Abstract
Acute pancreatitis (AP) is a rare but life-threatening complication of SLE. The current study evaluated the clinical characteristics and risk factors for the mortality of patients with SLE-related AP in a cohort of South China. Methods. Inpatient medical records of SLE-related AP were retrospectively reviewed. Results. 27 out of 4053 SLE patients were diagnosed as SLE-related AP, with an overall prevalence of 0.67%, annual incidence of 0.56‰ and mortality of 37.04%. SLE patients with AP presented with higher SLEDAI score (21.70 ± 10.32 versus 16.17 ± 7.51, P = 0.03), more organ systems involvement (5.70 ± 1.56 versus 3.96 ± 1.15, P = 0.001), and higher mortality (37.04% versus 0, P = 0.001), compared to patients without AP. Severe AP (SAP) patients had a significant higher mortality rate compared to mild AP (MAP) (75% versus 21.05%, P = 0.014). 16 SLE-related AP patients received intensive GC treatment, 75% of them exhibited favorable prognosis. Conclusion. SLE-related AP is rare but concomitant with high mortality in South Chinese people, especially in those SAP patients. Activity of SLE, multiple-organ systems involvement may attribute to the severity and mortality of AP. Appropriate glucocorticosteroid (GC) treatment leads to better prognosis in majority of SLE patients with AP.
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic, autoimmune, inflammatory disease characterized by the presence of a plethora of autoantibodies, immune complex formation, and multiple organ system involvement. Gastrointestinal (GI) manifestations are common in SLE patients, but acute pancreatitis is rare [1–6]. It was reported that 19.2%–50% of SLE patients presented with gastrointestinal symptoms [7–11], whereas pancreatitis occurred in about 0.7%–8.2% of patients with SLE [7, 8, 11, 12] and the annual incidence was approximately 0.4–1.1‰ [3–5]. Our knowledge about SLE-related acute pancreatitis (AP) is mostly based on individual case reports or small case series. Despite its rarity, AP can be a life-threatening complication of SLE if not treated appropriately. Prevalence of SLE is relatively high in Chinese people, which is 0.7~1/1000 in comparison to 0.51/1000 in United States [13]. But so far very few case reports on SLE-related AP in Chinese population have been published. The current study aims to clarify the clinical characteristics, severity, mortality, and outcome of SLE-related acute pancreatitis in south China.
2. Materials and Methods
A retrospective review of inpatient medical records between January 2000 and January 2012 was performed at the First Affiliated Hospital of Sun Yat-Sen University in South China. 4053 patients were classified as SLE during the past 12 years who fulfilled at least four of the American College of Rheumatology (ACR) revised classification criteria for SLE (1997) [14]. A diagnosis of acute pancreatitis (AP) was established by the presence of typical clinical symptoms (including abdominal pain, nausea, and vomiting) and confirmed by more than a three-fold elevation of serum amylase or lipase or evidence of imaging findings-computer tomography [CT] scan or ultrasonography (USG) [15]. Among these SLE patients, 27 were with dual simultaneous diagnosis of AP, and another 23 age- and gender-matched SLE patients without AP were randomly selected. Review of the clinical files of these 50 SLE patients was performed and data was extracted.
The SLE Disease Activity Index (SLEDAI) [16] was used to evaluate SLE activity during AP, and patients were defined as active SLE if the SLEDAI score was equal to or greater than 6. The Systemic Lupus International Collaborating Clinics/ACR (SLICC/ACR) damage index [17] was used to ascertain organ damage in SLE. The Atlanta criteria [18] were used to classify the severity of acute pancreatitis. Severe acute pancreatitis (SAP) was defined as the presence of at least three of Ranson's criteria and eight or more Acute Physiology and Chronic Health Evaluation II (APACHE II) score, or with the evidence of organ failure (systolic blood pressure < 90 mmHg, PaO2 ≤ 60 mmHg on room air, creatinine > 2 mg/dL, gastrointestinal bleeding > 500 mL/24 h, DIC or severe hypocalcemia ≤ 7.5 mg/dL) or local complications (i.e., pancreatic necrosis, abscess, or pseudocyst). The positivity of CT scan was defined as diffuse or segmental enlargement of the pancreas, illegibility of peripancreas fat, low/high density area in contrast, and peripancreas effusion [19]. The positivity of USG was defined as pancreatic enlargement, decreased echodensity, and possible fluid collections [20].
Demographic information including gender, age at SLE onset, duration between the onset of SLE and AP, history of alcohol consumption, gallstone, metabolic abnormalities (hypertriglyceridemia and hypercalcemia), clinical symptoms, laboratory findings, medications (especially corticosteroid, and immunosuppressive agents (ISA)) and outcome were documented. Acute pancreatitis related to mechanical obstruction (choledocholithiasis), toxic-metabolic etiologies (alcohol intake, drugs, hypercalcemia, or hypertriglyceridemia), infection, or trauma were ruled out in every case [21].
