Abstract
Purpose.
The current treatments against inflammatory angiogenesis are steroids and anti-VEGF-A, such as dexamethasone and bevacizumab, respectively. However, the therapeutic windows for dexamethasone and bevacizumab against inflammatory angiogenesis are unknown.
Methods.
To investigate the therapeutic windows for dexamethasone and bevacizumab, we used the corneal pocket assay. IL-1β pellets were implanted in corneas of BALB/c mice that were then treated with dexamethasone or bevacizumab at different time points. Angiogenesis (area, number of vessels, and sprouting) was quantitated at various time points after implantation. Nuclear Factor-κB (NF-κB) signaling and leukocyte accumulation in inflammatory angiogenesis were examined by Western blotting, by immunohistochemistry, and in the authors' novel leukocyte transmigration assay.
Results.
Dexamethasone inhibited IL-1β–induced angiogenesis when treatment started 4 days after IL-1β implantation, while bevacizumab only inhibited angiogenesis by day 2 after implantation. Both bevacizumab and dexamethasone inhibited angiogenic sprouting. Interestingly, bevacizumab did not affect NF-κB activation, which is one of the main signaling targets for steroid action. The authors' new imaging approach revealed that bevacizumab and steroid treatment blocked leukocyte infiltration into implanted corneas.
Conclusions.
VEGF-A inhibition affected angiogenic sprouting, while it was not effective against matured vessels. Both dexamethasone and bevacizumab inhibited leukocyte transmigration from angiogenic vessels; however, dexamethasone had a larger therapeutic window. These insights improve the treatment strategy in angiogenic disorders.
Steroids and anti-VEGF agents are standard in the treatment of inflammatory angiogenic diseases; however, the effective window for these agents is unknown. This work provided novel insights that will help in optimizing treatments and protecting patients from overtreatment.
Introduction
Angiogenesis in the eye is the main cause of blindness in diseases such as age-related macular degeneration (AMD), diabetic retinopathy (DR), retinopathy of prematurity, or keratitis.
In studies of angiogenesis, treatment is often applied at the same time as the stimulus that causes angiogenesis. However, in the clinic, the antiangiogenic therapy usually starts at a later time, when pathology is established. Therefore, it is critical to understand the timing at which and the phase in which these therapies are effective. Experimental results that compare the timing of the anti-inflammatory or angiostatic therapies are scarce.
Inflammatory angiogenesis is primarily treated with steroids, which inhibit several pathways.1,2 Recently a study from this group showed that angiostatic steroids inhibit inflammatory angiogenesis by affecting Nuclear Factor-κB (NF-κB) signaling, as well as CD11b(+) cell infiltration. When applied at the same time, steroids inhibit VEGF-A and other angiogenic factors, such as CXC chemokines.2 Whether application of steroids at later time points is effective has not been examined.
Bevacizumab (Avastin) is a humanized anti-VEGF-A monoclonal antibody that is used for treatments of human cancer and ocular angiogenic diseases.3,4 Bevacizumab also inhibits inflammatory angiogenesis, with infrequent side effects.5 The frequent use of VEGF inhibitor in cancer or ocular angiogenic disorder has advanced our understanding of its actions as well as adverse effects.6 Some tumor cases are refractory against anti-VEGF-A therapy with variable efficacy.7–9 However, the efficacy of anti-VEGF-A therapy, or whether it induces drug resistance in inflammatory angiogenesis, is unknown.
Angiogenesis is a complex and highly regulated process that includes the steps of sprouting, maintenance, and regression. Distinct molecules play important roles in each of these angiogenic steps. In a mouse model of multistage tumorigenesis, distinct antiangiogenic drugs are effective at different stages of tumor angiogenesis.10 During vessel maturation, various angiogenic factors control sprouting, pruning, and maturation.11 These data indicate that the vascular phenotype undergoes dynamic changes during angiogenesis. However, the efficacy of angiostatic therapy in inflammatory angiogenesis is not well explored.
Materials and Methods
Animals
All animal experiments were approved by the Animal Care Committee of the Massachusetts Eye and Ear Infirmary. Male 6- to 10-week-old BALB/cN mice were purchased from Taconic (BALB) (Hudson, NY) or Kyudo Co., Ltd. (Saga, Japan). All animal experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Corneal Micropocket Assay in Mice
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). Poly-HEMA pellets (0.3 μL, P3932; Sigma Chemical Co., St. Louis, MO) containing 30 ng IL-1β (401 mL; R&D Systems, Minneapolis, MN) were prepared and implanted into the corneas. IL-1β pellets were positioned at approximately 1-mm distance to the corneal limbus. After implantation, bacitracin ophthalmic ointment (E. Fougera & Co., Melville, NY) was applied to each eye to prevent infection. Dexamethasone (5 mg/kg) (D2915; Sigma) was injected intraperitoneally daily, starting 2, 4, or 6 days after implantation, and continued until the 13th day. Bevacizumab (5 mg/mL) or dexamethasone (0.1% Santeson ophthalmic solution; Santen, Osaka, Japan) was topically applied to the eyes four times a day. Two, 4, 6, 10, and 14 days after the implantation, digital images of the corneal vessels were obtained and recorded using OpenLab software version 2.2.5 (Improvision Inc., Lexington, MA) with standardized illumination and contrast. The quantitative analysis of neovascularization in the mouse corneas was performed using Scion Image software (version 4.0.2; Scion Corp., Frederick, MD).
