Abstract
This work summarizes our current understanding of the elongation and termination/recycling phases of eukaryotic protein synthesis. We focus here on recent advances in the field. In addition to an overview of translation elongation, we discuss unique aspects of eukaryotic translation elongation including eEF1 recycling, eEF2 modification, and eEF3 and eIF5A function. Likewise, we highlight the function of the eukaryotic release factors eRF1 and eRF3 in translation termination, and the functions of ABCE1/Rli1, the Dom34:Hbs1 complex, and Ligatin (eIF2D) in ribosome recycling. Finally, we present some of the key questions in translation elongation, termination, and recycling that remain to be answered.
Eukaryotes have unique features of translation elongation (e.g., eEF1 recycling, eEF2 modification, and eIF5A function), termination (e.g., the use of eRF1 and eRF3), and ribosome recycling.
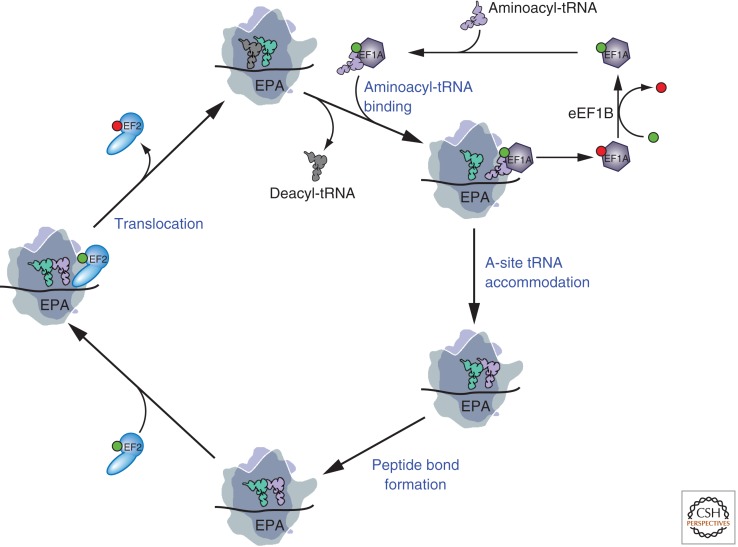
The mechanism of translation elongation is well conserved between eukaryotes and bacteria (Rodnina and Wintermeyer 2009), and, in general, studies on the mechanism of translation elongation have focused on bacterial systems. Following translation initiation, an 80S ribosome is poised on a messenger RNA (mRNA) with the anticodon of Met-tRNAi in the P site base-paired with the start codon. The second codon of the open reading frame (ORF) is present in the A (acceptor) site of the ribosome awaiting binding of the cognate aminoacyl-tRNA. The eukaryotic elongation factor eEF1A, the ortholog of bacterial EF-Tu, binds aminoacyl-tRNA in a GTP-dependent manner and then directs the tRNA to the A site of the ribosome (Fig. 1). Codon recognition by the tRNA triggers GTP hydrolysis by eEF1A, releasing the factor and enabling the aminoacyl-tRNA to be accommodated into the A site. Recent high-resolution structures of the bacterial ribosome bound to EF-Tu and aminoacyl-tRNA revealed distortion of the anticodon stem and at the junction between the acceptor and D stems that enables the aminoacyl-tRNA to interact with both the decoding site on the small subunit and with EF-Tu. It is thought that the energetic penalty for this distortion is paid for by the perfect codon–anticodon match and the attendant stabilizing interactions that occur between the A site and cognate tRNA to promote high-fidelity decoding (Schmeing et al. 2009, 2011). These interactions might exceed those involving 16S rRNA bases A1492, A1493, and G530 with the minor groove of the codon–anticodon helix (Ogle et al. 2001) to include residues in ribosomal proteins and other regions of the tRNA (Jenner et al. 2010). The recent structures of the ribosome bound to EF-Tu and aminoacyl-tRNA also revealed that the conserved nucleotide A2662 (Thermus thermophilus numbering) in the sarcin–ricin loop of 23S rRNA in the large subunit interacts with the conserved catalytic His residue in the G domain enabling the His residue to coordinate and position the water molecule required for GTP hydrolysis (Voorhees et al. 2010). It is expected that these mechanisms of initial aminoacyl-tRNA binding, codon recognition, and GTPase activation will be shared between bacteria and eukaryotes.
Figure 1.
Model of the eukaryotic translation elongation pathway. In this model the large ribosomal subunit is drawn transparent to visualize tRNAs, factors, and mRNA binding to the decoding center at the interface between the large and small subunits and tRNAs interacting with the peptidyl transferase center in the large subunit. Starting at the top, an eEF1A·GTP·aminoacyl-tRNA ternary complex binds the aminoacyl-tRNA to the 80S ribosome with the anticodon loop of the tRNA in contact with the mRNA in the A site of the small subunit. Following release of eEF1A·GDP, the aminoacyl-tRNA is accommodated into the A site, and the eEF1A·GDP is recycled to eEF1A·GTP by the exchange factor eEF1B. Peptide bond formation is accompanied by transition of the A- and P-site tRNAs into hybrid states with the acceptors ends of the tRNAs moving to the P and E sites, respectively. Binding of eEF2·GTP promotes translocation of the tRNAs into the canonical P and E sites, and is followed by release of eEF2·GDP, which unlike eEF1A does not require an exchange factor. The ribosome is now ready for the next cycle of elongation with release of the deacylated tRNA from the E site and binding of the appropriate eEF1A·GTP·aminoacyl-tRNA to the A site. Throughout, GTP is depicted as a green ball and GDP as a red ball; also, the positions of the mRNA, tRNAs, and factors are drawn for clarity and are not meant to specify their exact places on the ribosome.
Following accommodation of the aminoacyl-tRNA into the A site, peptide bond formation with the P-site peptidyl-tRNA occurs rapidly. The peptidyl transferase center (PTC), consisting primarily of conserved ribosomal RNA (rRNA) elements on the large ribosomal subunit, positions the substrates for catalysis. Recent crystal structures of the Saccharomyces cerevisiae 80S ribosome and the T. thermophila 60S subunit revealed that the rRNA structure of the PTC is nearly superimposable between the eukaryotic and bacterial ribosomes (Ben-Shem et al. 2010, 2011; Klinge et al. 2011), supporting the idea that the mechanism of peptide bond formation, the heart of protein synthesis, is universally conserved.
Following peptide bond formation, ratcheting of the ribosomal subunits triggers movement of the tRNAs into so-called hybrid P/E and A/P states with the acceptor ends of the tRNAs in the E and P-sites and the anticodon loops remaining in the P and A sites, respectively. Translocation of the tRNAs to the canonical E and P sites requires the elongation factor eEF2 in eukaryotes, which is the ortholog of bacterial EF-G. Binding of the GTPase eEF2 or EF-G in complex with GTP is thought to stabilize the hybrid state and promote rapid hydrolysis of GTP. Conformational changes in eEF2/EF-G accompanying GTP hydrolysis and Pi release are thought to alternatively unlock the ribosome allowing tRNA and mRNA movement and then lock the subunits in the posttranslocation state. Pi release is also coupled to release of the factor from the ribosome. A structure of EF-G bound to a posttranslocation bacterial ribosome revealed the interaction of EF-G domain IV with the mRNA, P-site tRNA, and decoding center on the small ribosomal subunit (Gao et al. 2009), consistent with the notion that EF-G and eEF2 function, at least in part, to prevent backward movement of the tRNAs in the unlocked state of the ribosome.
In the posttranslocation state of the ribosome, a deacylated tRNA occupies the E site and the peptidyl-tRNA is in the P site. The A site is vacant and available for binding of the next aminoacyl-tRNA in complex with eEF1A. Although it has been proposed that E-site tRNA release is coupled to binding of aminoacyl-tRNA in the A site (Nierhaus 1990), recent single molecule and ensemble kinetic analyses indicate that release of the E-site tRNA is not strictly coupled to binding of aminoacyl-tRNA in the A site (Semenkov et al. 1996; Uemura et al. 2010; Chen et al. 2011).
