Abstract
Our understanding of the molecular and pathophysiological mechanisms underlying the disease process in patients with β-thalassemia intermedia has substantially increased over the past decade. Earlier studies observed that patients with β-thalassemia intermedia experience a clinical-complications profile that is different from that in patients with β-thalassemia major. In this article, a variety of clinical morbidities are explored, and their associations with the underlying disease pathophysiology and risk factors are examined. These involve several organs and organ systems including the vasculature, heart, liver, endocrine glands, bone, and the extramedullary hematopoietic system. The effects of some therapeutic interventions on the development of clinical complications are also discussed.
Although less severe than β-thalassemia major, β-thalassemia intermedia seriously impacts almost every organ system. Complications are often due to primary iron overload and hypercoagulability.
Distinction between the various phenotypes of β-thalassemia relies primarily on the clinical severity of the disease, which should be assessed both at initial presentation and over a period of close follow-up (Rund and Rachmilewitz 2005). The term “β-thalassemia intermedia” (TI) was first suggested to describe patients who had clinical manifestations that are too severe to be termed “β-thalassemia minor” yet too mild to be termed “β-thalassemia major” (TM) (Sturgeon et al. 1955). Patients with TI usually present to medical attention in later childhood or even adulthood. They show mild to moderate anemia and a hemoglobin level ranging between 7 and 10 g/dL, which is sustainable without the need for regular transfusion therapy (Camaschella and Cappellini 1995). Unfortunately, many patients with TI are set on a life of unnecessary regular transfusions, that is, similar to patients with TM, particularly if they present during a period of intercurrent infection requiring a few transfusions. It is essential to evaluate the patient carefully over the first few months after the genetic diagnosis of β-thalassemia is established and not to embark on any treatment modality, especially regular transfusion therapy, too hastily. The patient’s well-being, particularly with respect to activity, growth, development, and the early appearance of skeletal changes or other morbidities, is the factor to be taken into consideration before the phenotype is clearly established and the treatment modality is selected (Steinberg et al. 2009; Taher et al. 2011).
In this article, we provide an overview of novel insights regarding the variety of clinical morbidities experienced by TI patients and examine their associations with the underlying disease pathophysiology and risk factors. We primarily focus on complications due to primary iron overload and the hypercoagulable state of TI, while also highlighting pertinent aspects of other clinical complications.
MOLECULAR UNDERSTANDING AT A GLANCE
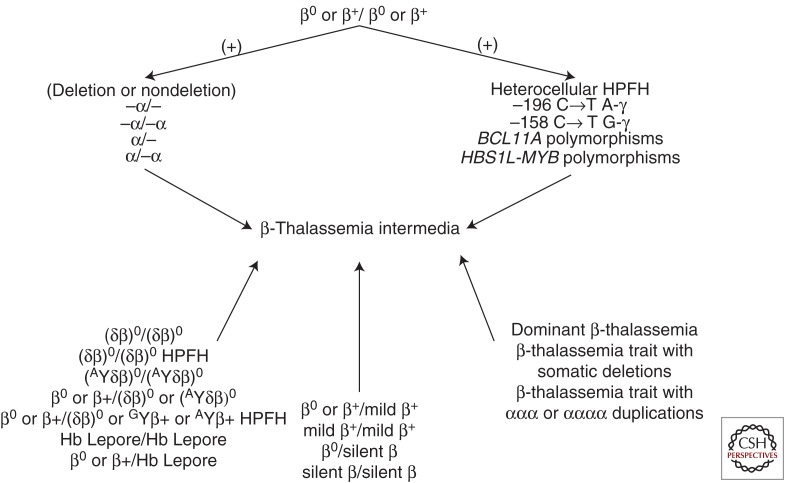
Although the term TI lacks specific molecular correlates and the diagnosis remains largely clinical, a genotype/phenotype association has been described (Galanello and Cao 1998). Most TI patients are homozygotes or compound heterozygotes for β-thalassemia, meaning that both β-globin loci are affected and the disease has a recessive genetic pattern (Galanello and Cao 1998). Less commonly, only a single β-globin locus is affected, the other being completely normal; thus, in these instances, TI is dominantly inherited (Weatherall and Clegg 2001). The phenotype of TI may also result from the increased production of α-globin chains by a triplicated or quadruplicated α-genotype associated with β-heterozygosity (Steinberg et al. 2009). In TI, the genetic basis for phenotypic diversity is best explained in terms of primary, secondary, and tertiary genetic modifiers (Weatherall 2001). The primary modifiers represent the broad diversity of mutations that affect the β-globin genes, ranging from mild promoter mutations that cause a slight reduction in β-globin chain production to the many different mutations that result in the β0-thalassemias, that is, a complete absence of β-globin product (see Thein 2012). Compound heterozygosity for these different mutations can provide a broad spectrum of clinical phenotypes. The secondary genetic modifiers are those that are involved directly in modifying the degree of globin-chain imbalance in β-thalassemia. The coinheritance of α-thalassemia has this effect, and, because there are numerous different molecular forms of α-thalassemia of different severity, this interaction provides further scope for a wide range of different β-thalassemia phenotypes. Similarly, the degree of globin chain imbalance can be reduced by the more effective synthesis of the γ-chains of fetal hemoglobin after birth. There are several genes involved in modifying the γ-chain response, some that are encoded in the β-globin gene cluster, others that are on different chromosomes (Fig. 1) (Sankaran et al. 2010). The tertiary modifiers are those that are not related to globin chain production but that may have an important effect on the complications of the disease (Weatherall 2004).
Figure 1.
Main genetic profiles that could lead to the β-thalassemia intermedia phenotype. Hb, hemoglobin; HPFH, hereditary persistence of fetal hemoglobin.
PATHOPHYSIOLOGY AND CLINICAL MORBIDITY
Current evidence highlights that transfusion independence in TI does not come without its own side effects. Although TI patients sustain levels of anemia that are generally adequate for growth and development without blood transfusions, several other pathogenic mechanisms remain in play. Ineffective erythropoiesis and a secondary drive for increased intestinal iron absorption, extramedullary hematopoiesis (EMH), intra- and extravascular hemolysis, and hypercoagulability have all been described (Taher et al. 2006a). Knowledge of the various clinical morbidities that could emanate from these underlying mechanisms continues to expand, and it is now apparent that TI patients experience a spectrum of morbidities that remain different from those commonly observed in TM (Taher et al. 2006a).
Iron Overload and Target Organ Toxicity
Current models for iron metabolism in TI suggest that the combination of ineffective erythropoiesis, anemia, and hypoxia leads to a compensatory increase in serum levels of erythropoietin, as well as a decrease in serum levels of hepcidin, which control the concentration of ferroportin on the intestinal epithelium (Weizer-Stern et al. 2006; Gardenghi et al. 2010; Melchiori et al. 2010; Tanno and Miller 2010; Taher et al. 2011). Three proposed regulators of hepcidin production are growth differentiation factor 15, secreted by erythroid precursors, twisted gastrulation factor 1, and hypoxia-inducible transcription factors (Tanno et al. 2007; Taher et al. 2009a). Regardless of the signaling mechanism, the end result is suppression of hepcidin levels, increased intestinal iron absorption, and increased release of recycled iron from macrophages within the reticuloendothelial system. This, in turn, leads to relatively low levels of serum ferritin and preferential portal and hepatocyte iron loading (Origa et al. 2007; Taher et al. 2008a, 2009a). Although iron accumulation in patients with TI occurs more slowly than in patients with TM who are regularly transfused, it can reach levels much higher than normal thresholds, especially as patients advance in age (Taher et al. 2008a, 2010a). Considerably elevated liver iron concentration (LIC) and high levels of circulating toxic iron species like non-transferrin-bound iron (NTBI) have been documented in transfusion-independent patients with TI (Pakbaz et al. 2007; Taher et al. 2008a, 2009b).
Iron overload has important clinical consequences in patients with TI. Because iron accumulation primarily occurs in hepatocytes, it can predispose patients to liver fibrosis and cirrhosis, and potentially, hepatocellular carcinoma (Borgna-Pignatti et al. 2004; Taher et al. 2009a; Mancuso 2010; Restivo Pantalone et al. 2010). An association between elevated LIC and the occurrence of endocrinopathy, bone disease, and vascular morbidity has also been reported in TI (Musallam et al. 2011b). In a study of 168 patients with TI and a mean LIC of 8.4 mg Fe/g dry weight (dw), a 1-mg increase in LIC was independently associated with a significantly increased risk of developing thrombosis, pulmonary hypertension (PHT), hypothyroidism, hypogonadism, and osteoporosis. The level associated with a significantly increased risk of developing vascular morbidity was ≥7 mg Fe/g dw, and the level for endocrine and bone morbidity was ≥6 mg Fe/g dw. These observations were made in both transfusion-naive patients and in patients who received previous transfusions. Moreover, it was apparent that elevated LIC was associated with a steeper increase in the rate of age-related vascular morbidity and earlier onset of endocrine and bone disease compared with patients with low LIC (Musallam et al. 2011b). Recent studies, to be further illustrated in the subsequent section, have also documented a high incidence of silent brain infarction, large cerebral vessel disease, and decreased neuronal function, primarily in the temporal and parietal lobes, among splenectomized patients with TI (Karimi et al. 2010a; Taher et al. 2010d; Musallam et al. 2011a, 2012a). Large-vessel cerebrovascular disease significantly correlated with higher NTBI levels (Musallam et al. 2011a), and decreased neuronal function was observed more frequently in patients with elevated LIC (>15 mg Fe/g dw) (Musallam et al. 2012a).