2.1. Statistical Analysis
Statistical analysis was done using the SPSS program 13.0 and Prism software version 5.0. The Mann-Whitney U test was used for continuous variables and the chi-square or Fisher's exact test for categorical variables. Survival rates were estimated using the Kaplan-Meier method. A P value <0.05 was considered statistically significant in all comparisons.
3. Results
3.1. Demographic and Clinical Characteristics of SLE-Related Acute Pancreatitis
27 out of 4053 SLE patients were diagnosed as SLE-related AP during the past 12 years, with an overall prevalence of 0.67% and annual incidence of 0.56‰. One patient developed 2 episodes of pancreatitis and the other 26 patients had only one episode at the time of hospitalization. The demographic and clinical features of each SLE-related AP patient were shown in Table 1.
Table 1.
The demographic and clinical characteristics of each SLE patient with AP.
| Case | Age at SLE onset (y) | Duration between onset of SLE and AP (m) | SLEDAI score at onset of AP | Number of involved organs concomitant with AP | GC treatment after onset of AP | Outcome |
|---|---|---|---|---|---|---|
| 1 | 23 | 0.5 | 9 | 5 | Increased dose | In remission |
| 2 | 16 | 48 | 12 | 4 | Stop | In remission |
| 3 | 18 | 12 | 14 | 6 | Increased dose | Died |
| 4 | 22 | 72 | 14 | 7 | Increased dose | In remission |
| 5 | 16 | 36 | 16 | 7 | Increased dose | In remission |
| 6 | 57 | 24 | 17 | 4 | Increased dose | In remission |
| 7 | 36 | 0.5 | 17 | 6 | Initial treatment | Died |
| 8 | 48 | 180 | 18 | 7 | maintaining | Died |
| 9 | 14 | 36 | 21 | 4 | maintaining | Died |
| 10 | 14 | 12 | 23 | 7 | Increased dose | Died |
| 11 | 19 | 2 | 23 | 6 | Increased dose | In remission |
| 12 | 14 | 2 | 27 | 7 | Increased dose | Died |
| 13 | 46 | 1 | 25 | 4 | Increased dose | In remission |
| 14 | 22 | 0.25 | 18 | 3 | Decreased dose | In remission |
| 15 | 51 | 240 | 18 | 6 | Decreased dose | In remission |
| 16 | 42 | 4 | 19 | 7 | Increased dose | In remission |
| 17 | 20 | 84 | 18 | 5 | Decreased dose | Died |
| 18 | 39 | 24 | 13 | 5 | Increased dose | In remission |
| 19 | 15 | 2 | 33 | 6 | Decreased dose | Died |
| 20 | 15 | 36 | 41 | 7 | Increased dose | In remission |
| 21 | 26 | 3 | 41 | 8 | Increased dose | Died |
| 22 | 39 | 1 | 27 | 6 | Increased dose | In remission |
| 23 | 20 | 72 | 10 | 3 | maintaining | In remission |
| 24 | 36 | 12 | 38 | 6 | maintaining | Died |
| 25 | 16 | 2 | 8 | 3 | Increased dose | In remission |
| 26 | 30 | 48 | 47 | 8 | Increased dose | In remission |
| 27 | 14 | 72 | 19 | 6 | maintaining | In remission |
3.2. Comparison of Demographic and Clinical Features in SLE Patients with and without SLE-Related AP
The majority of patients (92.59%, 25/27) were females and the mean age at SLE onset was 26.96 ± 13.30 years (ranged from 14 to 57 years). Time interval between the onset of SLE and AP ranged from 1 week to 20 years, and more than half of the patients (51.85%, 14/27) developed AP within the first year of the onset of SLE. All these 27 patients were classified as active SLE with average SLEDAI score of 21.70 ± 10.32 at the onset of AP. The clinical features related to acute pancreatitis in these 27 SLE patients were nonspecific. Abdominal pain (92.59%), fever (77.78%) and nausea/vomiting (74.07%), were the most frequent manifestations and other symptoms included diarrhea (44.44%), loss of appetite (44.44%) and GI tract hemorrhage (14.81%).
Other organ system involvement was found in all SLE-related AP patients with an average number of 5.70 ± 1.56 (ranged from 3 to 8 organs), including hematological system, kidney, liver, serositis, mucocutaneous involvement, respiratory system, arthritis, and central nervous system.
Clinical features and laboratory findings were compared between these two groups and the results were shown in Table 2. SLE patients with AP presented with higher SLEDAI score (21.70 ± 10.32 versus 16.17 ± 7.51, P = 0.03), more organ system involvement (5.70 ± 1.56 versus 3.96 ± 1.15, P = 0.001), higher frequence of fever (77.78% versus 39.13%, P = 0.006), hepatological and hematological disorders (82.61% versus 34.78%, P = 0.01; 100% versus 60.87%, P = 0.001), serositis (62.96% versus 26.09%, P = 0.01), elevated CRP (81.82% versus 47.62%, P = 0.02), positive anti-La antibody (33.33% versus 0, P = 0.003), and higher mortality (37.04% versus 0, P = 0.001) compared to SLE patients without AP.