Whole-Mount Immunofluorescence
The whole-mount immunohistochemistry for implanted corneas has been previously described.12,13 After vascular endothelial cells were stained with 10 μg/mL rhodamine-labeled ConA perfusion (RL-1002; Vector Laboratories, Burlingame, CA), the animals' eyes were enucleated and fixed with 4% paraformaldehyde for 30 minutes at 4°C. For whole-mount preparation, the corneas were microsurgically exposed by removing other portions of the eye. Radial cuts were then made in the cornea. Tissues were washed with PBS three times for 5 minutes and then placed in methanol for 20 minutes. Tissues were incubated overnight at 4°C with anti-mouse CD31 mAb (5 μg/mL, 550,274; BD Pharmingen, San Diego, CA) in PBS containing 10% goat serum and 1% Triton X-100. Tissues were washed four times for 20 minutes in PBS followed by incubation with Alexa Fluor 488 goat anti-rat IgG (20 μg/mL, A11006; Invitrogen, Carlsbad, CA) overnight at 4°C. Corneal flat mounts were prepared on glass slides using a mounting medium (TA-030-FM, Mountant Permafluor; Lab Vision Corporation, Fremont, CA). The flat mounts were examined by fluorescence microscopy, and digital images were recorded using OpenLab software (version 2.2.5; Improvision Inc.) with standardized illumination and contrast. The portion of neovasculature tip at the indicated days after the cytokine implantation was calculated as the following ratio: distance from angiogenic tip to limbal vessel/distance from bottom portion of the implanted pellets to limbal vessel.
Western Blot Analysis
At day 3 after 30 ng IL-1β implantation, corneas in mice treated with dexamethasone, IgG, or bevacizumab from day 2 were harvested and lysed in a mammalian cell lysis kit (MCL1; Sigma) containing phosphatase Inhibitor Cocktail 1 (P2850; Sigma) and phosphatase Inhibitor Cocktail 2 (P2850; Sigma). Blots were incubated with anti-IκB-α (1:1000, 9242; Cell Signaling), anti-phospho-IκB-α (1:1000, 9241; Cell Signaling, Beverly, MA), or anti-ß-tubulin (1:1000, ab11308; Abcam, Cambridge, UK) and visualized with a secondary antibody coupled to horseradish peroxidase (Amersham, Arlington Heights, IL) and an enhanced chemiluminescence system.
Immunohistochemistry
The eyes were harvested and snap-frozen in optimal cutting temperature (OCT) compound (Sakura Finetechnical, Tokyo, Japan). Sections (10 μm) were prepared, air-dried, and fixed in ice-cold acetone for 10 minutes. The sections were blocked with 3% Nonfat-Dried Milk bovine working solution (M7409; Sigma) and stained with anti-mouse CD11b (1:50, 550,282; BD Pharmingen). After an overnight incubation, sections were washed and stained for 20 minutes with Alexa Fluor 488 goat anti-rabbit IgG (10 μg/mL, A11034; Invitrogen).
Ex Vivo Leukocyte Transmigration Assay
The experimental mice were anesthetized at the indicated day after cytokine implantation. Five hundred microliters acridine orange (AO) (0.2 mg/mL) was injected intravenously. The animals were perfused with 10 μg/mL rhodamine-labeled ConA lectin in PBS (pH 7.4) at 2 hours after AO injection. ConA was used to label vascular endothelial cells and leukocytes in blood vessels. Under deep anesthesia, the chest cavity was opened and a 24-gauge perfusion needle was placed into the aorta. Drainage was achieved by opening the right atrium. The animals were then perfused with 10 mL PBS to wash out blood cells in the vessels. After PBS perfusion, the animals were perfused with 5 mL rhodamine-labeled ConA; residual unbound Con A was removed with 1 mL PBS. Immediately after perfusion, the corneas were carefully removed and flat mounts prepared using a mounting medium (TA-030-FM, Mountant Permafluor; Lab Vision Corporation). Transmigrated leukocytes in inflamed areas (800 μm × 800 μm) around implanted pellets were counted manually.
Statistical Analysis
All values are expressed as mean ± SEM. Student's t-test was used for statistical analysis. Differences between the experimental groups were considered statistically significant or highly significant when the probability value, P, was <0.05 or <0.01, respectively.