How iron overload contributes to vascular morbidity in TI remains unclear. Iron-derived reactive oxygen species are implicated in the pathogenesis of several vascular disorders including atherosclerosis, microangiopathic hemolytic anemia, vasculitis, and reperfusion injury (Balla et al. 2003; Malyutin et al. 2012). It has been shown that the presence of NTBI in serum can cause oxidative vessel injury (Auer et al. 2002). Free radicals act directly on the endothelial cells and have a close interaction with lipid peroxidation, causing a modification of low-density lipoprotein and facilitating its deposition, with the consequent formation of atherosclerotic plaques (Aessopos et al. 2009). Thus, iron-mediated endothelial dysfunction and a secondary atherosclerotic process may explain arterial disease in TI patients and echo recent studies supporting the idea that TI patients show a pro-atherogenic biochemical phenotype (Hahalis et al. 2011; Lai et al. 2011). Moreover, iron overload may aggravate ineffective erythropoiesis and the secondary release into the circulation of damaged red blood cells (RBC) with thrombogenic potential, thus putting patients at risk of venous thrombosis (Borenstain-Ben Yashar et al. 1993; Gardenghi et al. 2010).
Several studies have reported absence of cardiac iron overload on T2* magnetic resonance imaging (MRI), even in TI patients who show significant hepatic iron loading (Mavrogeni et al. 2008; Origa et al. 2008; Roghi et al. 2010; Taher et al. 2010e); however, myocardial siderosis has been documented in small subgroups of older TI patients at autopsy (Lombardo et al. 1995; Voskaridou et al. 2004; Au et al. 2009). Moreover, damage to cardiac tissue may result from exposure to NTBI without accumulation of toxic iron species within myocytes (Glickstein et al. 2006; Taher et al. 2010e). This suggests that even without evidence of cardiac siderosis, TI patients may still be at risk for iron-related cardiac dysfunction.
Hypercoagulability and Thromboembolic Disease
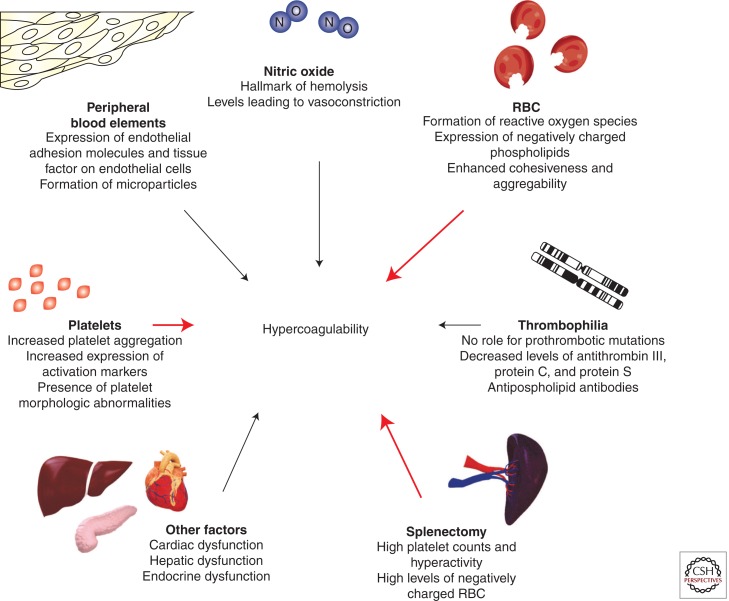
The hypercoagulable state in patients with TI has been attributed to several factors (Fig. 2) (Eldor and Rachmilewitz 2002; Cappellini et al. 2010) and can be present from childhood (Eldor et al. 1999). It is often a combination of these factors that leads to thromboembolic events (Ataga et al. 2007; Musallam and Taher 2011). Patients with TI have chronically activated platelets and enhanced platelet aggregation (Winichagoon et al. 1981), as confirmed by the increased expression of CD62P (P-selectin) and CD63, markers of in vivo platelet activation (Del Principe et al. 1993; Ruf et al. 1997). It has been shown that TI patients have four to 10 times higher metabolites of prostacyclin (PG I2) and thromboxane A2, both markers of hemostatic activity, than healthy individuals (Eldor et al. 1991). Splenectomized TI patients also have high platelet counts (Atichartakarn et al. 2003a; Cappellini et al. 2005), but with a shorter life span due to enhanced consumption (Eldor et al. 1989). A recent study showed that increased platelet adhesion is a common finding in splenectomized β-thalassemia patients, which is induced by mechanisms involving both platelets and RBC and could potentially predict clinical thrombosis (Goldschmidt et al. 2008).
Figure 2.
Factors contributing to the hypercoagulable state in β-thalassemia intermedia. RBC, red blood cells. (From Cappellini et al. 2010; reprinted, with permission.)
The role of the RBCs in the hypercoagulability of TI has received great attention. The oxidation of globin subunits in thalassemia erythroid cells leads to the formation of hemichromes (Rund and Rachmilewitz 2005), which precipitate instigating heme disintegration and the eventual release of toxic NTBI species (Hershko et al. 1978). The free iron, in turn, catalyzes the formation of reactive oxygen species, leading to oxidation of membrane proteins and formation of red cell senescence antigens like phosphatidylserine (Kuypers and de Jong 2004), which cause the thalassemic RBCs to become rigid, deformed, and to aggregate, resulting in premature cell removal (Tavazzi et al. 2001). Thalassemic RBCs with negatively charged phospholipids increase thrombin generation (Borenstain-Ben Yashar et al. 1993; Helley et al. 1996), as evidenced by studies using annexin V, a protein with high affinity and specificity for anionic phospholipids (Helley et al. 1996). Splenectomized patients have a substantially higher number of these negatively charged RBCs and, in turn, show higher thrombin generation (Cappellini et al. 2000; Atichartakarn et al. 2002). TI patients were also found to have higher levels of pro-coagulant microparticles of RBC, leukocytic, and endothelial origins compared with controls (Habib et al. 2008); the contribution of these fragments to thromboembolic events in TI is under investigation.
The presence of other peripheral blood elements in thalassemics such as E-selectin (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), von Willebrand factor (VWF), and vascular cell adhesion molecule-1 (VCAM-1) indicates that endothelial injury or activation may be an aspect of the disease, aiding in the recruitment of white blood cells and RBCs, and promoting thrombosis (Butthep et al. 1995, 1997). In fact, studies have shown that RBCs from TI patients show increased adhesion to cultured endothelial cells (Hovav et al. 1999). Inherited thrombophilia does not have a role in the hypercoagulable state of TI (Iolascon et al. 2001; Zalloua et al. 2003), but high levels of anti-phospholipid antibodies and low proteins C and S levels have been documented (Taher et al. 2008b). The presence of cardiac, hepatic, or endocrine dysfunction in older patients with severe iron overload may also contribute to hypercoagulability (Taher et al. 2008b).
Data on the incidence of thromboembolic events in TI are limited. In one study including nine Italian pediatric thalassemia centers, 4% of 683 patients with TM and 9.6% of 52 patients with TI had experienced a thromboembolic event (Borgna-Pignatti et al. 1998). In a cohort study including 83 splenectomized patients with TI followed for >10 yr, 29% of patients experienced a venous thromboembolic event (Cappellini et al. 2000). Conventional risk factors (described in the non-thalassemic population) for venous thrombosis were usually absent in such patients (Gillis et al. 1999), further highlighting the unique pathophysiology of hypercoagulability in TI. The largest study to date examined data from 8860 thalassemia patients in the Mediterranean area and Iran and observed that thromboembolic event occurred 4.38 times more frequently in TI than TM, with more venous events occurring in TI and more arterial events occurring in TM (Taher et al. 2006b). It was found that 14% of mortalities in the whole group were attributed to thromboembolic events. Age above 20 yr, splenectomy, and personal or family history of thromboembolic events were identified as the main risk factors for thrombosis in TI. Furthermore, the study showed that 68% of TI patients who had a thrombotic event had an average hemoglobin level of <9 g/dL, and only 33% were receiving regular blood transfusions, whereas 94% were splenectomized. Moreover, patients receiving aspirin therapy had a significantly lower rate of recurrent thromboembolic events (Taher et al. 2006b).