Although these basic mechanisms of translation elongation and peptide bond formation are conserved between bacteria and eukaryotes, several features of translation elongation are unique in eukaryotes. Moreover, recent studies have characterized additional factors that may function in translation elongation.
eEF1 RECYCLING
Following GTP hydrolysis both EF-Tu and eEF1A are released from the ribosome in complex with GDP. The spontaneous rate of GDP dissociation from these factors is slow, and a guanine nucleotide exchange factor is required to recycle the inactive GDP-bound factor to its active GTP-bound state. In the case of EF-Tu, the single polypeptide factor EF-Ts promotes nucleotide exchange. In contrast, the eukaryotic factor eEF1B, composed of two to four subunits, catalyzes guanine nucleotide exchange on eEF1A. Interestingly, despite the strong homology between eEF1A and EF-Tu, the catalytic eEF1Bα subunit does not resemble EF-Ts and the two proteins promote guanine nucleotide exchange by distinct mechanisms (Rodnina and Wintermeyer 2009). Whereas EF-Ts binds to the EF-Tu G domain and indirectly destabilizes Mg2+ binding leading to GDP release, eEF1Bα inserts an essential Lys residue into the Mg2+ and γ-phosphate binding site to directly destabilize Mg2+ binding. It is unclear why eukaryotes use a more elaborate exchange factor, although it might provide a means to regulate translation elongation (Sivan et al. 2011).
eEF2 MODIFICATION
As noted above, eEF2 and EF-G promote translocation by binding to the ribosome and inserting domain IV of the factor into the decoding center of the small subunit. Interestingly, a conserved His residue (His699 in yeast eEF2) at the tip of domain IV of eEF2 is posttranslationally modified to diphthamide. The diphthamide modification is formed in two steps and requires the action of five proteins, DPH1-5. Despite the universal conservation of the diphthamide modification in eukaryotes and archaea, diphthamide is nonessential for cell viability. The DPH1-5 genes can be deleted in yeast, and derivatives of CHO cells that fail to make diphthamide grow normally (Liu et al. 2004). However, knockout mice lacking DPH1(Ovca1), DPH3, or DPH4 were either embryonic lethal or showed severe developmental defects (Chen and Behringer 2004; Liu et al. 2006; Webb et al. 2008), perhaps suggesting a critical role for diphthamide at a specific time during development. Currently, the only known function of diphthamide is to serve as a site of ADP-ribosylation by diphtheria toxin and related toxins. Modification of eEF2 inactivates the factor and blocks translation. As it seems improbable that cells would retain diphthamide solely to enable pathogen inhibition of protein synthesis, it is presumed that the diphthamide modification somehow enhances eEF2 function. Consistent with this idea, amino acid substitutions at His699 in yeast eEF2 have been reported to block or impair yeast cell growth (Kimata and Kohno 1994). Moreover, a yeast strain expressing the eEF2-H699N mutant displayed reduced translation and increased sensitivity to translational inhibitors; but, as expected, the mutant was resistant to diphtheria toxin (Ortiz et al. 2006). Interestingly, both the eEF2-H699N mutant and a dph5 mutant that fails to make diphthamide displayed enhanced −1 ribosomal frameshifting in vivo (Ortiz et al. 2006). This latter finding indicates a positive role for diphthamide in translation; however, additional studies are needed to define the function of this modification. Given its location at the tip of eEF2 domain IV, it is appealing to speculate that diphthamide may contact the mRNA, tRNA, or rRNA in the decoding center of the ribosome to promote or enhance the fidelity of translocation.
In addition to the constitutive modification of eEF2 by diphthamide, the factor is also phosphorylated in mammalian cells by a novel Ca2+-activated protein kinase eEF2K. Phosphorylation of eEF2 is thought to block translation by impairing factor binding to the ribosome (Carlberg et al. 1990). Interestingly, despite its apparent role in blocking total protein synthesis, eEF2 phosphorylation in neurons has been linked to enhanced localized translation of activity-regulated cytoskeleton-associated protein Arc/Arg3.1, an immediate early gene induced by sensory inputs and learning that functions in postsynaptic endocytosis (Park et al. 2008; Waung et al. 2008). Although mRNA specificity in this regulation might reflect localized phosphorylation of eEF2 in the cell, this result bears resemblance to previous reports of enhanced translation of a subset of mRNAs in cells treated with the translation inhibitor cycloheximide (Walden and Thach 1986). In this latter case it was proposed that inhibition of general translation enhances the translation of the normally uncompetitive mRNAs by freeing up a limiting factor required for their translation. Accordingly, it will be interesting to further examine the factor requirements for Arc/Arg3.1 mRNA translation. Moreover, the recently described ribosomal profiling technique (Ingolia et al. 2009) may provide a further test to this model and identify additional mRNAs whose translation is enhanced under conditions where global translation elongation is inhibited.
FUNGAL SPECIFIC FACTOR eEF3
In addition to the canonical eEF1 and eEF2, translation elongation in all yeast and higher fungi that have been examined requires an additional factor eEF3. eEF3 is an ATPase and contains two ATP-binding cassettes (ABCs). Thus, whereas yeast eEF1 and eEF2 can functionally replace their mammalian counterparts to promote translation with mammalian ribosomes in vitro, mammalian eEF1 and eEF2 must be supplemented with eEF3 to promote protein synthesis with yeast ribosomes (Skogerson and Engelhardt 1977). Consistent with this ribosome specificity for eEF3, the factor binds to ribosomes and a cryo-EM structure of eEF3 bound to posttranslocation yeast 80S ribosomes has been reported (Andersen et al. 2006). Ribosome binding by eEF3 was enhanced in the presence of the nonhydrolyzable ATP analog ADPNP, consistent with the idea that ATP hydrolysis is required for eEF3 dissociation from the ribosome.
In contrast to eEF1A and eEF2, which bind to the ribosomal A site, eEF3 spans across the top of the two subunits contacting the central protuberance of the 60S subunit and the head of the 40S subunit (Andersen et al. 2006). Moreover, a chromodomain inserted within the second ABC domain of eEF3 was found to bind near the ribosomal E site. It has been proposed that eEF3 may facilitate release of deacylated tRNA from the E site following translocation (Triana-Alonso et al. 1995; Andersen et al. 2006). Both genetic and physical interactions have been reported between eEF1A and eEF3 (Anand et al. 2003, 2006); however, further study is needed to determine whether this interaction is associated with the proposed coupling between the ribosomal A and E sites.
It is unclear why translation with yeast ribosomes requires eEF3 but translation with other eukaryotic or bacterial ribosomes does not. No close eEF3 homologs can be found in the genome sequences of other organisms, so it is unlikely that an eEF3-like protein has been overlooked in studies of bacterial or mammalian translation. Although there have been reports of ribosome-associated ATPase activities in various eukaryotic and bacterial systems, it is not clear whether these activities are associated with protein synthesis, and the lack of eEF3-like proteins in other organisms suggests that these reported activities are not related to eEF3 function. Comparison of the crystal structures of the yeast 80S ribosome with the structures of bacterial ribosomes does not reveal a unique feature that would indicate the need for an additional elongation factor. It is noteworthy that the yeast 80S ribosome structure contains an additional nonribosomal protein Stm1p. The Stm1p was bound to the head of the 40S subunit and then snaked through the mRNA entry tunnel of the ribosome (Ben-Shem et al. 2011). Interestingly, Stm1p appears to inhibit the function of 80S ribosomes (Balagopal and Parker 2011) and to oppose eEF3 function (Van Dyke et al. 2009). In yeast lacking Stm1p, eEF3 binding to ribosomes is enhanced; and overexpression of eEF3 impairs the growth of the cells lacking Stm1p (Van Dyke et al. 2009). Further genetic, biochemical, and structural studies are needed to determine the function of eEF3 in translation elongation and to resolve its unique requirement in fungi.
eIF5A/EF-P
Recent studies have revealed an additional factor requirement for translation elongation. The factor eIF5A was originally characterized for its ability to stimulate the transfer of methionine from Met-tRNAi in the 80S initiation complex to the aminoacyl-tRNA analog puromycin (Kemper et al. 1976). Because this methionyl-puromycin synthesis assay monitors formation of the first peptide bond, the factor that stimulated the assay was denoted as an initiation factor. Interestingly, the structurally related bacterial protein EF-P was likewise identified by its ability to stimulate the synthesis of methionyl-puromycin using a reconstituted in vitro translation system from E. coli (Glick and Ganoza 1975). Further examination of the function of eIF5A in globin mRNA translation revealed that eIF5A lowered the Mg2+ optimum for protein synthesis in assays lacking spermidine; however, this stimulatory effect of eIF5A was not seen in assays containing spermidine where the optimum Mg2+ concentration was already low (Schreier et al. 1977).