Results
Time Course of Inflammatory Angiogenic Phenotypes
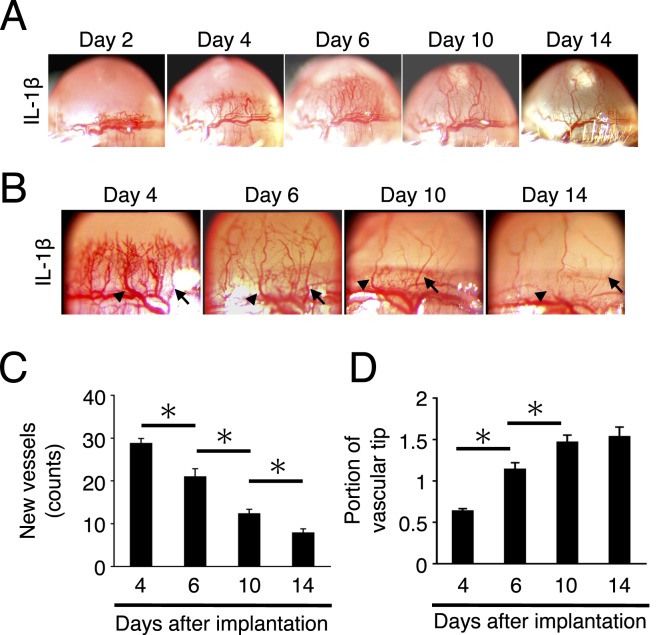
The time course of IL-1β–induced corneal angiogenesis was examined until day 14. In line with a prior report from this group,14 on day 2 limbus vessels dilated, and on day 4 the sprouting new vessels reached the site of pellet implantation (Fig. 1A). Main trunks of these angiogenic vessels kept on growing beyond the bottom of the pellet until day 14. The regression of some branches started from day 4, and some small vessels lost bloodstreams around day 10 (Fig. 1B). These ghost vessels were not visibly perfused when examined by slit lamp on days 10 and 14 (Fig. 1B). Quantitative analysis showed that the number of corneal vessels reached a maximum on day 4 and then decreased until day 14 (Fig. 1C). The portion of neovascular tips reached around 1.5-fold height compared with the bottom portion of the implanted pellets (Fig. 1D). These data indicate that vascular sprouting occurred on day 2, followed by vascular pruning that started after day 4, and then the vessels keep on growing toward the IL-1β pellets until day 10 to 14.
Figure 1. .
Time course of inflammatory angiogenesis. (A) Photomicrographs of corneas of BALB/c mice on days 2, 4, 6, 10, and 14 after IL-1β implantation. (B) Neovascularization in IL-1β–implanted corneas on days 4, 6, 10, and 14 at high magnification. Arrow and arrowhead indicate the same vessels at different time points. Between matured angiogenic vessels, most angiogenic vessels regressed. (C) Quantitation of the number of angiogenic vessels in IL-1β–implanted corneas on days 4, 6, 10, and 14 after implantation. (D) Quantitation of the portion of IL-1β–induced angiogenic tips on days 4, 6, 10, and 14 after implantation (n = 9–10). *P < 0.01.
Therapeutic Window of Steroids in Inflammatory Angiogenesis
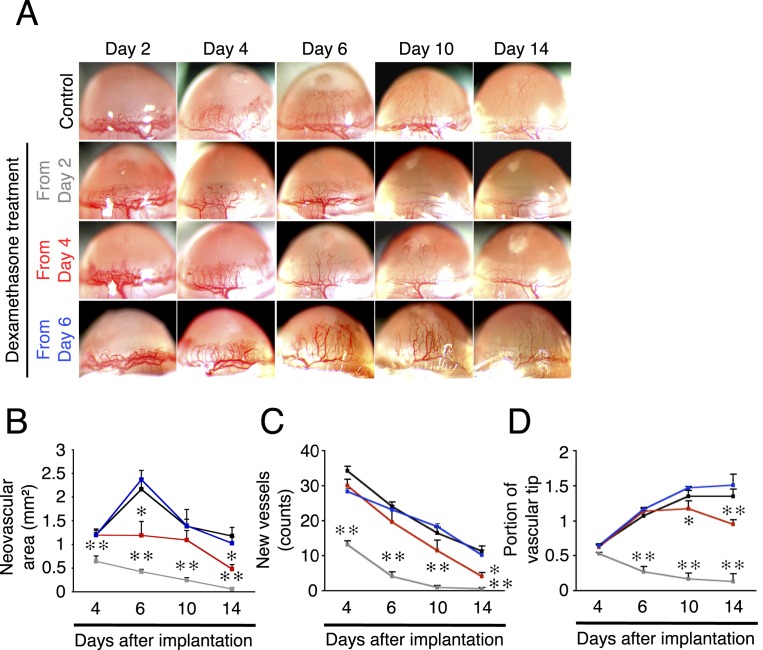
Next was an examination of the effect of dexamethasone, a synthetic corticosteroid analogue, on IL-1β–induced corneal angiogenesis starting at different days after implantation. When systemic dexamethasone treatment was started from day 2 after IL-1β implantation, corneal angiogenesis on day 4 was significantly less than in the control group (Figs. 2A–D). In these animals there were no angiogenic vessels (either perfused or nonperfused) on days 10 and 14 (Figs. 2A–D). In animals treated 4 days after implantation, corneal angiogenesis (vascular area, number of vessels, and portion of vascular tips) was significantly less compared with the control group on day 14 (Figs. 2A–D). These data indicate that dexamethasone enhances regression of the angiogenic vessels, even if the therapy starts after angiogenic sprouting. When treatment started from day 6, dexamethasone did not change the IL-1β–induced corneal angiogenesis compared with untreated until day 14 (Figs. 2A–D). For better comparability with the topical anti-VEGF therapy, the effect of topical dexamethasone on IL-1β implantation was studied. The results confirmed a similar angiostatic impact with the systemic treatment (Supplemental Figs. 1A–D, http://www.iovs.org/content/53/7/3296/suppl/DC1). These data suggest that the inhibitory targets of dexamethasone are affected from day 2 to day 4, but not day 6, in this inflammatory angiogenesis model.
