The closely related enteric bacteria Escherichia coli and Salmonella typhimurium are among the best understood microorganisms, and much of our knowledge of prokaryotic physiology and genetics has derived from their study. Despite their utility as model prokaryotes, however, it is clear that E. coli and S. typhimurium do not represent all aspects of microbial physiology and behavior. One such underrepresented area is that of the microbial cell-cell interaction. There are a variety of microorganisms that have evolved elaborate means by which individual cells communicate and coordinate their actions. Often, these bacteria produce and release extracellular signal molecules that allow them to gauge their own population density and respond by altering expression of specific genes, a process generally described as quorum sensing. Examples of intercellular communication systems include oligopeptide-based signaling used by a variety of Gram-positive bacteria, A factor production during fruiting body development in Myxocccus xanthus, butanolide control of antibiotic biosynthesis in Streptomyces spp., and a volatile fatty acid methyl ester signal that regulates virulence in the plant pathogen Ralstonia solanacearum (see refs. 1–4). In Gram-negative bacteria, by far the most common form of quorum sensing is mediated by production and subsequent perception of acylated homoserine lactones (acyl HSLs) (5, 6).
Research on quorum sensing in diverse bacteria has shed light on the mechanisms by which cohorts of bacteria orchestrate their efforts during pathogenesis and symbiosis with host organisms, respond to nutrient deprivation, and control multicellular behavior. Identification of an E. coli quorum sensor has been an attractive, but elusive, goal and although there is suggestive evidence for such a system (see below), it has remained ill defined. In fact, E. coli has been quite productively used as the heterologous host of choice for studies of acyl HSL-based signaling, with no apparent signal interference (for an example see ref. 7). In a report published in this issue of the Proceedings, Surette and Bassler (8) use the bioluminescent, quorum-sensing marine bacterium Vibrio harveyi to identify signaling molecules produced by E. coli and S. typhimurium. Their findings represent a significant step toward elucidating an extracellular signaling mechanism in these bacteria.
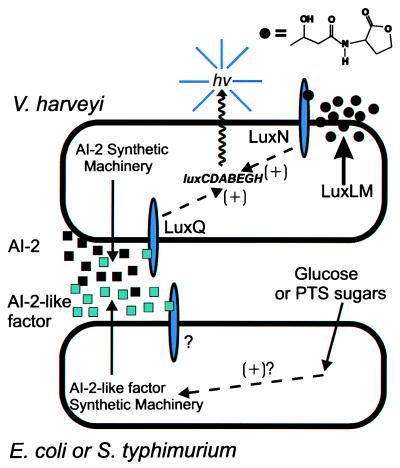
V. harveyi is a free-living marine bacterium that can be found in seawater and also is often associated with the intestines of fish and other animals. Bioluminescence (lux) genes are regulated by an elaborate signaling mechanism, involving the integration of two discrete quorum-sensing systems that function via a pair of two component-type sensor kinases (Fig. 1). The first of these, system I, is involved in response to N-3-hydroxybutyryl homoserine lactone, an acyl HSL signal molecule (9, 10). In V. harveyi synthesis of hydroxybutyryl HSL requires the LuxL and LuxM gene products, one of which is presumably an acyl HSL synthase. Response to hydroxybutyryl HSL requires the LuxN gene product, a two-component-type sensor kinase. The second regulatory system (system II) functions through an as-yet-uncharacterized signal molecule(s) called AI-2. Perception of AI-2 is dependent on a second sensor kinase, LuxQ. The input from both the LuxN and LuxQ sensor kinases is integrated at the level of lux gene expression via a single response regulator called LuxO and an additional regulator called LuxR (11, 12). Either system I or system II alone is sufficient for induction of the lux genes (10). Recently, a pair of V. harveyi reporter strains defective in perception of either hydroxybutyryl HSL or AI-2 were used to analyze the strain specificity of bioluminescence gene regulation (13). This study refined and extended the earlier observation of Greenberg et al. (14) that V. harveyi would respond to factors produced by nonluminescent marine vibrios. It is now clear that hydroxybutyryl HSL (system I) is a highly specific autoinducer, used by V. harveyi, but few other bacteria. Conversely, signals that are recognized by system II, presumably AI-2 or similar molecules, are produced by a broad range of bacteria.
Figure 1.
Cell-to-cell communication systems in V. harveyi and their use in detection of signals in other bacteria. The structure of the LuxLM-derived signal, N-3-hydroxybutyryl homoserine lactone is shown (top right). The depiction of the V. harveyi-based cross-feeding assay used by Surette and Bassler (8) to detect AI-2-like factors in E. coli or S. typhimurium is not meant to imply that the V. harveyi reporter strain and the tested microbes normally communicate with each other in the natural environment.
With these observations in hand Surette and Bassler (8) have used the V. harveyi system I mutant that responds exclusively to AI-2, to demonstrate that cell-free culture fluids of E. coli and S. typhimurium also can contain high levels of AI-2-like factors. This study provides the most convincing evidence to date for extracellular signal production by these model microbes. However, this signal(s) and its synthesis have some interesting and unexpected features. First, the AI-2 like factor is produced in Luria-Bertani medium only when it is supplemented with glucose (0.5%). The inducing activity is not detectable in cultures grown in the absence of supplemental glucose, although other sugars transported by the phosphotransferase system (PTS) also stimulate production. Initial chemical analysis suggests that the factor is not an acyl HSL, but is an uncharged, polar molecule of less than 1 kDa, which is sensitive to treatment with base. Structural analysis of this AI-2-like factor is in progress.