This impact of spermidine on detection of eIF5A activity is noteworthy given the unique posttranslational modification of eIF5A. In all eukaryotes and archaea, a conserved Lys residue in eIF5A is posttranslationally modified in two steps to hypusine (Park et al. 2010). In the first step, an N-butylamine moiety is transferred from spermidine to the ε-amino group of the Lys side chain to form deoxyhypusine. A subsequent hydroxylation reaction completes the modification. Unmodified eIF5A fails to stimulate the formation of methionyl-puromycin (Park et al. 1991); consistent with this, the deoxyhypusine synthase gene that catalyzes the biosynthesis of the hypusine is essential in yeast. The Lys residue in eIF5A that is modified to hypusine is located in a loop at the top of domain I of the protein (Kim et al. 1998), and the corresponding residue in EF-P is either an Arg or Lys (Bailly and de Crecy-Lagard 2010; Navarre et al. 2010; Yanagisawa et al. 2010). Interestingly, in at least some bacteria expressing the Lys variant of EF-P, this residue is modified by the addition of a β-lysine (Bailly and de Crecy-Lagard 2010; Navarre et al. 2010; Yanagisawa et al. 2010), which resembles the hypusine side chain. Although this posttranslational modification is required for EF-P stimulation of methionyl-puromycin synthesis by bacterial ribosomes (Park et al. 2012), further studies are needed to define the mechanistic role of the EF-P modification.
In attempts to resolve the function of eIF5A in yeast, various labs have depleted or inactivated the factor and examined the impact on general protein synthesis using polysome analyses. Depletion of eIF5A using transcriptional shut-off or degron approaches has indicated defects either in translation initiation or elongation, perhaps reflecting differences in assay conditions or in the efficiency of eIF5A depletion (Zanelli et al. 2006; Gregio et al. 2009; Saini et al. 2009; Henderson and Hershey 2011). Inactivation of an eIF5A temperature-sensitive mutant resulted in polysome retention in the absence of the elongation inhibitor cycloheximide (Saini et al. 2009), indicative of a role for eIF5A throughout translation elongation. As eIF5A has been suggested to stimulate the translation of only a subset of mRNAs in the cell (Kang and Hershey 1994), it will be valuable to examine the impact of eIF5A inactivation on genome-wide translation using ribosomal profiling (Ingolia et al. 2009).
Further supporting a role for eIF5A in general translation elongation, inactivation of eIF5A in yeast caused increased ribosomal transit times (Gregio et al. 2009; Saini et al. 2009), and genetic analyses revealed functional interactions between eIF5A and eEF2 (Saini et al. 2009; Dias et al. 2012). Although eEF2 was found to cosediment with eIF5A in pull-down assays (Jao and Chen 2006; Zanelli et al. 2006), this interaction appears to be bridged by ribosomes and so further experiments will be needed to determine whether eEF2 and eIF5A can simultaneously bind to the same 80S complex.
Finally, using a reconstituted in vitro translation initiation and elongation system from yeast, addition of eIF5A was found to stimulate the rate of methionyl-puromycin synthesis and tripeptide synthesis by twofold (Saini et al. 2009). Interestingly, addition of eIF5A also stimulated the rate of peptide release in assays containing release factors eRF1 and eRF3 (Saini et al. 2009). In all of these assays eIF5A activity was fully dependent on its hypusine modification. These studies suggest that eIF5A stimulates the reactivity of peptidyl-tRNA in the ribosomal P site with either aminoacyl-tRNA or protein ligands that enter the A site. Consistently, a crystal structure of the bacterial 70S ribosome revealed EF-P binding in a site adjacent to the P-site bound Met-tRNAi (Blaha et al. 2009). Taken together, the unique spermidine-derived posttranslational modification of eIF5A, the impact of eIF5A and spermidine on the optimal Mg2+ concentration for peptide bond synthesis, and the binding site of EF-P adjacent to the P-site tRNA suggest that eIF5A/EF-P serves as an efficient polyamine delivery system to promote reactivity of the P-site tRNA. To further define the function of eIF5A in translation, it will be necessary to accurately map the binding site of eIF5A on the 80S ribosome and to determine the timing of E-site tRNA release and eIF5A binding during the translation elongation cycle. In addition, more in-depth kinetic studies to determine the steps in elongation, termination, and/or initiation affected by the factor would be valuable in elucidating its mechanism of action.
EUKARYOTIC TRANSLATION TERMINATION
Translation termination takes place when the end of the coding sequence is reached by the ribosome and a stop codon (UAA, UGA, or UAG) enters the A site. Termination in eukaryotes is catalyzed by two protein factors, eRF1 and eRF3, that appear to collaborate in the process (Stansfield et al. 1995; Zhouravleva et al. 1995; Alkalaeva et al. 2006). The class I factor, eRF1, is responsible for high-fidelity stop codon recognition and peptidyl-tRNA hydrolysis. The class II factor, eRF3, is a translational GTPase that is more closely related to EF-Tu than to EF-G (Atkinson et al. 2008). Although bacteria also possess both class I (RF1 and RF2) and class II (RF3) release factors with similar nomenclature, there are striking structural and mechanistic differences between the classes in eukaryotes and bacteria. Most importantly, the class I release factors are wholly different proteins with no apparent evolutionary relationship. These factors appear to have evolved after the divergence of the bacterial and eukaryotic lineages and are different evolutionary solutions to the problem of termination (and as we shall see, recycling).
Like RF1 and RF2, eRF1 is, broadly speaking, a tRNA-shaped protein factor composed of three domains (Song et al. 2000). The amino-terminal domain is responsible for codon recognition and contains a distal loop with a highly conserved NIKS motif that has been proposed to decode stop codons through codon:anticodon-like interactions. Chemical crosslinking experiments suggest that this loop is indeed in close proximity to the stop codon nucleotides (Chavatte et al. 2002). Other regions of eRF1 also appear to contribute to stop codon recognition including the YxCxxxF motif (Kolosov et al. 2005; Fan-Minogue et al. 2008; Bulygin et al. 2010). Overall, the findings in eukaryotes suggest that stop codon recognition is more complex than in the bacterial system.
The middle (M) domain of eRF1 is functionally analogous to the tRNA acceptor stem and as such extends into the PTC to promote peptide release (Song et al. 2000). Like bacterial RF1 and RF2, this domain contains a universally conserved Gly-Gly-Gln (GGQ) motif that appears to be essential in promoting the chemistry of peptide hydrolysis as detailed in the bacterial system (Frolova et al. 1999; Laurberg et al. 2008; Weixlbaumer et al. 2008). It is particularly interesting to note that these tripeptide motifs are an example of convergent evolution in the otherwise unrelated class I release factors. GGQ is clearly a successful chemical solution for catalyzing peptidyl-tRNA hydrolysis in the highly conserved, RNA-rich PTC of the ribosome.
The carboxyl terminus of eRF1 is involved in facilitating interactions with the class II release factor eRF3 (Merkulova et al. 1999; Kononenko et al. 2008; Cheng et al. 2009). eRF3 itself has a variable amino terminus (Ter-Avanesyan et al. 1993) and a more conserved carboxyl terminus that is directly involved in interactions with the M and C domains of eRF1. Although eRF3 is an essential gene, the carboxy-terminal fragment is sufficient in yeast to complement the deletion of eRF3 (Ter-Avanesyan et al. 1993; Kononenko et al. 2008; Cheng et al. 2009). The nonessential amino terminus has been implicated in binding interactions with PABP and Upf1 and in the prion properties of the factor [PSI+] (Paushkin et al. 1996; Hoshino et al. 1999; Cosson et al. 2002; Ivanov et al. 2008).
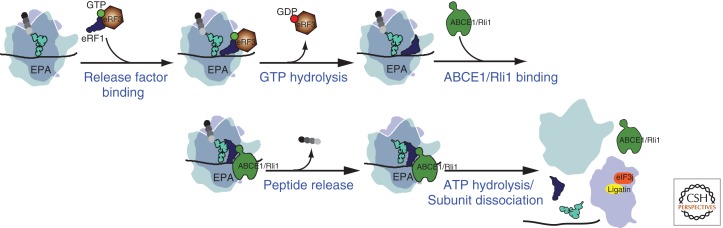
In vitro, eRF3 both accelerates peptide release and increases termination efficiency at stop codons in a manner that depends on GTP hydrolysis (Alkalaeva et al. 2006; Eyler and Green 2011). Dissociation of GTP from eRF3 is slowed by eRF1 binding off the ribosome and, as such, eRF1 has been proposed to play the role of a GTP dissociation inhibitor (TDI, for GTP dissociation inhibitor) (Pisareva et al. 2006). The ternary complex, eRF1:eRF3:GTP, next engages the ribosome, triggering GTP hydrolysis (Frolova et al. 1996) ultimately leading to the deposition of the M domain of eRF1 in the PTC. In this scenario, eRF3 is playing a role similar to that of EF-Tu (to which it is closely related) in controlling delivery of a tRNA-like molecule into the PTC. During the delivery process, the presence of a stop codon in the A site can be evaluated by eRF1 to achieve the reported high levels of discrimination (Salas-Marco and Bedwell 2005). These roles for eRF1/eRF3 are incorporated into a model in Figure 2. Interestingly, there is no eRF3 homolog in archaea, where instead aEF1α (the elongation factor equivalent to EF-Tu in bacteria and eEF1α in eukaryotes) is thought to take its role in the termination reaction mechanism (Saito et al. 2010). Finally, as previously alluded to, bacteria have no eRF3 homolog, but instead have a different class 2 release factor, RF3; this factor is more closely related to EF-G than to EF-Tu and does not appear to play an equivalent role to eRF3 in promoting the release reaction (Freistroffer et al. 1997; Zaher and Green 2011) or as discussed next, in the downstream events of recycling.