The OPTIMAL CARE (Overview on Practices in Thalassemia Intermedia Management Aiming for Lowering Complication Rates Across a Region of Endemicity) study evaluated 584 patients with TI at six comprehensive care centers (Lebanon, Italy, Iran, Egypt, United Arab Emirates, and Oman) for the associations between patient and disease characteristics, treatment received, and the rate of clinical complications (Taher et al. 2010c). Thromboembolic disease, mostly venous, was the fifth most common complication, affecting 14% of the patient population. Splenectomy was associated with a fivefold increased risk of thrombosis. Conversely, a positive history of transfusion and a hemoglobin level ≥9 g/dL were found to be protective against thrombosis (Taher et al. 2010c). A higher occurrence of thrombosis with advancing age was also observed (Taher et al. 2010a). A substudy of the OPTIMAL CARE determined that splenectomized TI patients who experience thrombosis are characterized by high nucleated RBC (≥300 × 106/L) and platelet counts (≥500 × 109/L) (Taher et al. 2010b), further confirming the dual role of platelets and RBCs in this setting (Goldschmidt et al. 2008). Moreover, they were more likely to have evidence of PHT and be transfusion-naive (Taher et al. 2010b). As such, it was suggested that splenectomized TI patients at risk of developing thrombosis may be identified early on by these laboratory markers, presence of PHT, and transfusion status (Taher et al. 2010b). The study further examined how long it took for a thrombosis to develop following splenectomy and found the median time to thrombosis to be 8 yr (Taher et al. 2010b). This delay indicates that thrombosis in splenectomized patients with TI is not an acute complication, but a manifestation of a chronic underlying process, further emphasizing the need for long-term treatment preventive strategies (Taher et al. 2010b).
The prevalence of overt strokes in TI patients with a history of thrombosis ranges between 5% and 9% (Taher et al. 2006b, 2010b; Karimi et al. 2008). Few case reports also describe a frequent occurrence of overt strokes in TI patients with moyamoya syndrome (Sanefuji et al. 2006; Marden et al. 2008; Goksel et al. 2010; Oberoi et al. 2010). However, a higher prevalence of silent strokes has been consistently documented. The earliest study was conducted in 1999 and showed a 37.5% rate of ischemic lesions on brain MRI in 16 patients with TI who were neurologically intact and had no conventional stroke-related risk factors (Manfre et al. 1999). More recently, a cross-sectional brain MRI study was conducted in Lebanon on 30 splenectomized adults with TI that were selected from a larger cohort of patients based on absence of neurological or gross cognitive signs or symptoms and any stroke-related risk factors. None of the patients were receiving anti-platelet or anticoagulant therapy. Eighteen patients (60%) had evidence of one or more ischemic lesions on brain MRI, all involving the subcortical white matter. Most patients had evidence of multiple lesions. The frontal subcortical white matter was nearly always involved, followed by the parietal and occipital subcortical white matter. The vast majority of patients (94%) had evidence of small to medium (<1.5 cm) lesions with only one patient showing evidence of a large lesion (>1.5 cm) (Taher et al. 2010d). It was noted that increasing age and transfusion naivety were both independently associated with a higher occurrence and multiplicity of lesions (Taher et al. 2010d). Around the same time, another cross-sectional study was conducted in Iran on 30 randomly selected TI adults who were splenectomized, had a hemoglobin level >7 g/dL, and a platelet count ≥500 × 109/L. The investigators noted eight patients (26.7%) with silent ischemic lesions (Karimi et al. 2010a). The variability in the observed frequency and multiplicity of silent stroke in the three studies could be primarily attributed to the strength of the magnetic field used (Tesla units). Although none of these studies included a control group, the incidence of silent strokes discovered incidentally on brain scans of healthy individuals of a similar age group (<50 yr) ranges from zero to a maximum of 11%, suggesting that the described changes are pathological rather than normal variations (Taher et al. 2010d).
Brain magnetic resonance angiography (MRA) and positron emission tomography-computed tomography (PET-CT) studies have also been recently conducted in TI. In one study including 29 asymptomatic, splenectomized adults, 27.6% had evidence of arterial stenosis on MRA. Two patients had more than one artery involved, and the internal carotid artery was the most commonly involved artery. Among the 12 identified stenotic lesions, two were severe (>75% stenosis), one was moderate (51%–75% stenosis), and the remaining nine were mild (≤50% stenosis). However, there was no association between large-vessel stenosis and silent strokes identified on MRI, leaving small arteriolar pathology as the most likely explanation for the latter, especially that cardiac emboli do not seem to play a role (Musallam et al. 2011a). Although PET-CT scanning was also not helpful in identifying silent strokes in patients with TI, it revealed that decreased neuronal function is a common finding (63.3%) in this patient population, that is, primarily left sided, multiple, and most commonly in the temporal and parietal lobes (Musallam et al. 2012a).
An important question is whether the observed silent brain abnormalities in TI are truly silent or do they require careful consideration and intervention. In the general population and in patients with sickle cell disease, silent strokes, arterial stenosis on MRA, and decreased neuronal function on PET-CT have all been associated with subsequent risk of overt stroke and neurocognitive decline (Taher et al. 2010d; Musallam et al. 2011a, 2012a).
Other Complications
Pulmonary Hypertension
Another complication of TI that was found to occur at a relatively high frequency, especially compared with patients with TM, is PHT (Aessopos et al. 2001, 2005, 2007; Taher et al. 2006a, 2010c; Amoozgar et al. 2011a). One limitation in most available studies, however, is the use of echocardiography instead of cardiac catheterization for the diagnosis of PHT, which may increase the rate of false-positive findings (Parent et al. 2011). Chronic anemia and hypoxia (Aessopos et al. 2001), iron overload (Isma’eel et al. 2008; Mokhtar et al. 2010; Karimi et al. 2011), splenectomy (Phrommintikul et al. 2006; Amoozgar et al. 2010; Karimi et al. 2011), hypercoagulability and microthrombotic disease of the pulmonary circulation (Singer et al. 2006; Karimi et al. 2011), and chronic hemolysis (Aessopos et al. 2007; Karimi et al. 2011) have all been implicated in the pathophysiology of PHT in TI. PHT is neither associated with myocardial siderosis (Roghi et al. 2010; Taher et al. 2010e) nor left ventricular dysfunction in TI, but is a leading cause of right-sided heart failure and thus warrants attention (Aessopos et al. 1995, 2005; Isma’eel et al. 2008). Sildenafil citrate, a potent inhibitor of cyclic guanosine monophosphate-specific phosphodiesterase-5 and a selective smooth muscle relaxant, showed promising results for the management of PHT in small studies in TI patients (Littera et al. 2002; Derchi et al. 2005). The drug is currently being evaluated in a large multicenter trial on patients with thalassemia including TI.
Extramedullary Hematopoietic Pseudotumors
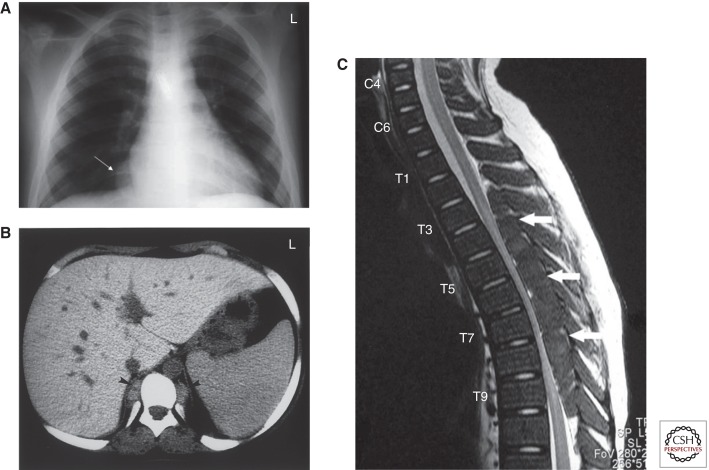
Ineffective red cell production by the bone marrow forces expansion of the hematopoietic tissue outside the marrow medulla and leads to hematopoietic compensatory involvement, mostly in the form of masses, in other regions in the body. This is called extramedullary hematopoiesis (EMH) (Taher et al. 2006a). Almost all body sites may be involved including the spleen, liver, lymph nodes, thymus, heart, breasts, prostate, broad ligaments, kidneys, adrenal glands, pleura, retroperitoneal tissue, skin, peripheral and cranial nerves, and the spinal canal. Among the various body regions reported, paraspinal involvement (11%–15% of cases) receives most attention because of the debilitating clinical consequences secondary to spinal cord compression (Fig. 3) (Haidar et al. 2010). Management options include blood transfusion therapy, which helps decrease the demand for EMH, radiotherapy of the pseudotumors, or fetal hemoglobin induction by hydroxcarbamide. Combinations of these modalities have also been used. There is no evidence as to the best treatment, and this remains individualized depending on the severity of symptoms, the size of the mass, clinical condition, and previous treatment (Chehal et al. 2003; Tai et al. 2006; Haidar et al. 2010). Surgery is not always possible because of the diffuse nature of the mass and the likelihood of recurrence. Furthermore, immediate total resection of extramedullary hematopoietic masses can lead to clinical decompensation and deterioration because these masses play a crucial role in maintaining an adequate hemoglobin level (Jackson et al. 1988).