Table 2.
Comparison of demographic and clinical features in SLE patients with and without AP.
| SLE with AP (n = 27) | SLE without AP (n = 23) | P | |
|---|---|---|---|
| Female (%) | 25 (92.59%) | 20 (86.96%) | 0.42 |
| Age on SLE diagnosis (y) | 26.96 ± 13.30 | 28.39 ± 9.98 | 0.26 |
| GCs dose (mg) | 61.19 ± 37.63 | 50.96 ± 28.82 | 0.18 |
| SLEDAI score | 21.70 ± 10.32 | 16.17 ± 7.51 | 0.03 |
| SLICC/ACR damage index | 1.19 ± 0.92 | 0.96 ± 1.19 | 0.11 |
| Mortality | 10 (37.04%) | 0 | 0.001 |
| Fever (%) | 21 (77.78%) | 9 (39.13%) | 0.006 |
| Neuropsychiatric (%) | 7 (25.93%) | 1 (4.35%) | 0.042 |
| Pulmonary (%) | 11 (40.74%) | 5 (21.74%) | 0.13 |
| Articular (%) | 16 (59.26%) | 16 (69.57%) | 0.32 |
| Mucocutaneous involvement (%) | 18 (66.67%) | 16 (69.57%) | 0.54 |
| Renal (%) | 24 (88.89%) | 20 (86.96%) | 0.59 |
| Hepatological (%) | 19 (82.61%) | 8 (34.78%) | 0.01 |
| Hematological (%) | 27 (100.00%) | 14 (60.87%) | 0.001 |
| Serositis (%) | 17 (62.96%) | 6 (26.09%) | 0.01 |
| Number of organs involved | 5.70 ± 1.56 | 3.96 ± 1.15 | 0.001 |
| Positive anti-dsDNA (%) | 24 (88.89%) | 19 (82.61%) | 0.41 |
| Positive anti-Sm (%) | 6/21 (28.57%) | 10 (43.48%) | 0.24 |
| Positive anti-Ro (%) | 13/21 (61.90%) | 14 (60.87%) | 0.60 |
| Positive anti-La (%) | 7/21 (33.33%) | 0 | 0.003 |
| Positive ACL-IgG (%) | 4/21 (19.05%) | 1/22 (4.55%) | 0.168 |
| Positive ACL-IgM (%) | 4/21 (19.05%) | 1/22 (4.55%) | 0.16 |
| Positive anti-β 2 GPI (%) | 3/21 (14.29%) | 2/22 (9.09%) | 0.48 |
| Low C3 (%) | 26/26 (100%) | 22 (95.65%) | 0.47 |
| Low C4 (%) | 21/26 (80.77%) | 20 (86.96%) | 0.42 |
| Elevated CRP (%) | 18/22 (81.82%) | 10/21 (47.62%) | 0.02 |
3.3. Comparison of Clinical Features between SAP and MAP Patients
According to Atlanta criteria, 27 SLE-related AP patients were divided into SAP group (severe acute pancreatitis, n = 8, 29.63%) and MAP group (mild acute pancreatitis, n = 19, 70.37%). The comparison of the demographic and clinical data between SAP and MAP patients as shown in Table 3. The results indicated that the age of onset of AP in SAP patients as significantly younger than MAP (19.63 ± 10.88 versus 30.05 ± 13.25, P = 0.016). SAP patients presented with significantly higher mortality (75% versus 21.05%, P = 0.014) and more abnormal hematologic findings (thrombocytopenia and leucopenia, 100% versus 52.63%, P = 0.026; 87.5% versus 31.58%, P = 0.013, resp.) compared to MAP. The Kaplan-Meier survival curves showed death rate within 30 days after onset of acute pancreatitis in SAP and MAP groups (Figure 1).
Table 3.