Figure 2.
Model of the eukaryotic translation termination and recycling pathways. In this model the large ribosomal subunit is drawn as transparent to visualize tRNAs, factors, and mRNA binding to the decoding center at the interface between the large and small subunit. Throughout, GTP is depicted as a green ball and GDP as a red ball; also, the positions of the mRNA, tRNAs, and factors are drawn for clarity and are not meant to specify their exact places on the ribosome. On recognition of a stop codon, the eRF1:eRF3:GTP ternary complex binds to the A site of the ribosome in a preaccommodated state, GTP hydrolysis occurs, and eRF3 is released. ABCE1/Rli1 binds and facilitates the accommodation of eRF1 into an optimally active configuration.
EUKARYOTIC TRANSLATION RECYCLING (AND SOME CONNECTIONS TO REINITIATION)
Recycling is the process that takes place once the completed polypeptide chain has been released. At this stage, the 80S ribosome still is bound to the mRNA, the now deacylated tRNA, and likely the class I release factor eRF1. X-ray structures from the bacterial system indicate that both pre- and posttermination complexes are found in a preratcheted (or classical) configuration (Korostelev et al. 2008; Laurberg et al. 2008; Weixlbaumer et al. 2008; Jin et al. 2010) and so this seems likely also to be the case in the eukaryotic system. At this stage the ribosomal subunits must be dissociated and the mRNA and deacylated tRNA released to regenerate the necessary components for subsequent rounds of translation. In this section we describe recent studies that have greatly increased our understanding of these events in eukaryotes.
It is worth noting that in some cases full dissociation of the ribosomal complex will occur following termination, whereas in other cases partial dissolution of the complex will allow for a class of events that is loosely termed “reinitiation.” Historically, reinitiation is a term used to describe a process wherein ribosomes translate two or more ORFs in a transcript without undergoing complete recycling between these events (Kozak 1984). Incomplete recycling could potentially also take place at the termination codon of an mRNA containing a single ORF, allowing scanning along the 3′ untranslated region (UTR) and facilitating transfer of the 40S subunit to the 5′ UTR and a subsequent round of translation of the same ORF. What seems to be most firmly established is that PABP, eIF4G, and eIF4E interact with one another specifically (Tarun and Sachs 1996), thus potentially bringing into close proximity the 5′ and 3′ ends of an mRNA. As outlined by Hinnebusch and Lorsch (2012), however, the mechanistic implications of these interactions are not yet fully understood. Such binding interactions could serve mostly to protect the mRNA from degradation or could additionally be important in promoting translation, decay, or other processes (Bernstein et al. 1989; Sachs and Davis 1989; Jacobson 1996). Although the field has long discussed the potentiating role of these interactions in promoting translation, more recent studies (also discussed by Hinnebusch and Lorsch 2012) have provided evidence that closed-loop mRNA formation via the PABP-eIF4G interaction is nonessential in vivo (Tarun et al. 1997), and may serve a redundant function in recruiting eIF4F to mRNA during initiation (Park et al. 2011). It will be important moving forward to determine the particular biochemical benefits that are specified by communication between the 5′ and 3′ ends of an mRNA.
THE ELUSIVE RECYCLING FACTOR IN EUKARYOTES
Recycling is reasonably well defined in bacterial systems and involves a specialized factor, ribosome recycling factor (RRF), that interacts with the posttermination ribosome complex following the stimulated dissociation of the class I release factor (RF1 or RF2) by RF3. RRF interacts with a ratcheted state of the ribosome (with a deacylated tRNA bound in the P/E state) and destabilizes intersubunit bridging interactions (Gao et al. 2005; Dunkle et al. 2011). EF-G:GTP promotes subunit dissociation and IF3 binds to the resulting small ribosomal subunit to stabilize the dissociation event and promote release of the deacylated tRNA and the mRNA (Hirokawa et al. 2005; Peske et al. 2005; Zavialov et al. 2005). At this stage, the dissociated ribosomal subunits are ready for the next round of initiation.
In eukaryotes, there is no homolog of RRF, and, as discussed above, the termination factors are both structurally and mechanistically distinct from their equivalents in bacteria. Unlike in bacteria, eRF3 does not appear to promote the departure of the class I release factor eRF1 (a known biochemical role for RF3 [Freistroffer et al. 1997], and indeed, current evidence suggests that eRF1 remains associated with the ribosomal complex following termination (Pisarev et al. 2007). This posttermination complex containing bound eRF1 and a deacylated tRNA, potentially in an unratcheted state, is what must be targeted by the recycling machinery in eukaryotes. Although initial reports argued that eIF3 might play an active role in recycling in higher eukaryotes (Pisarev et al. 2007), the steady-state nature of these studies left open the possibility that eIF3 merely functioned to stabilize dissociated subunits by directly binding to the subunit interface. Such a view is consistent with a similar role for IF3 in bacteria and with the fact that eIF3 does not possess any intrinsic capacity for coupling energy to the process of subunit dissociation. Subsequent studies by several groups identified the multifunctional ABC-family protein ABCE1 found in eukaryotes and archaea as a likely candidate for promoting ribosomal recycling (Pisarev et al. 2010; Barthelme et al. 2011). This cytosolic ATPase is highly conserved throughout the eukaryotic kingdom (Kerr 2004; Dean and Annilo 2005) and is essential in all organisms tested (Dong et al. 2004; Zhao et al. 2004; Andersen and Leevers 2007).
HOW DOES RECYCLING ACTUALLY WORK?
Mechanistic insights into ribosome recycling recently came unexpectedly from studies of several proteins implicated in the no-go decay (NGD) pathway, through which mRNAs with translating ribosomes stalled on them are degraded. These two factors, Dom34 and Hbs1, are related to the eukaryotic termination factors eRF1 and eRF3, respectively, and appear to be important in triggering the NGD response (Doma and Parker 2006). Biochemical studies in reconstituted systems (Shoemaker et al. 2010; Pisareva et al. 2011) established that these factors bind to the A site of ribosomal complexes to promote subunit dissociation. Although Dom34 is related to eRF1, it lacks the codon recognition motif (NIKS) that is responsible for discriminating between sense and stop codons, and lacks the full extension of the M domain (with the GGQ motif) that promotes peptide release (Lee et al. 2007; Graille et al. 2008). Consistent with this structural view, the Dom34:Hbs1 complex promotes subunit dissociation (and not peptide release) in a codon-independent manner (Shoemaker et al. 2010). These ideas subsequently led to the demonstration that the termination factors themselves (eRF1 and eRF3) trigger slow rates of subunit dissociation (Shoemaker et al. 2010). These data make some sense given that earlier studies had shown that eRF1 is retained following the termination reaction (Pisarev et al. 2007) and is required for eukaryotic recycling (Pisarev et al. 2010).
What then does ABCE1 (Rli1 in yeast) do to promote ribosome recycling? Although the canonical release factors do appear to possess some intrinsic ribosome recycling activity, the addition of Rli1 to this reaction substantially increases the rate of the observed reaction in vitro (Pisarev et al. 2010; Shoemaker and Green 2011) and this activity depends on ATP hydrolysis. As for related ABC-family ATPases, ABCE1/Rli1 is proposed to somehow convert the chemical energy from ATP hydrolysis into mechanical motions that can separate the subunits. Similarly, the Dom34-dependent subunit dissociation activity is also substantially promoted (∼20-fold) by the presence of Rli1 (Shoemaker and Green 2011). Biochemical studies on the equivalent complex (Pelota:ABCE1) in a mammalian in vitro reconstituted system (Pisareva et al. 2011) are markedly consistent with these studies in yeast with Dom34:Rli1.