Figure 3.
Paraspinal extramedullary hematopoietic pseudotumors. (A) Chest X-ray showing expanded anterior rib ends consistent with medullary hyperplasia. A paraspinal mass is seen in the right lower zone (white arrow). (B) Computed tomography scan showing inactive paraspinal extramedullary hematopoietic lesion with increased density compared with soft tissue, caused by iron deposition (black arrowheads). (C) Magnetic resonance image of cervical and thoracic spine. T2-weighted sagittal image showing thoracic cord compression by extramedullary intraspinal epidural hematopoietic mass from T2 to T10 (white arrows). (From Haidar et al. 2010; reprinted, with permission.)
Leg Ulcers
Leg ulcers are more common in older than in younger patients with TI. It is unclear why ulcers develop in some patients who are maintained at relatively low hemoglobin levels and have the same amount of fetal hemoglobin as others in whom ulcers do not develop. The skin at the extremities of elderly TI patients can be thin because of reduced tissue oxygenation making the subcutaneous tissue fragile and increasing the risk of ulceration after minimal trauma. These ulcers are often very painful and indolent, although blood transfusions may provide some relief (Taher et al. 2010c). Simple measures may be beneficial, such as keeping the patient’s legs and feet raised above the level of the heart for 1–2 h during the day or sleeping with the end of the bed raised. Pentoxifylline (Trental, Sanofi-Aventis), which alters the rheological properties of RBCs (Dettelbach and Aviado 1985), can accelerate the healing of ulcers. Hydroxycarbamide also has some benefit, either alone or in combination with erythropoietin (Eprex, Janssen-Cilag) (al-Momen 1991; Taher et al. 2010c). The use of an oxygen chamber can also provide moderate relief where tissue hypoxia may be an underlying cause of the ulceration (Gimmon et al. 1982).
Gallstones
Gallstones are more common in TI than in TM primarily because of increased hemolysis. Unrelated genetic factors such as uridine 5′-diphospho-α-d-glucose (UDPG) deficiency (Gilbert’s syndrome) have also been reported to increase gallstone formation in patients with thalassemia (Borgna-Pignatti et al. 2003). The gallbladder is always inspected during splenectomy, and cholecystectomy is performed, particularly if stones are considered symptomatic. This is undertaken to prevent cholecystitis, which can have serious consequences in the splenectomized patient.
Endocrine Disease and Pregnancy
Osteoporosis secondary to bone marrow expansion and 25-hydroxy vitamin D deficiency is highly prevalent in TI patients (Napoli et al. 2006; Taher et al. 2010c). Fractures and bone pain can be devastating consequences. Different regimens of vitamin D and calcium are frequently prescribed to patients with TI, but with careful monitoring of renal function (Borgna-Pignatti 2007; Taher et al. 2011). Although the efficacy and safety of bisphosphonates have been proven in patients with TM, data on patients with TI are limited (Voskaridou et al. 2008). Other endocrine complications can also occur in patients with TI secondary to anemia and iron overload (Taher et al. 2010c; Musallam et al. 2011b). Delayed puberty is common, but fertility is usually normal. In pregnant women with TI, experience reveals an increased risk of abortion, pre-term delivery, intrauterine growth restriction, Caesarean section delivery, and thromboembolic events (Nassar et al. 2008). Although the use of blood transfusions may be required to address these complications, the risk of alloimmunization in never-transfused women should always be taken into consideration. Splenomegaly can interfere with the enlargement of the uterus and can be complicated by hypersplenism. Splenectomy can therefore become necessary during gestation or after delivery. Anticoagulation should be considered especially in women with additional pro-thrombotic risk factors (Nassar et al. 2006).
GENERAL CONSIDERATIONS FOR MANAGEMENT
It is evident that without treatment, TI patients experience more frequent morbidity and poorer health-related quality of life (Taher et al. 2010a; Musallam et al. 2011c, 2011d). Currently, management of patients with TI is almost entirely based on clinical expertise and evidence derived from observational studies.
Transfusion Therapy
In patients with TI, the most challenging therapeutic decisions are whether and when to initiate transfusion therapy (Borgna-Pignatti 2007; Taher et al. 2011). Many patients require intermittent RBC transfusions because of intercurrent infection or pregnancy. Even if few transfusions have been administered in the acute situation, immediate commitment to a transfusion program should not be undertaken before the patient is observed in the non-emergency setting. Regular transfusion therapy is often indicated for growth failure, skeletal deformity, exercise intolerance, or when hemoglobin levels decline because of progressive splenomegaly (Borgna-Pignatti 2007; Borgna-Pignatti et al. 2010; Taher et al. 2011). Moreover, observational studies continue to confirm that transfused patients with TI experience fewer thromboembolic events, PHT, and silent brain infarcts compared with transfusion-naive patients (Taher et al. 2006b, 2010c,d, 2011; Aessopos et al. 2007; Karimi et al. 2011), which may be attributed to correction of the underlying ineffective erythropoiesis and resulting damaged RBCs with thrombogenic potential (Chen et al. 1996).
Iron Chelation
Iron overload in patients with TI can occur as a result of both increased intestinal absorption and transfusion therapy, but regardless of the source, iron overload can be monitored and chelation therapy initiated. Although iron chelator trials in TI patients are limited, the benefits of iron chelation therapy have been reported (Taher et al. 2010c). The initiation of chelation therapy in patients with TI depends primarily on the extent of iron overload and rate of accumulation, but, as with other aspects of the management of TI, clear disease-specific guidelines are not available. Patients with TI may have a positive iron balance by 5 yr of age (Cossu et al. 1981). The main challenge in TI patients is monitoring iron levels because serum ferritin measurement may underestimate the extent of iron overload (Origa et al. 2007). Studies have shown that for the same LIC, patients with TI show considerably lower serum ferritin levels than patients with TM, which is primarily attributed to shunting of iron from the reticuloendothelial system to the hepatocytes during primary iron overload (Taher et al. 2008a). Therefore, direct assessment of LIC either by biopsy or imaging every 1–2 yr is recommended, and chelation therapy should be initiated in patients with elevated indices of iron overload (Taher et al. 2009a, 2011).
Based on evidence that LIC levels of 6–7 mg Fe/g dw are associated with an increased occurrence of morbidity, decreasing LIC levels to ≤5 mg Fe/g dw is suggested (Musallam et al. 2011b). When LIC is reduced to desirable levels, low-dose chelation therapy may be of value to prevent further iron loading. If LIC measurement is not possible, a serum ferritin level of 400–500 µg/L is a reasonable alternative threshold for chelation therapy in this patient population. In most cases, intermittent iron chelation with careful periodic assessment is sufficient in patients with TI. The available chelators have been evaluated in small studies in TI patients (Cossu et al. 1981; Pippard and Weatherall 1988; Olivieri et al. 1992; Ladis et al. 2010; Voskaridou et al. 2010) and showed both efficacy and safety, although results from larger clinical trials are awaited (see Brittenham and Olivieri 2012).
Splenectomy
The role of splenectomy in the management of TI is complex. Clinical observations suggest that splenectomy in patients with TI can contribute to an increased susceptibility to venous thrombosis (Cappellini et al. 2000; Taher et al. 2006b), PHT (Atichartakarn et al. 2003b; Aessopos and Farmakis 2005; Karimi et al. 2011), silent brain infarcts (Karimi et al. 2010a; Taher et al. 2010d), and other complications (Taher et al. 2010c). Based on these considerations, a guarded approach to splenectomy is advised, and the procedure should be delayed unless considered vitally necessary, as in cases with poor growth and development or increased transfusion demand when chelation therapy is not possible, hypersplenism, or symptomatic splenomegaly (Taher et al. 2011).