Comparison of demographic and clinical characteristics between SLE-related severe acute pancreatitis (SAP) and mild acute pancreatitis (MAP).
| SAP (n = 8) | MAP (n = 19) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Female | 7 (87.50%) | 18 (94.74%) | 0.513 |
| Age of onset AP (y) | 19.63 ± 10.88 | 30.05 ± 13.25 | 0.016 |
| Interval between onset of SLE and AP (m) | 23.38 ± 28.25 | 44.36 ± 64.73 | 0.822 |
| Early AP (⩽1 year) | 5 (62.50%) | 9 (47.37%) | 0.678 |
| SLEDAI score at onset of AP | 22.13 ± 6.24 | 21.53 ± 11.77 | 0.44 |
| SLICC/ACR damage index | 1.25 ± 0.89 | 1.16 ± 0.96 | 0.854 |
| Number of organs involved | 5.75 ± 1.28 | 5.68 ± 1.70 | 0.893 |
| Intensive therapy of GC/ISA | 5 (62.50%) | 11 (57.89%) | 1 |
| Mortality | 6 (75%) | 4 (21.05%) | 0.014 |
|
| |||
| Clinical characteristics | |||
| Fever | 8 (100.00%) | 13 (68.42%) | 0.136 |
| Mucocutaneous involvement | 6 (75.00%) | 12 (63.16%) | 0.676 |
| Articular involvement | 3 (37.50%) | 10 (52.63%) | 0.678 |
| Serositis | 5 (62.50%) | 10 (52.63%) | 0.696 |
| Neuropsychiatric involvement | 2 (25.00%) | 5 (26.32%) | 1 |
| Renal involvement | 7 (87.50%) | 15 (78.95%) | 1.0 |
|
| |||
| Laboratory findings | |||
| Serum amylase∗ | 18.09 ± 18.15 | 7.46 ± 5.88 | 0.077 |
| Serum lipase∗ | 8.53 ± 3.14 | 7.63 ± 5.45 | 0.616 |
| Elevated serum transaminase | 7 (87.5%) | 11 (57.89%) | 0.201 |
| Thrombocytopenia | 8 (100%) | 10 (52.63%) | 0.026 |
| Leucopenia | 7 (87.50%) | 6 (31.58%) | 0.013 |
| Positive anti-dsDNA | 8 (100.00%) | 16 (84.21%) | 0.532 |
| Positive anti-Sm | 1/7 (14.29%) | 5/14 (35.71%) | 0.613 |
| Low C3 | 8/8 (100.00%) | 18/18 (100.00%) | 1 |
| Low C4 | 7/8 (87.50%) | 14/18 (77.78%) | 1 |
| Anti-Ro | 2/7 (28.57%) | 11/14 (78.57%) | 0.056 |
| Anti-La | 1/7 (14.29%) | 6/14 (42.86%) | 0.337 |
∗Times in excess of the upper limit of normal (ULN).
Figure 1.
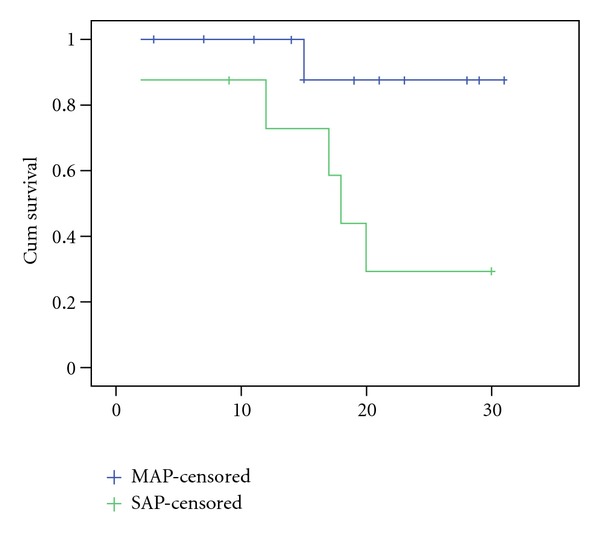
Kaplan-Meier survival curves for the time (days) from onset of SLE-related acute pancreatitis to death.
3.4. Comparison of Clinical Features between Pediatric- and Adult-Onset SLE-Related AP
SLE-related AP patients were divided into pediatric-onset group (under 18 years of age, n = 10) and adult-onset group (n = 17). Demographic and clinical characteristics were compared between these two groups. Pediatric-onset SLE-related AP had higher rate of severe AP (60% versus 11.76%, P = 0.014), higher serum amylase level (17.55 ± 16.09 versus 6.53 ± 5.42, P = 0.007), lower percentage of positive anti-Ro antibody (25% versus 84.62%, P = 0.01), and lower rate of anti-La antibody (0 versus 53.85%, P = 0.02) compared to adult-onset SLE-related AP. However, the difference in mortality was not statistically significant between pediatric and adult patients (50% versus 29.41%, P = 0.26).
3.5. Comparison of Clinical Features between Mortality and Nonmortality SLE Patients with AP
The risk factors for mortality were further analyzed. 27 SLE-related AP patients were divided into mortality group (n = 10) and nonmortality group (n = 17). The clinical manifestations were compared between these two groups and shown in Table 4. The mortality group had higher percentage of hypoalbuminemia (90% versus 47.06%, P = 0.031), hyperbilirubinemia (40% versus 5.88%, P = 0.047), hematuria (100% versus 41.18%, P = 0.002), and granular casts (70% versus 23.53%, P = 0.024) compared to nonmortality group. Severity of acute pancreatitis was the most powerful risk factor for mortality in SLE-related AP (OR 11.25, 95% CI (1.611, 78.57) and P = 0.014).