In addition to a role in recycling, Rli1 has also been shown to directly promote the rate of peptide release by eRF1:eRF3, in a manner that does not depend on ATP hydrolysis (Khoshnevis et al. 2010; Shoemaker and Green 2011). It seems reasonable to speculate that by promoting the release activity, Rli1 can help in staging the sequential events of termination and recycling. With both sets of factors, eRF1:eRF3 and Dom34:Hbs1, there are data to indicate that eRF3 or Hbs1 must be able to hydrolyze GTP in order for recycling to occur (Pisarev et al. 2010, Shoemaker et al. 2010; Pisareva et al. 2011; Shoemaker and Green 2011). Moreover, following GTP hydrolysis by these factors, their affinity for ribosomes is decreased and the factors are readily chased from the complex (Shoemaker and Green 2011). These data can be put together in a model (Fig. 2) in which under normal conditions, the eRF1:eRF3 complex recognizes stop codons and GTP hydrolysis by eRF3 permits departure of the GDP form of the factor (in a fashion akin to tRNA delivery by EF-Tu). Some form of accommodation takes place wherein the GGQ end of the release factor swings into the catalytic center of the large subunit. Peptide release is then catalyzed, stimulated by an ATP-independent activity of Rli1. Finally, ATP hydrolysis on Rli1 is coupled to subunit dissociation. Separated subunits then are bound by available initiation factors that prepare them for subsequent rounds of initiation or reinitiation (Pisarev et al. 2007). Deacylated tRNA and mRNA are likely dissociated from the isolated small subunits following recycling, with their departure enhanced by Ligatin (also known as eIF2D) and, to a lesser extent, by the pair of proteins MCT-1/DENR that are related in sequence to the different halves of Ligatin (Dmitriev et al. 2010; Skabkin et al. 2010). These factors may function by stabilizing the open state of the 40S subunit, from which tRNA would be expected to dissociate more rapidly. Release of tRNA and mRNA from recycled 40S subunits can also be stimulated in vitro by eIF1, eIF1A, and the j-subunit of eIF3 (Pisarev et al. 2007). It is currently unclear whether this mechanism involving conventional initiation factors or that involving Ligatin operates in vivo to complete the recycling process.
We note that the overall scheme we outline is similar in many ways to that for tRNA selection in bacteria, which is also facilitated by a G-protein factor, EF-Tu (Pape et al. 1998). The Dom34:Hbs1:Rli1 system shares many similarities with the tRNA selection pathway, although substrate recognition is less well understood. The most significant insights into substrate selection by these proteins came from studies showing a rather strict length dependence for the mRNA extending 3′ of the stall site on the ribosome; the less mRNA present in this position, the more potent the recycling by Pelota:Hbs1:ABCE1 in the mammalian system (Pisareva et al. 2011). These observations were broadly corroborated in the yeast system where it was further established that the length dependence was specifically conferred by the presence of Hbs1 (Shoemaker and Green 2011). The idea that NGD depends on recognition of truncated mRNAs is easily reconciled by literature indicating that the stalling of ribosomes during NGD (and also in nonstop decay [NSD]) eventually leads to endonucleolytic cleavage (Doma and Parker 2006). Once cleavage has occurred, 80S ribosomal complexes attached to truncated mRNAs are readily identified as targets for downstream events in NGD or NSD. Genome-wide approaches are likely to increase further our understanding of the structural features of ribosomal complexes that lead to the initiation of NGD.
STRUCTURAL INSIGHTS INTO EUKARYOTIC TERMINATION AND RECYCLING
Structural insights on termination and recycling have been significant during the past few years. Isolated partial structures of the complexed termination factors eRF1:eRF3 and their homologs Dom34:Hbs1 have both been determined recently (Cheng et al. 2009; Chen et al. 2010). The eRF1:eRF3 structure includes the full-length eRF1 species, but eRF3 lacks the nonessential amino-terminal domain as well as the GTPase domain; the Dom34:Hbs1 structure includes all domains of each protein. For both Schizosaccharomyces pombe and human eRF1:eRF3 complexes and for the Dom34:Hbs1 complex, the factors associate with each other via their carboxy-terminal domains. Moreover, in both cases, binding of the GTPase factors (eRF3/Hbs1) resulted in gross conformational changes in eRF1/Dom34, resulting in the latter more closely resembling the shape of a tRNA molecule.
Consistently, cryo-EM reconstructions show that eRF1:eRF3 and Dom34:Hbs1 bind to eukaryotic ribosomes in a manner similar to tRNA:eEF1A (Becker et al. 2011). The same mode of binding is also observed in archaea (Kobayashi et al. 2010), indicating conservation of these processes. Following GTP hydrolysis, either eRF3 or Hbs1 dissociates and Rli1/ABCE1 binds. The binding of this factor seems to facilitate the positioning of the central domain of Dom34 in the PTC (and thus by analogy, eRF1) (Becker et al. 2012); such positioning could readily explain the acceleration of peptide release promoted by Rli1 (Shoemaker and Green 2011). Subsequent ATP hydrolysis by Rli1 likely drives subunit dissociation, although a full molecular understanding of this process remains undefined. Beckmann and colleagues have further proposed that Rli1 drives Dom34 and/or eRF1 through the subunit interface region in a fashion similar to the movements of RRF promoted by EF-G in bacteria. If true, this motion could disrupt critical subunit bridges and lead directly to subunit splitting (Gao et al. 2005).
Interestingly, ABCE1/Rli1 was first studied in yeast as a factor involved in initiation. Rli1 is stably associated with the multifactor complex (MFC) free of ribosomes and with native 40S subunits in extracts. Depletion of the protein from cells leads to polysome runoff, characteristic of an initiation defect, and reduces association of MFC components with native 40S subunits without affecting MFC integrity. In addition, Rli1 promotes assembly of the 43S PIC (Dong et al. 2004), and results consistent with this conclusion were reported for mammalian ABCE1 (Chen et al. 2006). The defect in PIC formation might be an indirect consequence of defective recycling, although enrichment of larger rather than smaller polysomes would be expected from the failure to dissociate 80S posttermination complexes at stop codons, and the free 40S subunits in such cells should not be defective for MFC binding. Hence, an alternative view is that ABCE1/Rli1 operates at the interface between termination, recycling, and initiation by helping to recruit the MFC to the free 40S subunits generated by the recycling reaction. Rli1 also has been reported to function in ribosome biogenesis (Dong et al. 2004; Yarunin et al. 2005). It will be important to determine which of ABCE1/Rli1’s functions makes it an essential protein in vivo.
FUTURE DIRECTIONS
Many mechanistic questions remain to be addressed concerning elongation, termination, and recycling. For elongation, more in-depth kinetic analyses are needed to elucidate the function of eIF5A and its hypusine modification in translation initiation, elongation, and/or termination. To help define the role of eIF5A in elongation, it will be helpful to obtain additional insights regarding the timing of E-site tRNA release and eIF5A binding during the elongation cycle. Also, clarification of the role of eEF3 in fungal translation elongation may provide insights into bacterial and mammalian translation elongation that apparently lack a requirement for a comparable ATPase.
For termination, we lack substantial information on the communication between stop codon decoding and peptide release. Despite significant efforts, it remains to be fully determined how stop codons are recognized by eRF1 in the A site. Minimal binding energy is readily attributed to the relatively limited contacts thought to exist between the A-site codon and eRF1 in the decoding center, and yet these subtle differences in binding are somehow communicated to stimulate peptide release at an active site >75 Å away. Although direct comparison to the bacterial process is not warranted given the lack of conservation of the involved factors, the induced-fit mechanisms documented there (Youngman et al. 2007; He and Green 2010) likely play a similar role in the eukaryotic process. The effect of GTP hydrolysis on termination is also poorly understood. eRF1 alone can catalyze termination, but the rates of both termination and recycling are increased in the presence of eRF3. What conformational changes does eRF3 facilitate (either in eRF1 or the ribosome) that allow for more efficient catalysis? Furthermore, how does GTP hydrolysis by eRF3 (or Hbs1) commit or stage the ribosome for termination and coupling to recycling? These are some of the questions that will need to be addressed to decipher the mechanism of signal transduction between codon recognition, GTP hydrolysis, and peptide release in eukaryotes.
Finally, for recycling, how does Rli1/ABCE1 facilitate subunit dissociation? Rli1/ABCE1 contains two asymmetric ATPase sites and activity in at least one of these sites is required for cooperative function between Rli1 and eRF1 during recycling (Barthelme et al. 2011). The differential activity of these sites could be key in regulating the distinct functions of Rli1 in termination, recycling, and initiation. In vitro systems are well poised to address these questions, as many active site mutations in Rli1 are incompatible with in vivo analysis in this essential protein. It is worth noting that ABCE1/Rli1 is one of the few examples of an ATPase that directly engages the ribosome to promote a core translational event (the other being the fungal-specific elongation factor eEF3). Which other ATPases (e.g., Upf1 [Ghosh et al. 2010]) or factors (e.g., PABP [Hoshino et al. 1999; Cosson et al. 2002] or Tpa1 [Keeling et al. 2006]) in the cell might also directly engage the ribosome and translational GTPases to promote the core mechanisms of termination and recycling? Such insights will expand our view of the extensive network of interactions that dictate the translational output of the cell.