Figure 2. .
Time-dependent efficacy of steroids in inflammatory angiogenesis. Dexamethasone treatment started from day 2, 4, or 6 after IL-1β implantation in corneas of BALB/c mice. (A) Photomicrographs of IL-1β–implanted corneas of BALB/c mice on days 2, 4, 6, 10, and 14 after the implantation with or without dexamethasone treatment. (B–D) Quantitation of the angiogenic area (B), vessel number (C), or portion of vascular tips (D) in IL-1β–induced angiogenesis on days 4, 6, 10, and 14 after implantation without (black) or with dexamethasone treatment from day 2 (gray), day 4 (red), or day 6 (blue) (n = 3–8).
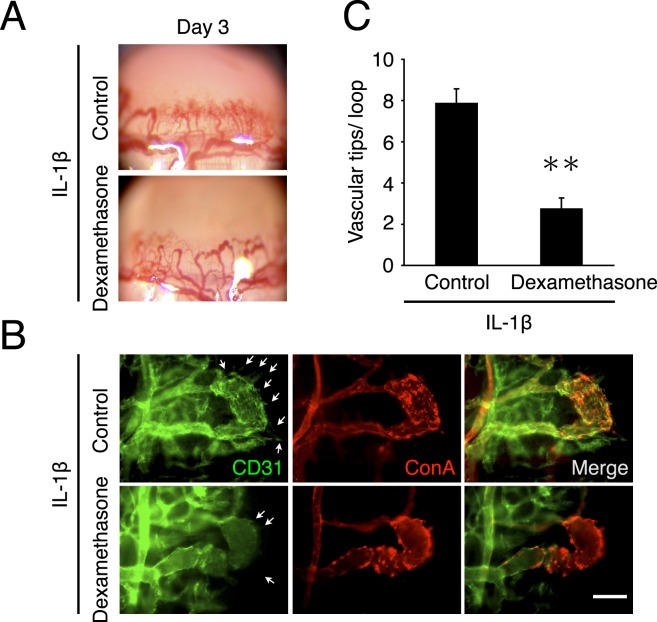
To confirm the inhibitory effect of dexamethasone treatment from day 2 for vascular sproutings, vascularized corneas were stained with CD31 antibodies (endothelial marker) and ConA (perfused vessels) on day 3 with or without dexamethasone treatment. Angiogenic sprouting in dexamethasone-treated corneas was inhibited compared with control (Figs. 3A, 3B). Quantitative analysis of the number of vascular sprouts showed that dexamethasone inhibited IL-1β–induced vascular sprouting from preexisting vessels in inflamed corneas (Fig. 3C).
Figure 3. .
Effect of steroids on inflammatory angiogenic sprouting. (A) Photomicrographs of IL-1β–implanted corneas of BALB/c mice on day 3 after implantation with or without dexamethasone treatment. (B) Double staining of corneal flat mounts for angiogenic endothelium (CD31, green) and perfused blood vessels (ConA, red) in IL-1β–implanted cornea of dexamethasone-treated mice on day 3. Arrows indicate angiogenic sprouts. Bar, 50 μm. (C) Quantitation of the number of angiogenic tips/angiogenic loops on day 3 with or without dexamethasone treatment from day 2 (n = 8). **P < 0.01.