Table 4.
Comparison of demographic and clinical characteristics between mortality and non-mortality group.
| Mortality (n = 10) | Non-mortality (n = 17) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Female | 10 (100%) | 15 (88.24%) | 0.387 |
| Age of onset AP (y) | 24.10 ± 12.02 | 28.65 ± 14.07 | 0.123 |
| SLEDAI score at onset of AP | 25.00 ± 9.40 | 19.76 ± 10.61 | 0.065 |
| Number of organs involved | 6.20 ± 1.14 | 5.41 ± 1.73 | 0.167 |
| Intensive therapy of GC/ISA | 4 (40%) | 12 (70.59%) | 0.124 |
|
| |||
| Clinical characteristics | |||
| Fever | 10 (100%) | 11 (64.71%) | 0.042 |
| Mucocutaneous involvement | 6 (60%) | 12 (70.59%) | 0.439 |
| Articular involvement | 6 (60%) | 7 (41.18%) | 0.293 |
| Serositis | 7 (70%) | 8 (47.06%) | 0.226 |
| Neuropsychiatric involvement | 4 (40 %) | 3 (17.65%) | 0.204 |
|
| |||
| Laboratory findings | |||
| Serum amylase∗ | 14.79 ± 17.34 | 8.15 ± 6.03 | 0.241 |
| Serum lipase∗ | 6.46 ± 3.51 | 8.20 ± 5.58 | 0.368 |
| Elevated serum transaminase | 8 (80%) | 10 (58.82%) | 0.244 |
| Hypoalbuminemia | 9 (90%) | 8 (47.06%) | 0.031 |
| Proteinuria | 10 (100%) | 12 (70.59%) | 0.077 |
| Hematuria | 10 (100%) | 7 (41.18%) | 0.002 |
| Granular casts | 7 (70%) | 4 (23.53%) | 0.024 |
| Hyperbilirubinemia | 4 (40%) | 1 (5.88%) | 0.047 |
| Positive anti-dsDNA | 10 (100%) | 14 (82.35%) | 0.232 |
| Positive anti-Sm | 2/8 (25%) | 4/13 (30.77%) | 0.59 |
| Low C3 | 9/9 (100%) | 17/17 (100%) | 1.0 |
| Low C4 | 7/9 (77.78%) | 14/17 (82.35%) | 0.58 |
| Anti-Ro | 3/8 (37.5%) | 10/13 (76.92%) | 0.09 |
| Anti-La | 1/8 (12.5%) | 6/13 (46.15%) | 0.133 |
∗Times in excess of the upper limit of normal (ULN).
4. Treatment and Outcome
Among these 27 SLE-associated AP patients, 26 were on steroid treatment before the onset of AP and the average dosage of GCs was 61.19 ± 37.63 mg/day (ranged from 10 mg/day to 120 mg/day). AP was considered as the initial presentation of SLE in one patient (patient 7 in Table 1), and standard GC treatment started after diagnosis. Additional immunosuppressive agents (ISA) were also administrated in 22 patients before the onset of AP, including 18 on hydroxychloroquine, 2 azathioprine, 8 methotrexate, 5 cyclophosphamide, and 1 FK506. After the episodes of AP, oral medicines were stopped because of fasting. Methotrexate or cyclophosphamide were continuously prescribed in 5 patients but switched to I.V. injection. 1 patient developed recurrent episode of AP when increasing the dosage of GC for the relapse of SLE, and GC treatment was stopped (patient 2) after onset of AP. 25 patients were continuously treated by GCs and/or ISA during their episode of AP. 16 patients were given aggressive treatment of GCs and/or ISA (12 patients obtained clinical and laboratory improvement (75%) and 4 died), 5 patients were treated with the maintenance dose of GCs and/or ISA (2 patients in remission (40%) and 3 died), and 4 patients were treated with decreased dose of GCs because of fever and concerning of potential infections (2 patients in remission (50%) and 2 died) (The results showed in Figure 2). Totally, 10 patients died and the overall mortality rate was 37.04% (10/27).
Figure 2.
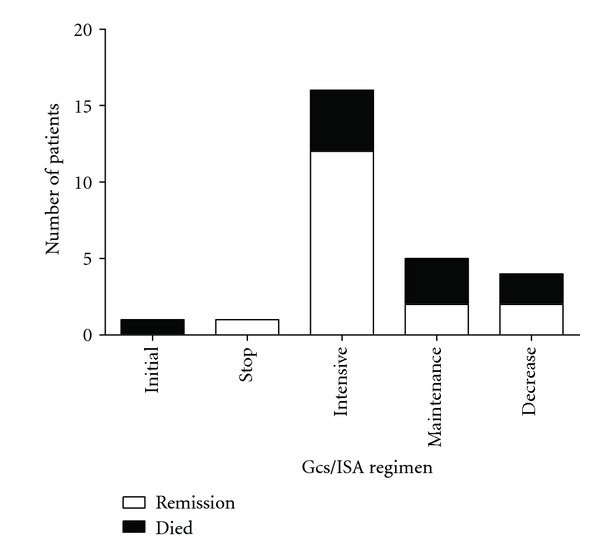
Treatment regimen and outcome of the SLE-related AP.