Footnotes
Editors: John W.B. Hershey, Nahum Sonenberg, and Michael B. Mathews
Additional Perspectives on Protein Synthesis and Translational Control available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Alkalaeva EZ, Pisarev AV, Frolova LY, Kisselev LL, Pestova TV 2006. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell 125: 1125–1136 [DOI] [PubMed] [Google Scholar]
- Anand M, Chakraburtty K, Marton MJ, Hinnebusch AG, Kinzy TG 2003. Functional interactions between yeast translation eukaryotic elongation factor (eEF) 1A and eEF3. J Biol Chem 278: 6985–6991 [DOI] [PubMed] [Google Scholar]
- Anand M, Balar B, Ulloque R, Gross SR, Kinzy TG 2006. Domain and nucleotide dependence of the interaction between Saccharomyces cerevisiae translation elongation factors 3 and 1A. J Biol Chem 281: 32318–32326 [DOI] [PubMed] [Google Scholar]
- Andersen DS, Leevers SJ 2007. The essential Drosophila ATP-binding cassette domain protein, pixie, binds the 40 S ribosome in an ATP-dependent manner and is required for translation initiation. J Biol Chem 282: 14752–14760 [DOI] [PubMed] [Google Scholar]
- Andersen CB, Becker T, Blau M, Anand M, Halic M, Balar B, Mielke T, Boesen T, Pedersen JS, Spahn CM, et al. 2006. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature 443: 663–668 [DOI] [PubMed] [Google Scholar]
- Atkinson GC, Baldauf SL, Hauryliuk V 2008. Evolution of nonstop, no-go and nonsense-mediated mRNA decay and their termination factor-derived components. BMC Evol Biol 8: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly M, de Crecy-Lagard V 2010. Predicting the pathway involved in post-translational modification of elongation factor P in a subset of bacterial species. Biol Direct 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal V, Parker R 2011. Stm1 modulates translation after 80S formation in Saccharomyces cerevisiae. RNA 17: 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelme D, Dinkelaker S, Albers SV, Londei P, Ermler U, Tampe R 2011. Ribosome recycling depends on a mechanistic link between the FeS cluster domain and a conformational switch of the twin-ATPase ABCE1. Proc Natl Acad Sci 108: 3228–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Armache JP, Jarasch A, Anger AM, Villa E, Sieber H, Motaal BA, Mielke T, Berninghausen O, Beckmann R 2011. Structure of the no-go mRNA decay complex Dom34-Hbs1 bound to a stalled 80S ribosome. Nat Struct Mol Biol 18: 715–720 [DOI] [PubMed] [Google Scholar]
- Becker T, Franckenberg S, Wickles S, Shoemaker CJ, Anger AM, Armache JP, Sieber H, Ungewickell C, Berninghausen O, Daberkow I, et al. 2012. Structural basis of highly conserved ribosome recycling in eukaryotes and archaea. Nature 482: 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A, Jenner L, Yusupova G, Yusupov M 2010. Crystal structure of the eukaryotic ribosome. Science 330: 1203–1209 [DOI] [PubMed] [Google Scholar]
- Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M 2011. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334: 1524–1529 [DOI] [PubMed] [Google Scholar]
- Bernstein P, Peltz SW, Ross J 1989. The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol Cell Biol 9: 659–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha G, Stanley RE, Steitz TA 2009. Formation of the first peptide bond: The structure of EF-P bound to the 70S ribosome. Science 325: 966–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulygin KN, Khairulina YS, Kolosov PM, Ven’yaminova AG, Graifer DM, Vorobjev YN, Frolova LY, Kisselev LL, Karpova GG 2010. Three distinct peptides from the N domain of translation termination factor eRF1 surround stop codon in the ribosome. RNA 16: 1902–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg U, Nilsson A, Nygard O 1990. Functional properties of phosphorylated elongation factor 2. Eur J Biochem 191: 639–645 [DOI] [PubMed] [Google Scholar]
- Chavatte L, Seit-Nebi A, Dubovaya V, Favre A 2002. The invariant uridine of stop codons contacts the conserved NIKSR loop of human eRF1 in the ribosome. EMBO J 21: 5302–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Behringer RR 2004. Ovca1 regulates cell proliferation, embryonic development, and tumorigenesis. Genes Dev 18: 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZQ, Dong J, Ishimura A, Daar I, Hinnebusch AG, Dean M 2006. The essential vertebrate ABCE1 protein interacts with eukaryotic initiation factors. J Biol Chem 281: 7452–7457 [DOI] [PubMed] [Google Scholar]
- Chen L, Muhlrad D, Hauryliuk V, Cheng Z, Lim MK, Shyp V, Parker R, Song H 2010. Structure of the Dom34-Hbs1 complex and implications for no-go decay. Nat Struct Mol Biol 17: 1233–1240 [DOI] [PubMed] [Google Scholar]
- Chen C, Stevens B, Kaur J, Smilansky Z, Cooperman BS, Goldman YE 2011. Allosteric vs. spontaneous exit-site (E-site) tRNA dissociation early in protein synthesis. Proc Natl Acad Sci 108: 16980–16985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Saito K, Pisarev AV, Wada M, Pisareva VP, Pestova TV, Gajda M, Round A, Kong C, Lim M, et al. 2009. Structural insights into eRF3 and stop codon recognition by eRF1. Genes Dev 23: 1106–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson B, Couturier A, Chabelskaya S, Kiktev D, Inge-Vechtomov S, Philippe M, Zhouravleva G 2002. Poly(A)-binding protein acts in translation termination via eukaryotic release factor 3 interaction and does not influence [PSI+] propagation. Mol Cell Biol 22: 3301–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Annilo T 2005. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Ann Rev Genomics Hum Genet 6: 123–142 [DOI] [PubMed] [Google Scholar]
- Dias CA, Gregio AP, Rossi D, Galvao FC, Watanabe TF, Park MH, Valentini SR, Zanelli CF 2012. eIF5A interacts functionally with eEF2. Amino Acids 42: 697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev SE, Terenin IM, Andreev DE, Ivanov PA, Dunaevsky JE, Merrick WC, Shatsky IN 2010. GTP-independent tRNA delivery to the ribosomal P-site by a novel eukaryotic translation factor. J Biol Chem 285: 26779–26787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doma MK, Parker R 2006. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440: 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Lai R, Nielsen K, Fekete CA, Qiu H, Hinnebusch AG 2004. The essential ATP-binding cassette protein RLI1 functions in translation by promoting preinitiation complex assembly. J Biol Chem 279: 42157–42168 [DOI] [PubMed] [Google Scholar]
- Dunkle JA, Wang L, Feldman MB, Pulk A, Chen VB, Kapral GJ, Noeske J, Richardson JS, Blanchard SC, Cate JH 2011. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332: 981–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler DE, Green R 2011. Distinct response of yeast ribosomes to a miscoding event during translation. RNA 17: 925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan-Minogue H, Du M, Pisarev AV, Kallmeyer AK, Salas-Marco J, Keeling KM, Thompson SR, Pestova TV, Bedwell DM 2008. Distinct eRF3 requirements suggest alternate eRF1 conformations mediate peptide release during eukaryotic translation termination. Mol Cell 30: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistroffer DV, Pavlov MY, MacDougall J, Buckingham RH, Ehrenberg M 1997. Release factor RF3 in E. coli accelerates the dissociation of release factors RF1 and RF2 from the ribosome in a GTP-dependent manner. EMBO J 16: 4126–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova L, Le Goff X, Zhouravleva G, Davydova E, Philippe M, Kisselev L 1996. Eukaryotic polypeptide chain release factor eRF3 is an eRF1- and ribosome-dependent guanosine triphosphatase. RNA 2: 334–341 [PMC free article] [PubMed] [Google Scholar]
- Frolova LY, Tsivkovskii RY, Sivolobova GF, Oparina NY, Serpinsky OI, Blinov VM, Tatkov SI, Kisselev LL 1999. Mutations in the highly conserved GGQ motif of class 1 polypeptide release factors abolish ability of human eRF1 to trigger peptidyl-tRNA hydrolysis. RNA 5: 1014–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Zavialov AV, Li W, Sengupta J, Valle M, Gursky RP, Ehrenberg M, Frank J 2005. Mechanism for the disassembly of the posttermination complex inferred from cryo-EM studies. Mol Cell 18: 663–674 [DOI] [PubMed] [Google Scholar]
- Gao YG, Selmer M, Dunham CM, Weixlbaumer A, Kelley AC, Ramakrishnan V 2009. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science 326: 694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Ganesan R, Amrani N, Jacobson A 2010. Translational competence of ribosomes released from a premature termination codon is modulated by NMD factors. RNA 16: 1832–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR, Ganoza MC 1975. Identification of a soluble protein that stimulates peptide bond synthesis. Proc Natl Acad Sci 72: 4257–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graille M, Chaillet M, van Tilbeurgh H 2008. Structure of yeast Dom34: A protein related to translation termination factor Erf1 and involved in No-Go decay. J Biol Chem 283: 7145–7154 [DOI] [PubMed] [Google Scholar]