Relatively Narrow Therapeutic Window of Anti-VEGF-A Therapy in Inflammatory Angiogenesis
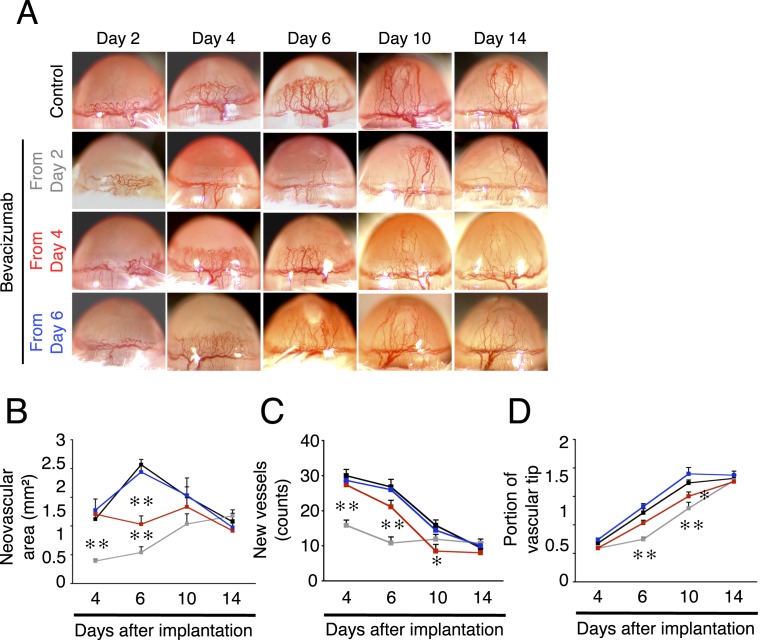
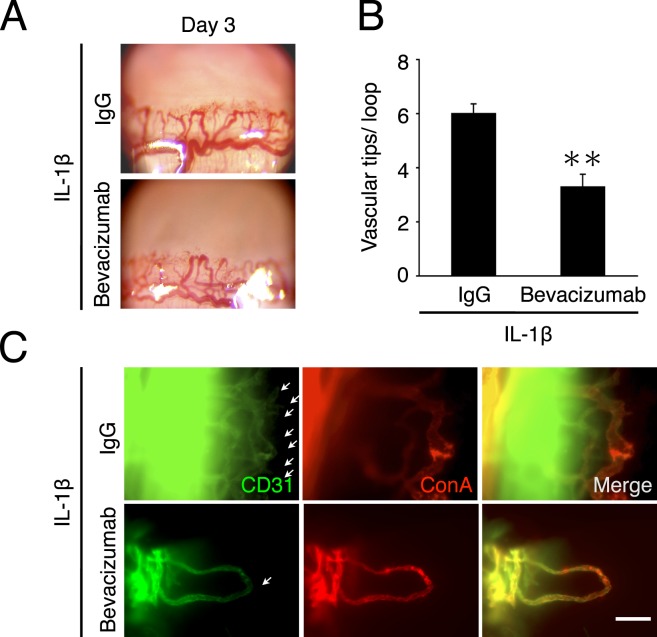
An antiangiogenic effect of dexamethasone could be in part through inhibition of VEGF-A.2 To examine the contribution of VEGF-A in inflammatory angiogenesis in the cornea, IL-1β–implanted corneas were treated with bevacizumab from day 2, 4, or 6 after implantation. When animals were treated with bevacizumab from day 2, reduced angiogenesis was observed on days 4 and 6 (Figs. 4A–D). However, on days 10 and 14 the portion of vascular tips did not differ from that in the IgG-treated control group (Figs. 4A–D). In animals in which bevacizumab treatment started from day 4, the angiogenic area but not vessel number and portion of vascular tips was less than in controls only on day 6. Interestingly, bevacizumab treatment from day 4 reduced vessel number and portion of vascular tips on day 10 but not day 6. Furthermore, in the group treated with bevacizumab from day 6, there was no significant inhibitory effect compared with control (Figs. 4A–D). These data indicate a role for VEGF-A in early stages of inflammatory angiogenesis. To investigate whether VEGF-A inhibition might modulate vascular sprouting, the number of angiogenic sprouts on day 3 with bevacizumab or IgG treatment was counted. Bevacizumab treatment significantly reduced the number of angiogenic sprouts compared with that in IgG-treated controls (Figs. 5A–C). This study yielded no observations of any adverse effects of bevacizumab, such as infection or corneal perforation, as inspected under light microscopy and as shown previously.15,16
Figure 4. .
Time-dependent efficacy of bevacizumab on inflammatory angiogenesis. Bevacizumab treatment was started from day 2, 4, or 6 after IL-1β implantation in BALB/c mouse corneas. (A) Photomicrographs of bevacizumab- or IgG-treated corneas of BALB/c mice on days 2, 4, 6, 10, and 14 after IL-1β implantation. (B–D) Quantitation of the angiogenic area (B), vessel number (C), or portion of vascular tips (D) on days 4, 6, 10, and 14 after IL-1β implantation and treatment with IgG (black) or bevacizumab from day 2 (gray), day 4 (red), or day 6 (blue) (n = 3–10).
Figure 5. .
Effect of bevacizumab on inflammatory angiogenic sprouting. (A) Photomicrographs of bevacizumab- or IgG-treated corneas of BALB/c mice on day 3 after IL-1β implantation. (B) Double staining of corneal flat mounts for angiogenic endothelium (CD31, green) and perfused blood vessels (ConA, red) in IL-1β–implanted corneas of bevacizumab- or IgG-treated mice on day 3. Arrows, angiogenic sprouts. Bar, 50 μm. (C) Quantitation of the number of angiogenic tips/angiogenic loops on day 3 with IgG or bevacizumab treatment from day 2 (n = 6–7). **P < 0.01.