5. Discussion
SLE-related AP is relatively rare compared to other organ injury involved in lupus. The incidence of clinical AP associated with SLE varies from 0.7 to 4% [5, 8, 12, 22], with the annual incidence of 0.4–1.1‰ [3, 4]. Most previous studies on this issue were individual case reports or small case series. So far, the Hopkins lupus cohort [12] reported the largest case series with 63 SLE-attribute pancreatitis out of 1740 SLE patients (3.5%), and a Taiwan series reported 40 out of 2976 SLE patients (1.34%). This study was the first report of the SLE-related AP in south China. In current cohort, 27 out of 4053 SLE patients were diagnosed as SLE-related AP, with the prevalence of 0.67%, and annual incidence of 0.56‰, which is comparable with the findings of previous literatures [3–5, 8, 12, 22].
The pathogenic mechanism of SLE-related AP is very complex and multifactors. Vascular damage (including vasculitis, intimal thickening, immune complex deposition, occlusion of arteries, and arterioles), autoantibody production, abnormal cellular immune response, and drug toxicity may be responsible for the development of pancreatitis [8]. In the current cohort, more than half patients (51.85%) developed acute pancreatitis within 1 year of the onset of SLE, and all 27 patients were active SLE with dramatically elevated SLEDAI scores and other simultaneous SLE manifestations, especially the hematologic and renal involvement. SLE patients with AP presented with higher SLEDAI scores compared to patients without AP. Previous studies [3, 4, 22, 23] also demonstrated that episodes of SLE-related pancreatitis significantly increased in the active SLE group. AP was considered as one of the clinical features of active SLE and was associated with the activity of the disease itself. These results indicated that SLE itself can be the primary etiologic factor or cofactor predisposing to AP.
SLICC/ACR damage index score represents disease burden in SLE patients. It was significantly higher in SLE patients with pancreatitis compared to SLE patients without pancreatitis in Hopkins cohort [12]. Although SLE-related AP had more organ system involvement in current study, the damage index score was low, and there was no significant difference between SLE patients with and without AP (1.19 ± 0.92 versus 0.96 ± 1.19, P = 0.11). The reason of the low-damage index score might lie in the relatively younger onset age, shorter duration of disease, and less-chronic organ damage.
Our study found that pediatric-onset AP tended to be more severe compared to adult-onset AP. SAP group had significant higher prevalence of thrombocytopenia and leucopenia than MAP group. Mortality patients has higher rate of hypoalbuminemia, hematuria, granular casts, and hyperbilirubinemia than nonmortality group, which indicated that multiple organ systems involvement, especially hematological, renal, and liver injury in SLE patients might be the major causes due to the severity and mortality of AP. In general population, the mortality rate of AP is about 3.8% ~ 10% [24–27]. Approximately 15~20% of all AP cases were SAP which accounted for a mortality rate of 16.3% ~ 30% [27–29]. SLE-related AP patients had much higher mortality. Wang et al. [23] reported that the mortality rate was 27.5% in all SLE-related AP and 78.57% in SAP. Richer et al. [30] reported that 57% of childhood-onset lupus with pancreatitis developed SAP with the mortality of 45%. In our cohort, the overall mortality rate of SLE-related AP was 37.04% compared to 0 in SLE patients without AP (P = 0.001), and mortality rate in SAP was 75%. The severity of AP might be the most important risk factor for the mortality of SLE-related AP patients (OR 11.25, 95% CI (1.611, 78.57), and P = 0.014).
In accordance with other literatures, the manifestations of SLE-related AP in this cohort were nonspecific and similar to non-SLE acute pancreatitis. Abdominal pain (92.59%), fever (77.78%), and nausea/vomiting (74.07%) were the most common symptoms. These symptoms could also be attributed to other gastrointestinal diseases or adverse reactions of medication and may lead to misdiagnosis in general practice. It was reported that the rate of misdiagnosis of AP in SLE was up to 88.6% [31]. Delayed diagnosis and improper treatment may contribute to unfavorable prognosis, even lifethreatening [32]. Likewise, the mortality rate of the Hopkins Lupus Cohort (3%) was considerably lower than average of other reported studies due to close monitoring, early diagnosis, and treatment [12]. So, AP should be paid more attention in any SLE patient with abdominal pain when mechanical obstruction or toxic-metabolic etiologies, infection, or trauma were ruled out.