Fetal Hemoglobin Induction
The clinical picture of TI could be greatly improved by an even partial reduction in the degree of the non-α to α-globin chain imbalance through reactivation of the γ-chain synthesis. Clinical experience suggests that induction of fetal hemoglobin production using hydroxycarbamide is associated with increases in total hemoglobin levels of ∼1 g/dL, a decrease in transfusion demand, and amelioration of certain disease complications like PHT, although a decrease in efficacy on long-term therapy is noted (Panigrahi et al. 2005; Karimi et al. 2010b,c, 2011; Rigano et al. 2010; Taher et al. 2010c; Amoozgar et al. 2011b). Treatment with hydroxycarbamide may also reduce the incidence of thrombotic events including silent strokes (M Karimi, S Haghpanah, MH Bagheri, et al., unpubl.). Hydroxycarbamide decreases plasma markers of thrombin generation and coagulation activation by reducing phospholipid expression on the surface of both RBCs and platelets and decreases RBC adhesion to thrombospondin. In addition to being a nitric oxide donor, hydroxycarbamide may also decrease hemostatic activation by its effect in decreasing the white blood cell count and particularly monocytes that express tissue factor (Ataga et al. 2007). Experience with other fetal hemoglobin-inducing agents for the management of TI is limited (Borgna-Pignatti 2007); however, the concept deserves further study, in light of the observation that patients with elevated fetal hemoglobin levels experience fewer morbidities including thrombosis (Musallam et al. 2012b).
Anticoagulation
The roles of anticoagulant or anti-platelet therapy for the prevention of vascular disease in TI patients have not been formally evaluated (Musallam and Taher 2011), although already on the basis of the available data (Karimi et al. 2010a, 2011; Taher et al. 2010b), there is a rationale to give aspirin to splenectomized patients with platelets >500 × 109/L and Coumadin to patients who experienced a thromboembolic event.
CONCLUDING REMARKS
The TI phenotype carries greater morbidity than previously recognized. Our knowledge of the various clinical morbidities that TI patients endure has substantially increased over the past decade. Findings confirm that TI should no longer be regarded as a mild form of thalassemia because patients experience serious manifestations involving almost every organ system. Studies that expand our understanding of the mechanisms and risk factors of disease, as well as clinical trials evaluating the roles of available treatments, will help establish management guidelines that transform patient care into optimal standards.
Footnotes
Editors: David Weatherall, Alan N. Schechter, and David G. Nathan
Additional Perspectives on Hemoglobin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Aessopos A, Farmakis D 2005. Pulmonary hypertension in β-thalassemia. Ann NY Acad Sci 1054: 342–349 [DOI] [PubMed] [Google Scholar]
- Aessopos A, Stamatelos G, Skoumas V, Vassilopoulos G, Mantzourani M, Loukopoulos D 1995. Pulmonary hypertension and right heart failure in patients with β-thalassemia intermedia. Chest 107: 50–53 [DOI] [PubMed] [Google Scholar]
- Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, Joussef J, Rombos J, Loukopoulos D 2001. Cardiac involvement in thalassemia intermedia: A multicenter study. Blood 97: 3411–3416 [DOI] [PubMed] [Google Scholar]
- Aessopos A, Farmakis D, Deftereos S, Tsironi M, Tassiopoulos S, Moyssakis I, Karagiorga M 2005. Thalassemia heart disease: A comparative evaluation of thalassemia major and thalassemia intermedia. Chest 127: 1523–1530 [DOI] [PubMed] [Google Scholar]
- Aessopos A, Kati M, Farmakis D 2007. Heart disease in thalassemia intermedia: A review of the underlying pathophysiology. Haematologica 92: 658–665 [DOI] [PubMed] [Google Scholar]
- Aessopos A, Tsironi M, Andreopoulos A, Farmakis D 2009. Heart disease in thalassemia intermedia. Hemoglobin 33: S170–S176 [DOI] [PubMed] [Google Scholar]
- al-Momen AK 1991. Recombinant human erythropoietin induced rapid healing of a chronic leg ulcer in a patient with sickle cell disease. Acta Haematol 86: 46–48 [DOI] [PubMed] [Google Scholar]
- Amoozgar H, Farhani N, Karimi M 2010. Risk factors for pulmonary hypertension in patients with thalassemia intermedia. Eur J Haematol 85: 549–551 [DOI] [PubMed] [Google Scholar]
- Amoozgar H, Farhani N, Karimi M 2011a. Early echocardiographic findings in β-thalassemia intermedia patients using standard and tissue Doppler methods. Pediatr Cardiol 32: 154–159 [DOI] [PubMed] [Google Scholar]
- Amoozgar H, Farhani N, Khodadadi N, Karimi M, Cheriki S 2011b. Comparative study of pulmonary circulation and myocardial function in patients with β-thalassemia intermedia with and without hydroxyurea, a case-control study. Eur J Haematol 87: 61–67 [DOI] [PubMed] [Google Scholar]
- Ataga KI, Cappellini MD, Rachmilewitz EA 2007. β-Thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. Br J Haematol 139: 3–13 [DOI] [PubMed] [Google Scholar]
- Atichartakarn V, Angchaisuksiri P, Aryurachai K, Onpun S, Chuncharunee S, Thakkinstian A, Atamasirikul K 2002. Relationship between hypercoagulable state and erythrocyte phosphatidylserine exposure in splenectomized haemoglobin E/β-thalassaemic patients. Br J Haematol 118: 893–898 [DOI] [PubMed] [Google Scholar]
- Atichartakarn V, Angchaisuksiri P, Aryurachai K, Chuncharunee S, Thakkinstian A 2003a. In vivo platelet activation and hyperaggregation in hemoglobin E/β-thalassemia: A consequence of splenectomy. Int J Hematol 77: 299–303 [DOI] [PubMed] [Google Scholar]
- Atichartakarn V, Likittanasombat K, Chuncharunee S, Chandanamattha P, Worapongpaiboon S, Angchaisuksiri P, Aryurachai K 2003b. Pulmonary arterial hypertension in previously splenectomized patients with β-thalassemic disorders. Int J Hematol 78: 139–145 [DOI] [PubMed] [Google Scholar]
- Au WY, Lam WW, Chu WW, Tam S, Wong WK, Lau J, Yeung YM, Liu HS, Liang R 2009. Organ-specific hemosiderosis and functional correlation in Chinese patients with thalassemia intermedia and hemoglobin H disease. Ann Hematol 88: 947–950 [DOI] [PubMed] [Google Scholar]
- Auer JW, Berent R, Weber T, Eber B 2002. Iron metabolism and development of atherosclerosis. Circulation 106: e7. [DOI] [PubMed] [Google Scholar]
- Balla J, Vercellotti GM, Nath K, Yachie A, Nagy E, Eaton JW, Balla G 2003. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transplant 18: v8–v12 [DOI] [PubMed] [Google Scholar]
- Borenstain-Ben Yashar V, Barenholz Y, Hy-Am E, Rachmilewitz EA, Eldor A 1993. Phosphatidylserine in the outer leaflet of red blood cells from β-thalassemia patients may explain the chronic hypercoagulable state and thrombotic episodes. Am J Hematol 44: 63–65 [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C 2007. Modern treatment of thalassaemia intermedia. Br J Haematol 138: 291–304 [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Carnelli V, Caruso V, Dore F, De Mattia D, Di Palma A, Di Gregorio F, Romeo MA, Longhi R, Mangiagli A, et al. 1998. Thromboembolic events in β thalassemia major: An Italian multicenter study. Acta Haematol 99: 76–79 [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Rigon F, Merlo L, Chakrok R, Micciolo R, Perseu L, Galanello R 2003. Thalassemia minor, the Gilbert mutation, and the risk of gallstones. Haematologica 88: 1106–1109 [PubMed] [Google Scholar]
- Borgna-Pignatti C, Vergine G, Lombardo T, Cappellini MD, Cianciulli P, Maggio A, Renda D, Lai ME, Mandas A, Forni G, et al. 2004. Hepatocellular carcinoma in the thalassaemia syndromes. Br J Haematol 124: 114–117 [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Marsella M, Zanforlin N 2010. The natural history of thalassemia intermedia. Ann NY Acad Sci 1202: 214–220 [DOI] [PubMed] [Google Scholar]