- Gregio AP, Cano VP, Avaca JS, Valentini SR, Zanelli CF 2009. eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun 380: 785–790 [DOI] [PubMed] [Google Scholar]
- He SL, Green R 2010. Visualization of codon-dependent conformational rearrangements during translation termination. Nat Struct Mol Biol 17: 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A, Hershey JW 2011. Eukaryotic translation initiation factor (eIF) 5A stimulates protein synthesis in Saccharomyces cerevisiae. Proc Natl Acad Sci 108: 6415–6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hinnebusch AG, Lorsch JR 2012. The mechanism of eukaryotic translation initiation: New insights and challenges. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a011544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa G, Nijman RM, Raj VS, Kaji H, Igarashi K, Kaji A 2005. The role of ribosome recycling factor in dissociation of 70S ribosomes into subunits. RNA 11: 1317–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino S, Imai M, Kobayashi T, Uchida N, Katada T 1999. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-Poly(A) tail of mRNA. Direct association of eRF3/GSPT with polyadenylate-binding protein. J Biol Chem 274: 16677–16680 [DOI] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS 2009. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324: 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov PV, Gehring NH, Kunz JB, Hentze MW, Kulozik AE 2008. Interactions between UPF1, eRFs, PABP and the exon junction complex suggest an integrated model for mammalian NMD pathways. EMBO J 27: 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A 1996. Poly(A) metabolism and translation: The closed-looped model. In Translational control (ed. Hershey JWB, Mathews M, Sonenberg N), pp. 505–548 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Jao DL, Chen KY 2006. Tandem affinity purification revealed the hypusine-dependent binding of eukaryotic initiation factor 5A to the translating 80S ribosomal complex. J Cell Biochem 97: 583–598 [DOI] [PubMed] [Google Scholar]
- Jenner L, Demeshkina N, Yusupova G, Yusupov M 2010. Structural rearrangements of the ribosome at the tRNA proofreading step. Nat Struct Mol Biol 17: 1072–1078 [DOI] [PubMed] [Google Scholar]
- Jin H, Kelley AC, Loakes D, Ramakrishnan V 2010. Structure of the 70S ribosome bound to release factor 2 and a substrate analog provides insights into catalysis of peptide release. Proc Natl Acad Sci 107: 8593–8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HA, Hershey JW 1994. Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J Biol Chem 269: 3934–3940 [PubMed] [Google Scholar]
- Keeling KM, Salas-Marco J, Osherovich LZ, Bedwell DM 2006. Tpa1p is part of an mRNP complex that influences translation termination, mRNA deadenylation, and mRNA turnover in Saccharomyces cerevisiae. Mol Cell Biol 26: 5237–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper WM, Berry KW, Merrick WC 1976. Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Bα and M2Bβ. J Biol Chem 251: 5551–5557 [PubMed] [Google Scholar]
- Kerr ID 2004. Sequence analysis of twin ATP binding cassette proteins involved in translational control, antibiotic resistance, and ribonuclease L inhibition. Biochem Biophys Res Commun 315: 166–173 [DOI] [PubMed] [Google Scholar]
- Khoshnevis S, Gross T, Rotte C, Baierlein C, Ficner R, Krebber H 2010. The iron-sulphur protein RNase L inhibitor functions in translation termination. EMBO Rep 11: 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Hung LW, Yokota H, Kim R, Kim SH 1998. Crystal structures of eukaryotic translation initiation factor 5A from Methanococcus jannaschii at 1.8 Å resolution. Proc Natl Acad Sci 95: 10419–10424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y, Kohno K 1994. Elongation factor 2 mutants deficient in diphthamide formation show temperature-sensitive cell growth. J Biol Chem 269: 13497–13501 [PubMed] [Google Scholar]
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Arpagaus S, Ban N 2011. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334: 941–948 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kikuno I, Kuroha K, Saito K, Ito K, Ishitani R, Inada T, Nureki O 2010. Structural basis for mRNA surveillance by archaeal Pelota and GTP-bound EF1α complex. Proc Natl Acad Sci 107: 17575–17579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosov P, Frolova L, Seit-Nebi A, Dubovaya V, Kononenko A, Oparina N, Justesen J, Efimov A, Kisselev L 2005. Invariant amino acids essential for decoding function of polypeptide release factor eRF1. Nucleic Acids Res 33: 6418–6425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko AV, Mitkevich VA, Dubovaya VI, Kolosov PM, Makarov AA, Kisselev LL 2008. Role of the individual domains of translation termination factor eRF1 in GTP binding to eRF3. Proteins 70: 388–393 [DOI] [PubMed] [Google Scholar]
- Korostelev A, Asahara H, Lancaster L, Laurberg M, Hirschi A, Zhu J, Trakhanov S, Scott WG, Noller HF 2008. Crystal structure of a translation termination complex formed with release factor RF2. Proc Natl Acad Sci 105: 19684–19689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M 1984. Selection of initiation sites by eucaryotic ribosomes: Effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res 12: 3873–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurberg M, Asahara H, Korostelev A, Zhu J, Trakhanov S, Noller HF 2008. Structural basis for translation termination on the 70S ribosome. Nature 454: 852–857 [DOI] [PubMed] [Google Scholar]
- Lee HH, Kim YS, Kim KH, Heo I, Kim SK, Kim O, Kim HK, Yoon JY, Kim HS, Kim do J, et al. 2007. Structural and functional insights into Dom34, a key component of no-go mRNA decay. Mol Cell 27: 938–950 [DOI] [PubMed] [Google Scholar]
- Liu S, Milne GT, Kuremsky JG, Fink GR, Leppla SH 2004. Identification of the proteins required for biosynthesis of diphthamide, the target of bacterial ADP-ribosylating toxins on translation elongation factor 2. Mol Cell Biol 24: 9487–9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wiggins JF, Sreenath T, Kulkarni AB, Ward JM, Leppla SH 2006. Dph3, a small protein required for diphthamide biosynthesis, is essential in mouse development. Mol Cell Biol 26: 3835–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkulova TI, Frolova LY, Lazar M, Camonis J, Kisselev LL 1999. C-terminal domains of human translation termination factors eRF1 and eRF3 mediate their in vivo interaction. FEBS Lett 443: 41–47 [DOI] [PubMed] [Google Scholar]
- Navarre WW, Zou SB, Roy H, Xie JL, Savchenko A, Singer A, Edvokimova E, Prost LR, Kumar R, Ibba M, et al. 2010. PoxA, yjeK, and elongation factor P coordinately modulate virulence and drug resistance in Salmonella enterica. Mol Cell 39: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus KH 1990. The allosteric three-site model for the ribosomal elongation cycle: Features and future. Biochemistry 29: 4997–5008 [DOI] [PubMed] [Google Scholar]
- Ogle JM, Brodersen DE, Clemons WM Jr, Tarry MJ, Carter AP, Ramakrishnan V 2001. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292: 897–902 [DOI] [PubMed] [Google Scholar]
- Ortiz PA, Ulloque R, Kihara GK, Zheng H, Kinzy TG 2006. Translation elongation factor 2 anticodon mimicry domain mutants affect fidelity and diphtheria toxin resistance. J Biol Chem 281: 32639–32648 [DOI] [PubMed] [Google Scholar]
- Pape T, Wintermeyer W, Rodnina MV 1998. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J 17: 7490–7497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Wolff EC, Smit-McBride Z, Hershey JW, Folk JE 1991. Comparison of the activities of variant forms of eIF-4D. The requirement for hypusine or deoxyhypusine. J Biol Chem 266: 7988–7994 [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. 2008. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59: 70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Nishimura K, Zanelli CF, Valentini SR 2010. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38: 491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EH, Zhang F, Warringer J, Sunnerhagen P, Hinnebusch AG 2011. Depletion of eIF4G from yeast cells narrows the range of translational efficiencies genome-wide. BMC Genomics 12: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Johansson HE, Aoki H, Huang B, Kim HY, Ganoza MC, Park MH 2012. Post-translational modification by β-lysylation is required for the activity of E. coli elongation factor P (EF-P). J Biol Chem 287: 2579–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD 1996. Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J 15: 3127–3134 [PMC free article] [PubMed] [Google Scholar]
