol. 2005;57:155–159. doi: 10.1016/j.etp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kähkönen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food. Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kaur S, Michael H, Arora S, Harkonem PL, Kumar S. The in vitro cytotoxic and apoptotic activity of Triphala-an Indian Herbal drug. J. Ethnopharmacol. 2005;67:249–251. doi: 10.1016/j.jep.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Kim H-R, Park B-K, Oh Y-M, et al. Green tea extract inhibits paraquat-induced pulmonary fibrosis by suppression of oxidative stress and Endothelin-l expression. Lung. 2006;184:287–295. doi: 10.1007/s00408-005-2592-x. [DOI] [PubMed] [Google Scholar]
- Lacerda AC, Rodrigues-Machado MdaG, Mendes PL, et al. Paraquat (PQ)-induced pulmonary fibrosis increases exercise metabolic cost, reducing aerobic performance in rats. J. Toxicol. Sci. 2009;34:671–679. doi: 10.2131/jts.34.671. [DOI] [PubMed] [Google Scholar]
- Lo YC, Lin YL, Yu KL, et al. San-Huang-Xie-Xin-Tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. J. Ethnopharmacol. 2005;101:68–74. doi: 10.1016/j.jep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Lock EA, Wilks MF. Paraquat. In: Krieger RI, editor. Handbook of Pesticide Toxicology. San Diego: Academic Press; 2001. pp. 1559–1603. [Google Scholar]
- Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An. Acad. Bras. Cienc. 2003;75:469–486. doi: 10.1590/s0001-37652003000400006. [DOI] [PubMed] [Google Scholar]
- Marrs TC, Adjei A. Paraquat. J. Med. Plant Res. 2003;1:203–266. [Google Scholar]
- Mustafa A, Gado AM, Al-Shabanah OA, Al-Bekairi AM. Protective effect of aminoguanidine against paraquat-induced oxidative stress in the lung of mice. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002;132:391–397. doi: 10.1016/s1532-0456(02)00095-9. [DOI] [PubMed] [Google Scholar]
- Novaes RD, Gonçalves RV, Marques DC, et al. Effect of bark extract of Bathysa cuspidata on hepatic oxidative damage and blood glucose kinetics in rats exposed to paraquat. Toxicol. Pathol. 2012;40:62–70. doi: 10.1177/0192623311425059. [DOI] [PubMed] [Google Scholar]
- Nunes LG. Phytochemistry prospection and mutagenicity evaluate in vitro of tree species vegetables: Strychnos Pseudoquina A. St.-Hill., Coutarea Hexandra (Jacq.) K. Schum and Bathysa cuspidata (A. St.-Hil.) Hook. Federal University of Viçosa; 2008. M.S. Thesis. Viçosa, Minas Gerais, Brazil. [Google Scholar]
- Nwabisi VC, Nwanze EAC. Biochemical studies on the toxicity of 1, 1′-dimethyl-4, 4′-bipyridylium dichloride in the rat. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1997;117:103–109. doi: 10.1016/s0742-8413(96)00166-1. [DOI] [PubMed] [Google Scholar]
- Park HK, Kim SJ, Kwon DY, Park JH, Kim YC. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci. 2010;87:181–186. doi: 10.1016/j.lfs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- Rocco PRM, Negri EM, Kurtz PM, et al. Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am. J. Respir. Crit. Care Med. 2001;164:1067–1071. doi: 10.1164/ajrccm.164.6.2007062. [DOI] [PubMed] [Google Scholar]
- Rocco PRM, Souza AB, Faffe DS, et al. Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am. J. Respir. Crit. Care Med. 2003;168:677–684. doi: 10.1164/rccm.200302-256OC. [DOI] [PubMed] [Google Scholar]
- Rocco PR, Facchinetti LD, Ferreira HC, et al. Time course of respiratory mechanics and pulmonary structural remodelling in acute lung injury. Respir. Physiol. Neurobiol. 2004;12:49–61. doi: 10.1016/j.resp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Sarban S, Kocyigit A, Yazar M, Isikan UE. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin. Biochem. 2005;38:981–986. doi: 10.1016/j.clinbiochem.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Satomi Y, Tsuchiya W, Mihara K, Ota M, Kasahara Y, Arahori F. Gene expression analysis of the lung following paraquat administration in rats using DNA. J. Toxicol. Sci. 2004;29:91–100. doi: 10.2131/jts.29.91. [DOI] [PubMed] [Google Scholar]
- Schoenberger CI, Rennard SI, Bitterman PB, Fukuda Y, Ferrans VJ, Crystal RG. Paraquat-induced pulmonary fibrosis. Role of the alveolitis in modulating the development of fibrosis. Am. Rev. Respir. Dis. 1984;129:168–173. doi: 10.1164/arrd.1984.129.1.168. [DOI] [PubMed] [Google Scholar]
- Silva MFR, Saldiva PHN. Paraquat poisoning: an experimental model of dose-dependent acute lung injury due to surfactant dysfunction. Braz. J. Med. Biol. Res. 1998;31:445–450. doi: 10.1590/s0100-879x1998000300018. [DOI] [PubMed] [Google Scholar]
- Sittipunt C. Paraquat poisoning. Respir. Care. 2005;50:383–385. [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc. Natl Acad. Sci. USA. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- Tsukamoto M, Tampo Y, Sawada M, Yonaha M. Paraquat-induced oxidative stress and dysfunction of the glutathione redox cycle in pulmonary microvascular endothelial cells. Toxicol. Appl. Pharmacol. 2002;178:82–89. doi: 10.1006/taap.2001.9325. [DOI] [PubMed] [Google Scholar]
- Valença SS, Bezerra FS, Romana-Souza B, Paiva RO, Costa AM, Porto LC. Supplementation with vitamins C and E improves mouse lung repair. J. Nutr. Biochem. 2008;19:604–611. doi: 10.1016/j.jnutbio.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Wagner H, Bladts S. In: Plant Drug Analysis: A Thin Layer Chromatography Atlas. Wagner H, Bladts S, editors. Berlin: Springer; 1996. pp. 62–73. [Google Scholar]
- Wang Y, Feinstein S, Manevich Y, Ho Y-S, Fisher AB. Peroxiredoxin 6 gene-targeted mice show increased lung injury with paraquat-induced oxidative stress. Antioxid. Redox Signal. 2006;8:229–237. doi: 10.1089/ars.2006.8.229. [DOI] [PubMed] [Google Scholar]