- *.Brittenham G, Olivieri N 2012. Cold Spring Harb Perspect Med (to be published) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butthep P, Bunyaratvej A, Funahara Y, Kitaguchi H, Fucharoen S, Sato S, Bhamarapravati N 1995. Alterations in vascular endothelial cell-related plasma proteins in thalassaemic patients and their correlation with clinical symptoms. Thromb Haemost 74: 1045–1049 [PubMed] [Google Scholar]
- Butthep P, Bunyaratvej A, Funahara Y, Kitaguchi H, Fucharoen S, Sato S, Bhamarapravati N 1997. Possible evidence of endothelial cell activation and disturbance in thalassemia: An in vitro study. Southeast Asian J Trop Med Public Health 28(Suppl): 141A–148A [PubMed] [Google Scholar]
- Camaschella C, Cappellini MD 1995. Thalassemia intermedia. Haematologica 80: 58–68 [PubMed] [Google Scholar]
- Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli G, Mannucci AP 2000. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol 111: 467–473 [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Grespi E, Cassinerio E, Bignamini D, Fiorelli G 2005. Coagulation and splenectomy: An overview. Ann NY Acad Sci 1054: 317–324 [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Motta I, Musallam KM, Taher AT 2010. Redefining thalassemia as a hypercoagulable state. Ann NY Acad Sci 1202: 231–236 [DOI] [PubMed] [Google Scholar]
- Chehal A, Aoun E, Koussa S, Skoury H, Taher A 2003. Hypertransfusion: A successful method of treatment in thalassemia intermedia patients with spinal cord compression secondary to extramedullary hematopoiesis. Spine (Phila Pa 1976) 28: E245–E249 [DOI] [PubMed] [Google Scholar]
- Chen S, Eldor A, Barshtein G, Zhang S, Goldfarb A, Rachmilewitz E, Yedgar S 1996. Enhanced aggregability of red blood cells of β-thalassemia major patients. Am J Physiol 270: H1951–H1956 [DOI] [PubMed] [Google Scholar]
- Cossu P, Toccafondi C, Vardeu F, Sanna G, Frau F, Lobrano R, Cornacchia G, Nucaro A, Bertolino F, Loi A, et al. 1981. Iron overload and desferrioxamine chelation therapy in β-thalassemia intermedia. Eur J Pediatr 137: 267–271 [DOI] [PubMed] [Google Scholar]
- Del Principe D, Menichelli A, Di Giulio S, De Matteis W, Cianciulli P, Papa G 1993. PADGEM/GMP-140 expression on platelet membranes from homozygous β thalassaemic patients. Br J Haematol 84: 111–117 [DOI] [PubMed] [Google Scholar]
- Derchi G, Forni GL, Formisano F, Cappellini MD, Galanello R, D’Ascola G, Bina P, Magnano C, Lamagna M 2005. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica 90: 452–458 [PubMed] [Google Scholar]
- Dettelbach HR, Aviado DM 1985. Clinical pharmacology of pentoxifylline with special reference to its hemorrheologic effect for the treatment of intermittent claudication. J Clin Pharmacol 25: 8–26 [DOI] [PubMed] [Google Scholar]
- Eldor A, Rachmilewitz EA 2002. The hypercoagulable state in thalassemia. Blood 99: 36–43 [DOI] [PubMed] [Google Scholar]
- Eldor A, Krausz Y, Atlan H, Snyder D, Goldfarb A, Hy-Am E, Rachmilewitz EA, Kotze HF, Heyns AD 1989. Platelet survival in patients with β-thalassemia. Am J Hematol 32: 94–99 [DOI] [PubMed] [Google Scholar]
- Eldor A, Lellouche F, Goldfarb A, Rachmilewitz EA, Maclouf J 1991. In vivo platelet activation in β-thalassemia major reflected by increased platelet-thromboxane urinary metabolites. Blood 77: 1749–1753 [PubMed] [Google Scholar]
- Eldor A, Durst R, Hy-Am E, Goldfarb A, Gillis S, Rachmilewitz EA, Abramov A, MacLouf J, Godefray YC, De Raucourt E, et al. 1999. A chronic hypercoagulable state in patients with β-thalassaemia major is already present in childhood. Br J Haematol 107: 739–746 [DOI] [PubMed] [Google Scholar]
- Galanello R, Cao A 1998. Relationship between genotype and phenotype. Thalassemia intermedia. Ann NY Acad Sci 850: 325–333 [DOI] [PubMed] [Google Scholar]
- Gardenghi S, Grady RW, Rivella S 2010. Anemia, ineffective erythropoiesis, and hepcidin: Interacting factors in abnormal iron metabolism leading to iron overload in β-thalassemia. Hematol Oncol Clin North Am 24: 1089–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S, Cappellini MD, Goldfarb A, Ciceri L, Fiorelli G, Rachmilewitz EA 1999. Pulmonary thromboembolism in thalassemia intermedia patients. Haematologica 84: 959–960 [PubMed] [Google Scholar]
- Gimmon Z, Wexler MR, Rachmilewitz EA 1982. Juvenile leg ulceration in β-thalassemia major and intermedia. Plast Reconstr Surg 69: 320–325 [DOI] [PubMed] [Google Scholar]
- Glickstein H, El RB, Link G, Breuer W, Konijn AM, Hershko C, Nick H, Cabantchik ZI 2006. Action of chelators in iron-loaded cardiac cells: Accessibility to intracellular labile iron and functional consequences. Blood 108: 3195–3203 [DOI] [PubMed] [Google Scholar]
- Goksel BK, Ozdogu H, Yildirim T, Oguzkurt L, Asma S 2010. β-Thalassemia intermedia associated with moyamoya syndrome. J Clin Neurosci 17: 919–920 [DOI] [PubMed] [Google Scholar]
- Goldschmidt N, Spectre G, Brill A, Zelig O, Goldfarb A, Rachmilewitz E, Varon D 2008. Increased platelet adhesion under flow conditions is induced by both thalassemic platelets and red blood cells. Thromb Haemost 100: 864–870 [PubMed] [Google Scholar]
- Habib A, Kunzelmann C, Shamseddeen W, Zobairi F, Freyssinet JM, Taher A 2008. Elevated levels of circulating procoagulant microparticles in patients with β-thalassemia intermedia. Haematologica 93: 941–942 [DOI] [PubMed] [Google Scholar]
- Hahalis G, Kalogeropoulos A, Terzis G, Tselepis AD, Kourakli A, Mylona P, Grapsas N, Alexopoulos D 2011. Premature atherosclerosis in non-transfusion-dependent β-thalassemia intermedia. Cardiology 118: 159–163 [DOI] [PubMed] [Google Scholar]
- Haidar R, Mhaidli H, Taher AT 2010. Paraspinal extramedullary hematopoiesis in patients with thalassemia intermedia. Eur Spine J 19: 871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helley D, Eldor A, Girot R, Ducrocq R, Guillin MC, Bezeaud A 1996. Increased procoagulant activity of red blood cells from patients with homozygous sickle cell disease and β-thalassemia. Thromb Haemost 76: 322–327 [PubMed] [Google Scholar]
- Hershko C, Graham G, Bates GW, Rachmilewitz EA 1978. Non-specific serum iron in thalassaemia: An abnormal serum iron fraction of potential toxicity. Br J Haematol 40: 255–263 [DOI] [PubMed] [Google Scholar]
- Hovav T, Goldfarb A, Artmann G, Yedgar S, Barshtein G 1999. Enhanced adherence of β-thalassaemic erythrocytes to endothelial cells. Br J Haematol 106: 178–181 [DOI] [PubMed] [Google Scholar]
- Iolascon A, Giordano P, Storelli S, Li HH, Coppola B, Piga A, Fantola E, Forni G, Cianciulli P, Perrotta S, et al. 2001. Thrombophilia in thalassemia major patients: Analysis of genetic predisposing factors. Haematologica 86: 1112–1113 [PubMed] [Google Scholar]
- Isma’eel H, Chafic AH, Rassi FE, Inati A, Koussa S, Daher R, Gharzuddin W, Alam S, Taher A 2008. Relation between iron-overload indices, cardiac echo-Doppler, and biochemical markers in thalassemia intermedia. Am J Cardiol 102: 363–367 [DOI] [PubMed] [Google Scholar]
- Jackson DV Jr, Randall ME, Richards F II 1988. Spinal cord compression due to extramedullary hematopoiesis in thalassemia: Long-term follow-up after radiotherapy. Surg Neurol 29: 389–392 [DOI] [PubMed] [Google Scholar]
- Karimi M, Khanlari M, Rachmilewitz EA 2008. Cerebrovascular accident in β-thalassemia major (β-TM) and β-thalassemia intermedia (β-TI). Am J Hematol 83: 77–79 [DOI] [PubMed] [Google Scholar]
- Karimi M, Bagheri H, Rastgu F, Rachmilewitz EA 2010a. Magnetic resonance imaging to determine the incidence of brain ischaemia in patients with β-thalassaemia intermedia. Thromb Haemost 103: 989–993 [DOI] [PubMed] [Google Scholar]
- Karimi M, Cohan N, Moosavizadeh K, Falahi MJ, Haghpanah S 2010b. Adverse effects of hydroxyurea in β-thalassemia intermedia patients: 10 years’ experience. Pediatr Hematol Oncol 27: 205–211 [DOI] [PubMed] [Google Scholar]