The Effect of Steroid or Anti-VEGF-A Therapy on NF-κB Signaling during Inflammatory Angiogenesis
The authors previously reported the important role of NF-κB signaling in IL-1β–induced inflammatory angiogenesis and reported that NF-κB signaling is one of the molecular targets for steroids.2,17 To examine NF-κB signaling during IL-1β–induced angiogenesis, Western blots were performed for phospho-IκB and IκB. IL-1β induced IκB phosphorylation on days 2 and 4 (Fig. 6A). Whether dexamethasone or bevacizumab affected IκB phosphorylation in inflammatory angiogenesis was examined. Interestingly, dexamethasone but not bevacizumab inhibited IκB phosphorylation (Figs. 6B, 6C). These data indicate differential signaling pathways for steroids and VEGF-A inhibition.
Figure 6. .
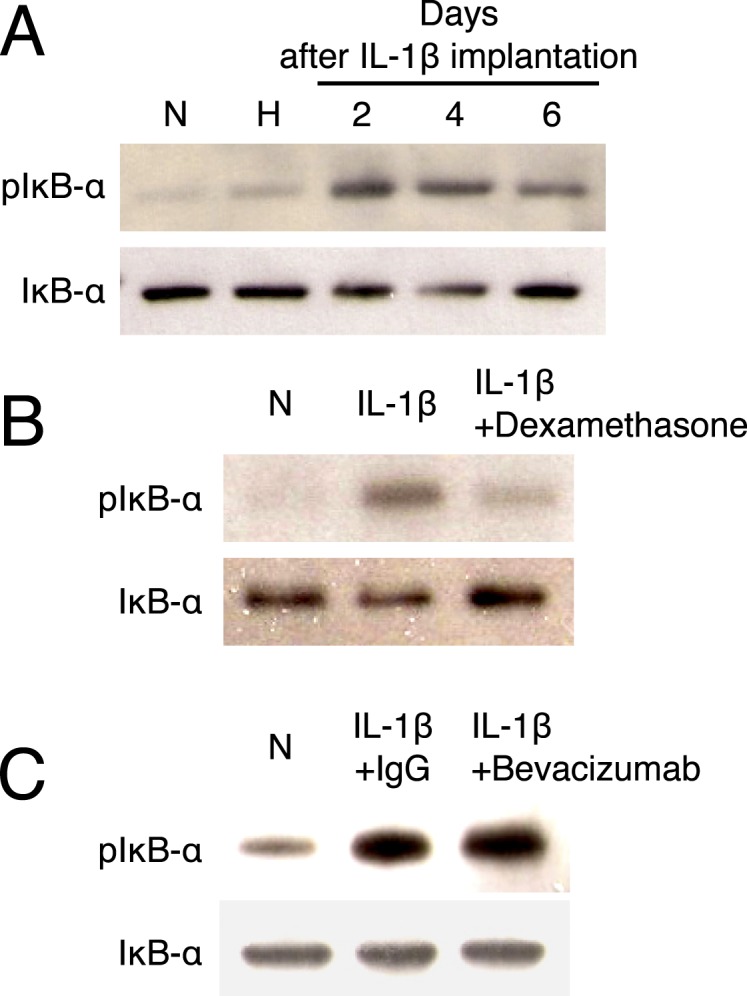
Impact of dexamethasone and bevacizumab on NF-κB signaling in IL-1β–induced angiogenesis. (A) Representative Western blots from IL-1β–implanted corneas for phospho-IκB and IκB, harvested on days 2, 4, and 6 (under reducing condition). N, untreated corneas; H, hydron-implanted corneas. (B) Representative Western blots of IL-1β–implanted corneas (day 3) for phospho-IκB and IκB with or without dexamethasone treatment (from day 2). (C) Representative Western blots of IgG- or bevacizumab-treated corneas (from day 2) on day 3 after IL-1β implantation for phospho-IκB and IκB.