An additional surprise is in the timing of factor production. Cultures begin synthesizing the AI-2-like signals in response to limiting glucose—the higher the concentration of glucose, the later the onset of synthesis. In either case, by the stationary phase of growth, much or all of the signal has been degraded. This relatively rapid turnover is not commonly observed for other regulatory extracellular factors, which are generally quite stable. This observation may provide an explanation for why AI-2-like factor production by E. coli or S. typhimurium has not been observed previously.
A final intriguing observation is that the common laboratory strain E. coli DH5α does not synthesize the factor. However, other laboratory strains and all of several clinical isolates of E. coli and S. typhimurium do synthesize the signal. The genetic basis of this difference is not yet known but one possibility is that domestication of E. coli has allowed loss of the ability to synthesize the factor. Regardless of the reason for the apparent loss of AI-2 production in E. coli DH5α, this strain provides a facile mutant background for rapid isolation of the wild-type gene(s) from strains of E. coli that do synthesize the factor.
The detection of AI-2-like extracellular signals in Luria- Bertani-glucose grown cultures of E. coli and S. typhimurium by the V. harveyi reporter strain, although suggestive, does not in itself provide direct evidence for a quorum-sensing function in these enteric bacteria. Rather, the presumptive target genes influenced by the AI-2-like signal must be identified. Previous investigations in E. coli identified SdiA, a member of the LuxR family of proteins (named for the LuxR protein from Vibrio fischeri—to complicate matters the V. harveyi LuxR protein described above is not a member of the LuxR family) that function as acyl-HSL responsive transcriptional regulators in many quorum-sensing Gram-negative bacteria (5, 6, 15). The sdiA gene product regulates expression of the ftsQAZ cell division locus, and therefore is thought to contribute to the control of cell division. The homologous sdiA gene in S. typhimurium regulates the expression of a number of different genes, some of which are thought to be involved in virulence (16). No corresponding acyl-HSL has been identified in E. coli or Salmonella. Analysis of E. coli cell-free culture fluids using sdiA-regulated fusion genes has identified a weak inducing activity that does not exhibit the chemical characteristics of an acyl-HSL (17). It will be intriguing to integrate the observations of Surette and Bassler (8) with these previous findings. It is possible that the SdiA signal and the AI-2 signal observed in the current study represent separate, discrete signaling pathways. However, it is not unlikely that the two signals will be related, perhaps even identical. If so, the lability of the AI-2 signal in late stage cultures might, in fact, explain the difficulty that has been encountered in purification of the SdiA factor. Clearly, purification and chemical characterization of the factors will help to settle this issue.
In V. harveyi it has been speculated that the acyl HSL signal, hydroxybutyryl HSL, functions as a species-specific cell density cue, and in contrast that the AI-2 factor might be a more general population signal (13). The detection of an AI-2-like signal in E. coli and S. typhimurium suggests that these microbes are capable of cell-cell communication in ways analogous to more overtly communicative microbes. If so, this factor may act as a intraspecies quorum-sensing signal, or a more general interbacterial signal for interaction with other microbes, which like V. harveyi, would respond to its presence. The current studies by Surette and Bassler (8) describe such a signal and show a clear path to identifying the signal structure(s) and the genes required for its synthesis. The question remains who, if anyone, responds to this signal?
Footnotes
The companion to this commentary is published on pages 7046–7050.
References
- 1.Flavier A B, Schell M A, Denny T P. Mol Microbiol. 1997;26:251–259. doi: 10.1046/j.1365-2958.1997.5661945.x. [DOI] [PubMed] [Google Scholar]
- 2.Horinouchi S, Beppu T. Annu Rev Microbiol. 1992;46:377–398. doi: 10.1146/annurev.mi.46.100192.002113. [DOI] [PubMed] [Google Scholar]
- 3.Dunny G M, Leonard B A B. Annu Rev Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin M. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuqua C, Winans S C, Greenberg E P. Annu Rev Microbiol. 1996;50:591–624. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 6.Fuqua C, Greenberg E P. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 7.Engebrecht J, Nealson K, Silverman M. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 8.Surette M G, Bassler B L. Proc Natl Acad Sci USA. 1998;95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J-G, Meighen E A. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- 10.Bassler B L, Wright M, Silverman M R. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 11.Bassler B L, Wright M, Silverman M R. Mol Microbiol. 1994;12:403–412. doi: 10.1111/j.1365-2958.1994.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 12.Swartzman E, Silverman M, Meighen E A. J Bacteriol. 1992;174:7490–7493. doi: 10.1128/jb.174.22.7490-7493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassler B L, Greenberg E P, Stevens A M. J Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg E P, Hastings J W, Ulitzur S. Arch Microbiol. 1979;120:87–91. [Google Scholar]
- 15.Wang X, de Boer P A J, Rothfield L I. EMBO J. 1991;10:3363–3372. doi: 10.1002/j.1460-2075.1991.tb04900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmer B M M, van Reeuwijk J, Timmers C D, Valentine P J, Heffron F. J Bacteriol. 1998;180:1185–1193. doi: 10.1128/jb.180.5.1185-1193.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitnikov D M, Shadel G S, Baldwin T O. Mol Gen Genet. 1996;252:622–625. doi: 10.1007/BF02172408. [DOI] [PubMed] [Google Scholar]