- Karimi M, Mohammadi F, Behmanesh F, Samani SM, Borzouee M, Amoozgar H, Haghpanah S 2010c. Effect of combination therapy of hydroxyurea with l-carnitine and magnesium chloride on hematologic parameters and cardiac function of patients with β-thalassemia intermedia. Eur J Haematol 84: 52–58 [DOI] [PubMed] [Google Scholar]
- Karimi M, Musallam KM, Cappellini MD, Daar S, El-Beshlawy A, Belhoul K, Saned MS, Temraz S, Koussa S, Taher AT 2011. Risk factors for pulmonary hypertension in patients with β thalassemia intermedia. Eur J Intern Med 22: 607–610 [DOI] [PubMed] [Google Scholar]
- Kuypers FA, de Jong K 2004. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol Biol (Noisy-le-grand) 50: 147–158 [PubMed] [Google Scholar]
- Ladis V, Berdousi H, Gotsis E, Kattamis A 2010. Deferasirox administration for the treatment of non-transfusional iron overload in patients with thalassaemia intermedia. Br J Haematol 151: 504–508 [DOI] [PubMed] [Google Scholar]
- Lai ME, Vacquer S, Carta MP, Spiga A, Cocco P, Angius F, Mandas A, Dessi S 2011. Thalassemia intermedia is associated with a proatherogenic biochemical phenotype. Blood Cells Mol Dis 46: 294–299 [DOI] [PubMed] [Google Scholar]
- Littera R, La Nasa G, Derchi G, Cappellini MD, Chang CY, Contu L 2002. Long-term treatment with sildenafil in a thalassemic patient with pulmonary hypertension. Blood 100: 1516–1517 [DOI] [PubMed] [Google Scholar]
- Lombardo T, Tamburino C, Bartoloni G, Morrone ML, Frontini V, Italia F, Cordaro S, Privitera A, Calvi V 1995. Cardiac iron overload in thalassemic patients: An endomyocardial biopsy study. Ann Hematol 71: 135–141 [DOI] [PubMed] [Google Scholar]
- Malyutin Z, Shai E, Dana M, Rachmilewitz E, Fibach E, Varon D 2012. Shorter carotid artery occlusion in a thalassemic mouse model: A potential role for oxidative stress affecting both RBCs and platelets. 17th European Haematology Association Congress, Amsterdam, The Netherlands, June 2012 [Google Scholar]
- Mancuso A 2010. Hepatocellular carcinoma in thalassemia: A critical review. World J Hepatol 2: 171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfre L, Giarratano E, Maggio A, Banco A, Vaccaro G, Lagalla R 1999. MR imaging of the brain: Findings in asymptomatic patients with thalassemia intermedia and sickle cell–thalassemia disease. AJR Am J Roentgenol 173: 1477–1480 [DOI] [PubMed] [Google Scholar]
- Marden FA, Putman CM, Grant JM, Greenberg J 2008. Moyamoya disease associated with hemoglobin Fairfax and β-thalassemia. Pediatr Neurol 38: 130–132 [DOI] [PubMed] [Google Scholar]
- Mavrogeni S, Gotsis E, Ladis V, Berdousis E, Verganelakis D, Toulas P, Cokkinos DV 2008. Magnetic resonance evaluation of liver and myocardial iron deposition in thalassemia intermedia and b-thalassemia major. Int J Cardiovasc Imaging 24: 849–854 [DOI] [PubMed] [Google Scholar]
- Melchiori L, Gardenghi S, Rivella S 2010. β-Thalassemia: HiJAKing ineffective erythropoiesis and iron overload. Adv Hematol 2010: 938640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtar GM, Adly AA, El Alfy MS, Tawfik LM, Khairy AT 2010. N-terminal natriuretic peptide and ventilation-perfusion lung scan in sickle cell disease and thalassemia patients with pulmonary hypertension. Hemoglobin 34: 78–94 [DOI] [PubMed] [Google Scholar]
- Musallam KM, Taher AT 2011. Thrombosis in thalassemia: Why are we so concerned? Hemoglobin 35: 503–510 [DOI] [PubMed] [Google Scholar]
- Musallam KM, Beydoun A, Hourani R, Nasreddine W, Raad R, Koussa S, Taher AT 2011a. Brain magnetic resonance angiography in splenectomized adults with β-thalassemia intermedia. Eur J Haematol 87: 539–546 [DOI] [PubMed] [Google Scholar]
- Musallam KM, Cappellini MD, Wood JC, Motta I, Graziadei G, Tamim H, Taher AT 2011b. Elevated liver iron concentration is a marker of increased morbidity in patients with β thalassemia intermedia. Haematologica 96: 1605–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam KM, Khoury B, Abi-Habib R, Bazzi L, Succar J, Halawi R, Hankir A, Koussa S, Taher AT 2011c. Health-related quality of life in adults with transfusion-independent thalassaemia intermedia compared to regularly transfused thalassaemia major: New insights. Eur J Haematol 87: 73–79 [DOI] [PubMed] [Google Scholar]
- Musallam KM, Taher AT, Duca L, Cesaretti C, Halawi R, Cappellini MD 2011d. Levels of growth differentiation factor-15 are high and correlate with clinical severity in transfusion-independent patients with β thalassemia intermedia. Blood Cells Mol Dis 47: 232–234 [DOI] [PubMed] [Google Scholar]
- Musallam KM, Nasreddine W, Beydoun A, Hourani R, Hankir A, Koussa S, Haidar M, Taher AT 2012a. Brain positron emission tomography in splenectomized adults with β-thalassemia intermedia: Uncovering yet another covert abnormality. Ann Hematol 91: 235–241 [DOI] [PubMed] [Google Scholar]
- Musallam KM, Sankaran VG, Cappellini MD, Duca L, Nathan DG, Taher AT 2012b. Fetal hemoglobin levels and morbidity in untransfused patients with β-thalassemia intermedia. Blood 119: 364–367 [DOI] [PubMed] [Google Scholar]
- Napoli N, Carmina E, Bucchieri S, Sferrazza C, Rini GB, Di Fede G 2006. Low serum levels of 25-hydroxy vitamin D in adults affected by thalassemia major or intermedia. Bone 38: 888–892 [DOI] [PubMed] [Google Scholar]
- Nassar AH, Usta IM, Taher AM 2006. β-Thalassemia intermedia and pregnancy: Should we anticoagulate? J Thromb Haemost 4: 1413–1414 [DOI] [PubMed] [Google Scholar]
- Nassar AH, Naja M, Cesaretti C, Eprassi B, Cappellini MD, Taher A 2008. Pregnancy outcome in patients with β-thalassemia intermedia at two tertiary care centers, in Beirut and Milan. Haematologica 93: 1586–1587 [DOI] [PubMed] [Google Scholar]
- Oberoi S, Bansal D, Singh P, Marwaha RK 2010. Stroke in a young boy with β-thalassemia intermedia secondary to moyamoya syndrome. J Pediatr Hematol Oncol 32: 568–570 [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Koren G, Matsui D, Liu PP, Blendis L, Cameron R, McClelland RA, Templeton DM 1992. Reduction of tissue iron stores and normalization of serum ferritin during treatment with the oral iron chelator L1 in thalassemia intermedia. Blood 79: 2741–2748 [PubMed] [Google Scholar]
- Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, Nemeth E 2007. Liver iron concentrations and urinary hepcidin in β-thalassemia. Haematologica 92: 583–588 [DOI] [PubMed] [Google Scholar]
- Origa R, Barella S, Argiolas GM, Bina P, Agus A, Galanello R 2008. No evidence of cardiac iron in 20 never- or minimally-transfused patients with thalassemia intermedia. Haematologica 93: 1095–1096 [DOI] [PubMed] [Google Scholar]
- Pakbaz Z, Fischer R, Fung E, Nielsen P, Harmatz P, Vichinsky E 2007. Serum ferritin underestimates liver iron concentration in transfusion independent thalassemia patients as compared to regularly transfused thalassemia and sickle cell patients. Pediatr Blood Cancer 49: 329–332 [DOI] [PubMed] [Google Scholar]
- Panigrahi I, Dixit A, Arora S, Kabra M, Mahapatra M, Choudhry VP, Saxena R 2005. Do α deletions influence hydroxyurea response in thalassemia intermedia? Hematology 10: 61–63 [DOI] [PubMed] [Google Scholar]
- Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, et al. 2011. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 365: 44–53 [DOI] [PubMed] [Google Scholar]
- Phrommintikul A, Sukonthasarn A, Kanjanavanit R, Nawarawong W 2006. Splenectomy: A strong risk factor for pulmonary hypertension in patients with thalassaemia. Heart 92: 1467–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippard MJ, Weatherall DJ 1988. Iron balance and the management of iron overload in β-thalassemia intermedia. Birth Defects Orig Artic Ser 23: 29–33 [PubMed] [Google Scholar]