Impact of Steroid and Anti-VEGF Therapy on Infiltration of Proangiogenic Leukocytes
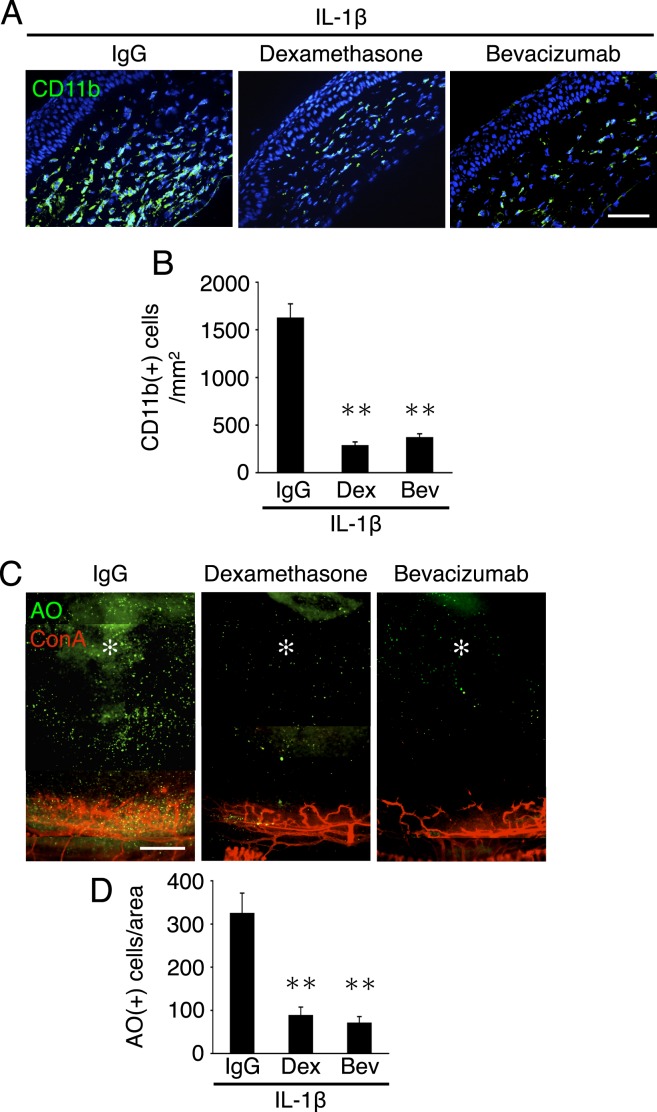
To examine the effect of dexamethasone and bevacizumab on leukocyte infiltration during inflammatory angiogenesis, the established immunohistochemistry was used and in addition a novel assay developed by this group for in vivo quantification of leukocyte infiltration into the cornea. For histology, CD11b(+) leukocytes, which a previous study from this group showed critically contribute to inflammatory angiogenesis, were stained.14 IL-1β implantation caused significant accumulation of CD11b(+) leukocytes. In comparison, IL-1β–implanted corneas that were treated with dexamethasone or bevacizumab had significantly less CD11b(+) cells in the stroma, while IgG treatment did not affect the number of accumulated leukocytes (Figs. 7A, 7B).
Figure 7. .
Impact of dexamethasone and bevacizumab on leukocyte infiltration in IL-1β–induced angiogenesis. (A) CD11b staining (green) of IL-1β–implanted corneal sections, treated with IgG, dexamethasone, or bevacizumab. CD11b staining shows CD11b(+) leukocytes in the cornea. (B) Quantitation of the number of CD11b-positive leukocytes (n = 8–10 sections from three or four mice). Bar, 50 μm. (C) Schematic of new leukocyte transmigration rate assay. (D) AO(+) leukocytes and Con A(+) angiogenic vessels in IL-1β–implanted corneas, 2 hours after AO injection, 3 days after pellet implantation. IgG, dexamethasone, or bevacizumab treatments started from day 2. (E) Quantitation of the number of AO(+) leukocytes in angiogenic areas of IL-1β–implanted corneas, 2 hours after AO injection, 3 days after pellet implantation (n = 3–5 from three mice). **P < 0.01.
To address the possibility that dexamethasone or bevacizumab might affect the survival or turnover of infiltrated leukocytes in inflammatory angiogenesis, leukocyte transmigration was next directly examined during inflammatory angiogenesis using this group's novel imaging assay (Fig. 7C). Systemic and topical dexamethasone as well as bevacizumab inhibited inflammatory leukocyte transmigration from angiogenic vessels into the corneal stroma (Figs. 7D, 7E, and Supplemental Figs. 2A, 2B, http://www.iovs.org/content/53/7/3296/suppl/DC1). These data indicate that VEGF-A inhibition as well as steroid treatment reduced leukocyte infiltration in inflammation, even though NF-κB signaling remained unaffected by VEGF-A inhibition.
Discussion
Inflammation is an integral part of various angiogenic diseases, including cancer progression and ocular diseases.14,18 The present authors previously reported that various molecules, including CXC chemokines, monocyte chemoattractant protein-1 (MCP-1), and adhesion molecules, contribute to inflammatory angiogenesis.2,14,19,20 A previous study from this group also showed that VEGF-A inhibition partially blocks inflammatory angiogenesis.21 However, it is unknown how or when VEGF-A contributes to angiogenesis during inflammation. This study showed that early angiogenic sprouting is inhibited by bevacizumab, as well as steroid treatment. However, it was found that bevacizumab did not enhance the regression of angiogenic vessels. VEGF-A thus appears essential for vascular sprouting, whereas VEGF-A's contribution to maturation or regression appears relatively less. This observation is in line with previous reports11 indicating that the phenotype of angiogenic vessels during inflammation and the relevant cytokines change during inflammatory angiogenesis.
The results suggest that early steroid administration is beneficial in suppressing the initial upregulation of inflammatory cytokines that occurs after injury.2,14 If steroids are used in this early stage, the beneficial effects appear to be highest for patients and outweigh the side effects.