Some immunosuppressants, such as corticosteroids, azathioprine, and cyclosporine have been implicated to cause pancreatitis in several case reports. Only 2 patients in our study took azathioprine but the medication was discontinued after the onset of AP. The current study couldn't verify the relationship between azathioprine and acute pancreatitis in SLE patients. There is still a controversy over steroid treatment in SLE-related AP. Increasingly accumulated evidence showed that steroids do not trigger acute pancreatitis or cause increased mortality on AP [22, 33, 34], but instead, they have a possible therapeutic effect on SLE-related pancreatitis [5, 35–37]. In Hopkins cohort, appropriate treatment with corticosteroids added a survival benefit in SLE-related AP. In current study, 16 SLE-related AP patients received intensive GC and/or ISA treatment, and 75% of them exhibited favorable prognosis.
In summary, SLE-related acute pancreatitis is rare but with high-mortality rate, which is even higher in those severe acute pancreatitis with multiple organ system involvement. Activity of SLE, hematological system, renal, and liver injury in SLE patients may attribute to the mortality of AP. Early diagnosis of acute pancreatitis in SLE patients, especially those with abdominal pain, and appropriate glucocorticosteroid treatment is beneficial for a better therapeutic outcome in the majority of patients.
Acknowledgments
This work was supported by the Guangdong Provincial Science and Technology Funds, China (2009B030801098, 2011B050300009, 2008B080703015).
Abbreviations
- AP:
Acute pancreatitis
- SAP:
Severe acute pancreatitis
- MAP:
Mild acute pancreatitis
- GC:
Glucocorticosteroid
- ISA:
Immunosuppressive agents.
References
- 1.Xu D, Yang H, Lai CC, et al. Clinical analysis of systemic lupus erythematosus with gastrointestinal manifestations. Lupus. 2010;19(7):866–869. doi: 10.1177/0961203310365883. [DOI] [PubMed] [Google Scholar]
- 2.Myung DS, Kim TJ, Lee SJ, et al. Lupus-associated pancreatitis complicated by pancreatic pseudocyst and central nervous system vasculitis. Lupus. 2009;18(1):74–77. doi: 10.1177/0961203308093462. [DOI] [PubMed] [Google Scholar]
- 3.Breuer GS, Baer A, Dahan D, Nesher G. Lupus-associated pancreatitis. Autoimmunity Reviews. 2006;5(5):314–318. doi: 10.1016/j.autrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Nesher G, Breuer GS, Temprano K, et al. Lupus-associated pancreatitis. Seminars in Arthritis and Rheumatism. 2006;35(4):260–267. doi: 10.1016/j.semarthrit.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Derk CT, DeHoratius RJ. Systemic lupus erythematosus and acute pancreatitis: a case series. Clinical Rheumatology. 2004;23(2):147–151. doi: 10.1007/s10067-003-0793-3. [DOI] [PubMed] [Google Scholar]
- 6.Essaadouni L, Samar E, Krati K. Pancreatitis as initial manifestation of systemic lupus erythematosus. Lupus. 2010;19(7):884–887. doi: 10.1177/0961203309356456. [DOI] [PubMed] [Google Scholar]
- 7.Firestein GS, Budd RC, Harris ED, Jr, McInnes IB, Ruddy S, Sergent JS. Kelley’s Textbook of Rheumatology. 8th edition. Philadelphia, Pa, USA: W. B. Saunders Company; 2008. [Google Scholar]
- 8.Noia JL, García FM, Ríos SS, Iglesias García J, Domínguez Muñoz JE. Pancreatitis and systemic lupus erythematosus. Revista Espanola de Enfermedades Digestivas. 2009;101(8):571–579. doi: 10.4321/s1130-01082009000800009. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Wang NS, Zhao BH, Tang LQ. Acute pancreatitis as an initial symptom of systemic lupus erythematosus: a case report and review of the literature. World Journal of Gastroenterology. 2005;11(30):4766–4768. doi: 10.3748/wjg.v11.i30.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hakeem MS, McMillen MA. Evaluation of abdominal pain in systemic lupus erythematosus. American Journal of Surgery. 1998;176(3):291–294. doi: 10.1016/s0002-9610(98)00155-x. [DOI] [PubMed] [Google Scholar]
- 11.Huang JL, Huang CC, Chen CY, Hung IJ. Acute pancreatitis: an early manifestation of systemic lupus erythematosus. Pediatric Emergency Care. 1994;10(5):291–293. [PubMed] [Google Scholar]
- 12.Makol A, Petri M. Pancreatitis in systemic lupus erythematosus: frequency and associated factors-a review of the Hopkins Lupus Cohort. Journal of Rheumatology. 2010;37(2):341–345. doi: 10.3899/jrheum.090829. [DOI] [PubMed] [Google Scholar]