- Restivo Pantalone G, Renda D, Valenza F, D’Amato F, Vitrano A, Cassara F, Rigano P, Di Salvo V, Giangreco A, Bevacqua E, et al. 2010. Hepatocellular carcinoma in patients with thalassaemia syndromes: Clinical characteristics and outcome in a long term single centre experience. Br J Haematol 150: 245–247 [DOI] [PubMed] [Google Scholar]
- Rigano P, Pecoraro A, Calzolari R, Troia A, Acuto S, Renda D, Pantalone GR, Maggio A, Di Marzo R 2010. Desensitization to hydroxycarbamide following long-term treatment of thalassaemia intermedia as observed in vivo and in primary erythroid cultures from treated patients. Br J Haematol 151: 509–515 [DOI] [PubMed] [Google Scholar]
- Roghi A, Cappellini MD, Wood JC, Musallam KM, Patrizia P, Fasulo MR, Cesaretti C, Taher AT 2010. Absence of cardiac siderosis despite hepatic iron overload in Italian patients with thalassemia intermedia: An MRI T2* study. Ann Hematol 89: 585–589 [DOI] [PubMed] [Google Scholar]
- Ruf A, Pick M, Deutsch V, Patscheke H, Goldfarb A, Rachmilewitz EA, Guillin MC, Eldor A 1997. In-vivo platelet activation correlates with red cell anionic phospholipid exposure in patients with β-thalassaemia major. Br J Haematol 98: 51–56 [DOI] [PubMed] [Google Scholar]
- Rund D, Rachmilewitz E 2005. β-Thalassemia. N Engl J Med 353: 1135–1146 [DOI] [PubMed] [Google Scholar]
- Sanefuji M, Ohga S, Kira R, Yoshiura T, Torisu H, Hara T 2006. Moyamoya syndrome in a splenectomized patient with β-thalassemia intermedia. J Child Neurol 21: 75–77 [DOI] [PubMed] [Google Scholar]
- Sankaran VG, Lettre G, Orkin SH, Hirschhorn JN 2010. Modifier genes in Mendelian disorders: The example of hemoglobin disorders. Ann NY Acad Sci 1214: 47–56 [DOI] [PubMed] [Google Scholar]
- Singer ST, Kuypers FA, Styles L, Vichinsky EP, Foote D, Rosenfeld H 2006. Pulmonary hypertension in thalassemia: Association with platelet activation and hypercoagulable state. Am J Hematol 81: 670–675 [DOI] [PubMed] [Google Scholar]
- Steinberg MH, Forget BG, Higgs DR, Weatherall DJ 2009. Disorders of hemoglobin: Genetics, pathophysiology, and clinical management. Cambridge University Press, New York [Google Scholar]
- Sturgeon P, Itano HA, Bergren WR 1955. Genetic and biochemical studies of intermediate types of Cooley’s anaemia. Br J Haematol 1: 264–277 [DOI] [PubMed] [Google Scholar]
- Taher A, Isma’eel H, Cappellini MD 2006a. Thalassemia intermedia: Revisited. Blood Cells Mol Dis 37: 12–20 [DOI] [PubMed] [Google Scholar]
- Taher A, Isma’eel H, Mehio G, Bignamini D, Kattamis A, Rachmilewitz EA, Cappellini MD 2006b. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost 96: 488–491 [PubMed] [Google Scholar]
- Taher A, El Rassi F, Isma’eel H, Koussa S, Inati A, Cappellini MD 2008a. Correlation of liver iron concentration determined by R2 magnetic resonance imaging with serum ferritin in patients with thalassemia intermedia. Haematologica 93: 1584–1586 [DOI] [PubMed] [Google Scholar]
- Taher AT, Otrock ZK, Uthman I, Cappellini MD 2008b. Thalassemia and hypercoagulability. Blood Rev 22: 283–292 [DOI] [PubMed] [Google Scholar]
- Taher A, Hershko C, Cappellini MD 2009a. Iron overload in thalassaemia intermedia: Reassessment of iron chelation strategies. Br J Haematol 147: 634–640 [DOI] [PubMed] [Google Scholar]
- Taher A, Musallam KM, El Rassi F, Duca L, Inati A, Koussa S, Cappellini MD 2009b. Levels of non-transferrin-bound iron as an index of iron overload in patients with thalassaemia intermedia. Br J Haematol 146: 569–572 [DOI] [PubMed] [Google Scholar]
- Taher AT, Musallam KM, El-Beshlawy A, Karimi M, Daar S, Belhoul K, Saned MS, Graziadei G, Cappellini MD 2010a. Age-related complications in treatment-naive patients with thalassaemia intermedia. Br J Haematol 150: 486–489 [DOI] [PubMed] [Google Scholar]
- Taher AT, Musallam KM, Karimi M, El-Beshlawy A, Belhoul K, Daar S, Saned M, Cesaretti C, Cappellini MD 2010b. Splenectomy and thrombosis: The case of thalassemia intermedia. J Thromb Haemost 8: 2152–2158 [DOI] [PubMed] [Google Scholar]
- Taher AT, Musallam KM, Karimi M, El-Beshlawy A, Belhoul K, Daar S, Saned MS, El-Chafic AH, Fasulo MR, Cappellini MD 2010c. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: The OPTIMAL CARE study. Blood 115: 1886–1892 [DOI] [PubMed] [Google Scholar]
- Taher AT, Musallam KM, Nasreddine W, Hourani R, Inati A, Beydoun A 2010d. Asymptomatic brain magnetic resonance imaging abnormalities in splenectomized adults with thalassemia intermedia. J Thromb Haemost 8: 54–59 [DOI] [PubMed] [Google Scholar]
- Taher AT, Musallam KM, Wood JC, Cappellini MD 2010e. Magnetic resonance evaluation of hepatic and myocardial iron deposition in transfusion-independent thalassemia intermedia compared to regularly transfused thalassemia major patients. Am J Hematol 85: 288–290 [DOI] [PubMed] [Google Scholar]
- Taher AT, Musallam KM, Cappellini MD, Weatherall DJ 2011. Optimal management of β thalassaemia intermedia. Br J Haematol 152: 512–523 [DOI] [PubMed] [Google Scholar]
- Tai SM, Chan JS, Ha SY, Young BW, Chan MS 2006. Successful treatment of spinal cord compression secondary to extramedullary hematopoietic mass by hypertransfusion in a patient with thalassemia major. Pediatr Hematol Oncol 23: 317–321 [DOI] [PubMed] [Google Scholar]
- Tanno T, Miller JL 2010. Iron loading and overloading due to ineffective erythropoiesis. Adv Hematol 2010: 358283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, et al. 2007. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med 13: 1096–1101 [DOI] [PubMed] [Google Scholar]
- Tavazzi D, Duca L, Graziadei G, Comino A, Fiorelli G, Cappellini MD 2001. Membrane-bound iron contributes to oxidative damage of β-thalassaemia intermedia erythrocytes. Br J Haematol 112: 48–50 [DOI] [PubMed] [Google Scholar]
- *.Thein SL 2012. Molecular basis of β thalassemia. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskaridou E, Douskou M, Terpos E, Papassotiriou I, Stamoulakatou A, Ourailidis A, Loutradi A, Loukopoulos D 2004. Magnetic resonance imaging in the evaluation of iron overload in patients with β thalassaemia and sickle cell disease. Br J Haematol 126: 736–742 [DOI] [PubMed] [Google Scholar]
- Voskaridou E, Christoulas D, Antoniadou L, Terpos E 2008. Continuous increase in erythropoietic activity despite the improvement in bone mineral density by zoledronic acid in patients with thalassemia intermedia-induced osteoporosis. Acta Haematol 119: 40–44 [DOI] [PubMed] [Google Scholar]
- Voskaridou E, Plata E, Douskou M, Papadakis M, Delaki EE, Christoulas D, Terpos E 2010. Treatment with deferasirox (Exjade) effectively decreases iron burden in patients with thalassaemia intermedia: Results of a pilot study. Br J Haematol 148: 332–334 [DOI] [PubMed] [Google Scholar]
- Weatherall DJ 2001. Phenotype–genotype relationships in monogenic disease: Lessons from the thalassaemias. Nat Rev Genet 2: 245–255 [DOI] [PubMed] [Google Scholar]
- Weatherall D 2004. 2003 William Allan Award address. The thalassemias: The role of molecular genetics in an evolving global health problem. Am J Hum Genet 74: 385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall DJ, Clegg JB 2001. The thalassaemia syndromes. Blackwell Science, Oxford [Google Scholar]
- Weizer-Stern O, Adamsky K, Amariglio N, Rachmilewitz E, Breda L, Rivella S, Rechavi G 2006. mRNA expression of iron regulatory genes in β-thalassemia intermedia and β-thalassemia major mouse models. Am J Hematol 81: 479–483 [DOI] [PubMed] [Google Scholar]
- Winichagoon P, Fucharoen S, Wasi P 1981. Increased circulating platelet aggregates in thalassaemia. Southeast Asian J Trop Med Public Health 12: 556–560 [PubMed] [Google Scholar]
- Zalloua PA, Shbaklo H, Mourad YA, Koussa S, Taher A 2003. Incidence of thromboembolic events in Lebanese thalassemia intermedia patients. Thromb Haemost 89: 767–768 [PubMed] [Google Scholar]