In various diseases, it is desirable to suppress the angiogenic activity, including vascular leakage. “Normalization” of angiogenic vessels may be beneficial in some diseases.22 However, in the eye, even matured vessels cannot be tolerated, as they would impair vision. Thus, especially in the eye, the earliest possible angiostatic therapy would be of value.
Glaucoma and diabetes are among the side effects of steroids. In patients, anti-VEGF therapy can cause cerebrovascular incidents, with debilitating consequences.23 Short-term usage of either antiangiogenic drug may significantly reduce the incidence of side effects. Reducing the duration and amount of the drug treatment to the period in which the drug unfolds its desired effects also minimizes the number of side effects. The results of the present study help to optimize the treatment regimen for patients with angiogenic diseases.
NF-κB is a key signaling molecule in inflammation that especially regulates leukocyte transmigration.24 Surprisingly, VEGF-A inhibition completely reduced IL-1β–induced leukocyte transmigration without affecting NF-κB signaling. This indicates that VEGF-A induces leukocyte transmigration directly, without going through the NF-κB signaling pathway.2,14 Recent work shows that VEGF-A inhibition changes vascular phenotype, for instance causing loss of fenestration.25 Because NF-κB regulation affects immunity adversely,26 early VEGF-A inhibitor treatment might be beneficial in inflammatory angiogenesis without affecting immunity.
Supplementary Material
Acknowledgments
We thank Mitsuru Arima, Keijiro Ishikawa, Yasuharu Takada, and Michiyo Takahara for technical assistance. We thank the Malaysian Palm Oil Board and the American Health Assistance Foundation.
Footnotes
Supported by National Institutes of Health Grant AI050775 (AH-M), an overseas Research Fellowship Award from Bausch & Lomb, a Fellowship Award from the Japan Eye Bank Association, and a Tear Film and Ocular Surface Society Young Investigator Fellowship (to SN under the mentorship of AH-M).
Disclosure: S. Nakao, None; S. Zandi, None; N. Lara-Castillo, None; M. Taher, None; T. Ishibashi, None; A. Hafezi-Moghadam, None
References
- 1. Folkman J, Ingber DE. Angiostatic steroids. Method of discovery and mechanism of action. Ann Surg. 1987;206:374–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakao S, Hata Y, Miura M, et al. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am J Pathol. 2007;171:1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342 [DOI] [PubMed] [Google Scholar]
- 4. Arimura N, Otsuka H, Yamakiri K, et al. Vitreous mediators after intravitreal bevacizumab or triamcinolone acetonide in eyes with proliferative diabetic retinopathy. Ophthalmology. 2009;116:921–926 [DOI] [PubMed] [Google Scholar]
- 5. Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545–2552 [DOI] [PubMed] [Google Scholar]
- 6. Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920 [DOI] [PubMed] [Google Scholar]
- 8. Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309 [DOI] [PubMed] [Google Scholar]
- 10. Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812 [DOI] [PubMed] [Google Scholar]
- 11. Darland DC, D'Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakao S, Maruyama K, Zandi S, et al. Lymphangiogenesis and angiogenesis: concurrence and/or dependence? Studies in inbred mouse strains. FASEB J. 2010;24:504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakao S, Zandi S, Hata Y, et al. Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: an endogenous trapping mechanism links lymph- and angiogenesis. Blood. 2011;117:1081–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakao S, Kuwano T, Tsutsumi-Miyahara C, et al. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bock F, Onderka J, Rummelt C, et al. Safety profile of topical VEGF neutralization at the cornea. Invest Ophthalmol Vis Sci. 2009;50:2095–2102 [DOI] [PubMed] [Google Scholar]
- 16. Chalam KV, Agarwal S, Brar VS, Murthy RK, Sharma RK. Evaluation of cytotoxic effects of bevacizumab on human corneal cells. Cornea. 2009;28:328–333 [DOI] [PubMed] [Google Scholar]
- 17. Watari K, Nakao S, Fotovati A, et al. Role of macrophages in inflammatory lymphangiogenesis: enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun. 2008;377:826–831 [DOI] [PubMed] [Google Scholar]
- 18. Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22:559–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakao S, Kuwano T, Ishibashi T, Kuwano M, Ono M. Synergistic effect of TNF-alpha in soluble VCAM-1-induced angiogenesis through alpha 4 integrins. J Immunol. 2003;170:5704–5711 [DOI] [PubMed] [Google Scholar]
- 20. Nakao S, Noda K, Zandi S, et al. VAP-1-mediated M2 macrophage infiltration underlies IL-1beta- but not VEGF-A-induced lymph- and angiogenesis. Am J Pathol. 2011;178:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuwano T, Nakao S, Yamamoto H, et al. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004;18:300–310 [DOI] [PubMed] [Google Scholar]
- 22. Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–316 [DOI] [PubMed] [Google Scholar]
- 23. Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology. 2009;116:362 [DOI] [PubMed] [Google Scholar]
- 24. Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909 [PubMed] [Google Scholar]
- 25. Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baeuerle PA, Baichwal VR. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–137 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.