- Peske F, Rodnina MV, Wintermeyer W 2005. Sequence of steps in ribosome recycling as defined by kinetic analysis. Mol Cell 18: 403–412 [DOI] [PubMed] [Google Scholar]
- Pisarev AV, Hellen CU, Pestova TV 2007. Recycling of eukaryotic posttermination ribosomal complexes. Cell 131: 286–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarev AV, Skabkin MA, Pisareva VP, Skabkina OV, Rakotondrafara AM, Hentze MW, Hellen CU, Pestova TV 2010. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol Cell 37: 196–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisareva VP, Pisarev AV, Hellen CU, Rodnina MV, Pestova TV 2006. Kinetic analysis of interaction of eukaryotic release factor 3 with guanine nucleotides. J Biol Chem 281: 40224–40235 [DOI] [PubMed] [Google Scholar]
- Pisareva VP, Skabkin MA, Hellen CU, Pestova TV, Pisarev AV 2011. Dissociation by Pelota, Hbs1 and ABCE1 of mammalian vacant 80S ribosomes and stalled elongation complexes. EMBO J 30: 1804–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnina MV, Wintermeyer W 2009. Recent mechanistic insights into eukaryotic ribosomes. Curr Opin Cell Biol 21: 435–443 [DOI] [PubMed] [Google Scholar]
- Sachs AB, Davis RW 1989. The Poly(A) binding protein is required for Poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell 58: 857–867 [DOI] [PubMed] [Google Scholar]
- Saini P, Eyler DE, Green R, Dever TE 2009. Hypusine-containing protein eIF5A promotes translation elongation. Nature 459: 118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kobayashi K, Wada M, Kikuno I, Takusagawa A, Mochizuki M, Uchiumi T, Ishitani R, Nureki O, Ito K 2010. Omnipotent role of archaeal elongation factor 1α (EF1α) in translational elongation and termination, and quality control of protein synthesis. Proc Natl Acad Sci 107: 19242–19247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Marco J, Bedwell DM 2005. Discrimination between defects in elongation fidelity and termination efficiency provides mechanistic insights into translational readthrough. J Mol Biol 348: 801–815 [DOI] [PubMed] [Google Scholar]
- Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FVt, Weir JR, Ramakrishnan V 2009. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 326: 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeing TM, Voorhees RM, Kelley AC, Ramakrishnan V 2011. How mutations in tRNA distant from the anticodon affect the fidelity of decoding. Nat Struct Mol Biol 18: 432–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier MH, Erni B, Staehelin T 1977. Initiation of mammalian protein synthesis: Purification and characterization of seven initiation factors. J Mol Biol 116: 727–753 [DOI] [PubMed] [Google Scholar]
- Semenkov YP, Rodnina MV, Wintermeyer W 1996. The “allosteric three-site model” of elongation cannot be confirmed in a well-defined ribosome system from Escherichia coli. Proc Natl Acad Sci 93: 12183–12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker CJ, Green R 2011. Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc Natl Acad Sci 108: E1392–E1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker CJ, Eyler DE, Green R 2010. Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science 330: 369–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan G, Aviner R, Elroy-Stein O 2011. Mitotic modulation of translation elongation factor 1 leads to hindered tRNA delivery to ribosomes. J Biol Chem 286: 27927–27935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skabkin MA, Skabkina OV, Dhote V, Komar AA, Hellen CU, Pestova TV 2010. Activities of Ligatin and MCT-1/DENR in eukaryotic translation initiation and ribosomal recycling. Genes Dev 24: 1787–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogerson L, Engelhardt D 1977. Dissimilarity in protein chain elongation factor requirements between yeast and rat liver ribosomes. J Biol Chem 252: 1471–1475 [PubMed] [Google Scholar]
- Song H, Mugnier P, Das AK, Webb HM, Evans DR, Tuite MF, Hemmings BA, Barford D 2000. The crystal structure of human eukaryotic release factor eRF1-mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell 100: 311–321 [DOI] [PubMed] [Google Scholar]
- Stansfield I, Jones KM, Kushnirov VV, Dagkesamanskaya AR, Poznyakovski AI, Paushkin SV, Nierras CR, Cox BS, Ter-Avanesyan MD, Tuite MF 1995. The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J 14: 4365–4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarun SZ, Sachs AB 1996. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J 15: 7168–7177 [PMC free article] [PubMed] [Google Scholar]
- Tarun SZ, Wells SE, Deardorff JA, Sachs AB 1997. Translation initiation factor eIF4G mediates in vitro poly (A) tail-dependent translation. Proc Natl Acad Sci 94: 9046–9051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Avanesyan MD, Kushnirov VV, Dagkesamanskaya AR, Didichenko SA, Chernoff YO, Inge-Vechtomov SG, Smirnov VN 1993. Deletion analysis of the SUP35 gene of the yeast Saccharomyces cerevisiae reveals two non-overlapping functional regions in the encoded protein. Mol Microbiol 7: 683–692 [DOI] [PubMed] [Google Scholar]
- Triana-Alonso FJ, Chakraburtty K, Nierhaus KH 1995. The elongation factor unique in higher fungi and essential for protein biosynthesis is an E site factor. J Biol Chem 270: 20473–20478 [DOI] [PubMed] [Google Scholar]
- Uemura S, Aitken CE, Korlach J, Flusberg BA, Turner SW, Puglisi JD 2010. Real-time tRNA transit on single translating ribosomes at codon resolution. Nature 464: 1012–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke N, Pickering BF, Van Dyke MW 2009. Stm1p alters the ribosome association of eukaryotic elongation factor 3 and affects translation elongation. Nucleic Acids Res 37: 6116–6125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V 2010. The mechanism for activation of GTP hydrolysis on the ribosome. Science 330: 835–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden WE, Thach RE 1986. Translational control of gene expression in a normal fibroblast. Characterization of a subclass of mRNAs with unusual kinetic properties. Biochemistry 25: 2033–2041 [DOI] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM 2008. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59: 84–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TR, Cross SH, McKie L, Edgar R, Vizor L, Harrison J, Peters J, Jackson IJ 2008. Diphthamide modification of eEF2 requires a J-domain protein and is essential for normal development. J Cell Sci 121: 3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weixlbaumer A, Jin H, Neubauer C, Voorhees RM, Petry S, Kelley AC, Ramakrishnan V 2008. Insights into translational termination from the structure of RF2 bound to the ribosome. Science 322: 953–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T, Sumida T, Ishii R, Takemoto C, Yokoyama S 2010. A paralog of lysyl-tRNA synthetase aminoacylates a conserved lysine residue in translation elongation factor P. Nat Struct Mol Biol 17: 1136–1143 [DOI] [PubMed] [Google Scholar]
- Yarunin A, Panse VG, Petfalski E, Dez C, Tollervey D, Hurt EC 2005. Functional link between ribosome formation and biogenesis of iron-sulfur proteins. EMBO J 24: 580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman EM, He SL, Nikstad LJ, Green R 2007. Stop codon recognition by release factors induces structural rearrangement of the ribosomal decoding center that is productive for peptide release. Mol Cell 28: 533–543 [DOI] [PubMed] [Google Scholar]
- Zaher HS, Green R 2011. A primary role for release factor 3 in quality control during translation elongation in Escherichia coli. Cell 147: 396–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli CF, Maragno AL, Gregio AP, Komili S, Pandolfi JR, Mestriner CA, Lustri WR, Valentini SR 2006. eIF5A binds to translational machinery components and affects translation in yeast. Biochem Biophys Res Commun 348: 1358–1366 [DOI] [PubMed] [Google Scholar]
- Zavialov AV, Hauryliuk VV, Ehrenberg M 2005. Splitting of the posttermination ribosome into subunits by the concerted action of RRF and EF-G. Mol Cell 18: 675–686 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fang LL, Johnsen R, Baillie DL 2004. ATP-binding cassette protein E is involved in gene transcription and translation in Caenorhabditis elegans. Biochem Biophys Res Commun 323: 104–111 [DOI] [PubMed] [Google Scholar]
- Zhouravleva G, Frolova L, Le Goff X, Le Guellec R, Inge-Vechtomov S, Kisselev L, Philippe M 1995. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J 14: 4065–4072 [DOI] [PMC free article] [PubMed] [Google Scholar]