- 13.Forabosco P, Gorman JD, Cleveland C, et al. Meta-analysis of genome-wide linkage studies of systemic lupus erythematosus. Genes and Immunity. 2006;7(7):609–614. doi: 10.1038/sj.gene.6364338. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism. 1997;40(9):p. 1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Ranson JHC, Shamamian P. Diagnostic standards for acute pancreatitis. World Journal of Surgery. 1997;21(2):136–142. doi: 10.1007/s002689900205. [DOI] [PubMed] [Google Scholar]
- 16.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis & Rheumatism. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 17.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis and Rheumatism. 1996;39(3):363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 18.Bradley EL, Frey CF. A clinically based classification system for acute pancreatitis: summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Archives of Surgery. 1993;128(5):586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 19.Silverstein W, Isikoff MB, Hill MC, Barkin J. Diagnostic imaging of acute pancreatitis: prospective study using CT and sonography. American Journal of Roentgenology. 1981;137(3):497–502. doi: 10.2214/ajr.137.3.497. [DOI] [PubMed] [Google Scholar]
- 20.Sarti DA, King W. The ultrasonic findings in inflammatory pancreatic disease. Seminars in Ultrasound. 1980;1(3):178–191. [Google Scholar]
- 21.Whitcomb D. Acute pancreatitis. The New England Journal of Medicine. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Ramos V, Duarte-Rojo A, Villa AR, et al. Systemic lupus erythematosus as a cause and prognostic factor of acute pancreatitis. Journal of Rheumatology. 2004;31(4):707–712. [PubMed] [Google Scholar]
- 23.Wang CH, Yao TC, Huang YL, Ou LS, Yeh KW, Huang JL. Acute pancreatitis in pediatric and adult-onset systemic lupus erythematosus: a comparison and review of the literature. Lupus. 2011;20(5):443–452. doi: 10.1177/0961203310387179. [DOI] [PubMed] [Google Scholar]
- 24.Fagenholz PJ, Castillo CFD, Harris NS, Pelletier AJ, Camargo CA., Jr Increasing United States hospital admissions for acute pancreatitis, 1988–2003. Annals of Epidemiology. 2007;17(7):491–e1. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Cavallini G, Frulloni L, Bassi C, et al. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Digestive and Liver Disease. 2004;36(3):205–211. doi: 10.1016/j.dld.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Mann DV, Hershman MJ, Hittinger R, Glazer G. Multicentre audit of death from acute pancreatitis. British Journal of Surgery. 1994;81(6):890–893. doi: 10.1002/bjs.1800810632. [DOI] [PubMed] [Google Scholar]
- 27.Fu CY, Yeh CN, Hsu JT, Jan YY, Hwang TL. Timing of mortality in severe acute pancreatitis: experience from 643 patients. World Journal of Gastroenterology. 2007;13(13):1966–1969. doi: 10.3748/wjg.v13.i13.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron TH, Morgan DE. Acute necrotizing pancreatitis. New England Journal of Medicine. 1999;340(18):1412–1417. doi: 10.1056/NEJM199905063401807. [DOI] [PubMed] [Google Scholar]
- 29.Isenmann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. British Journal of Surgery. 1999;86(8):1020–1024. doi: 10.1046/j.1365-2168.1999.01176.x. [DOI] [PubMed] [Google Scholar]
- 30.Richer O, Ulinski T, Lemelle I, et al. Abdominal manifestations in childhood-onset systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2007;66(2):174–178. doi: 10.1136/ard.2005.050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Zeng XJ, Dong Y, et al. Digestive system involvement in systemic lupus erythematosus. Chinese Journal of Digestive Diseases. 1999;19(1):42–44. [Google Scholar]
- 32.Nwaneri UR, Callender CO, Stevens JE. Lupus pancreatitis. Journal of the National Medical Association. 1995;87(8):575–576. [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds JC, Inman RD, Kimberly RP. Acute pancreatitis in systemic lupus erythematosus: report of twenty cases and a review of the literature. Medicine. 1982;61(1):25–32. doi: 10.1097/00005792-198201000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg WM, Lewis JH. Steroid-induced pancreatitis: does it really exist? Gastroenterology. 1981;81(4):799–808. [PubMed] [Google Scholar]
- 35.Saab S, Corr MP, Weisman MH. Corticosteroids and systemic lupus erythematosus pancreatitis: a case series. Journal of Rheumatology. 1998;25(4):801–806. [PubMed] [Google Scholar]
- 36.Kapoor D, Mendez E, Espinoza LR. Corticosteroids and SLE pancreatitis. Journal of Rheumatology. 1999;26(4):1011–1012. [PubMed] [Google Scholar]
- 37.Petri M. Pancreatitis in systemic lupus erythematosus: still in search of a mechanism. Journal of Rheumatology. 1992;19(7):1014–1016. [PubMed] [Google Scholar]


