Abstract
SZ117 is a monoclonal antibody against matrix metalloproteinase-2 (MMP-2) and exhibits anti-tumor angiogenic effect. In this study, we observed that SZ117 bound to a 280 kDa protein, which was detected in tumor cell-derived Matrigel and various tumor cells. Using immunoprecipitation, mass spectrometry analysis, and Western blot analysis, we identified the 280 kDa protein as filamin A and found that filamin A and its degraded products, notably a 53 kDa fragment, were released from a variety of tumor cells. This suggests that SZ117 is useful in the study of the pathogenesis of filamin A and that blockage of filamin A by SZ117 might contribute to the anti-tumor angiogenic effect of the monoclonal antibody.
Introduction
Tumor cells make all mechanisms for growth and metastasis possible, including production and release of various growth factors, proteases, and matrix proteins. These promote tumor angiogenesis and recruit a variety of cells to the tumor tissue for build up of the tumor microenvironment, which nurtures tumor growth and metastasis.(1–4) Hence, targeting these tumor cell-derived proteins and factors with monoclonal antibodies is a reasonable strategy for anti-tumor therapy.(5) In this context, monoclonal antibodies, such as Avastin, have been used to treat patients with advanced colon cancer and prolong the life of cancer patients for 4∼6 months.(6,7) More recently, we reported that SZ117, a monoclonal antibody against matrix metalloproteinase-2 (MMP-2), was able to block MMP2 activity and inhibit tumor cell-mediated angiogenesis,(8) whereas the mechanism underlying the inhibitory effect of the antibody is enigmatic.
In this investigation, we found that monoclonal antibody SZ117 recognized a 280 kDa protein in tumor cell-derived Matrigel and various tumor cells and that the 280 kDa protein was identified as filamin A, suggesting that SZ117 is also a filamin A antibody. Furthermore, we observed that filamin A and its degraded fragments were produced and released from a variety of tumor cells. Since filamin A has been reported to be implicated with tumor vascular remodeling and invasion in various cancers,(9,10) monoclonal antibody SZ117 is useful not only in anti-tumor angiogenesis but also in the study of filamin A-mediated tumor pathogenesis.
Materials and Methods
Materials
Matrigel matrix was purchased from BD Biosciences (basement membrane, #354234; San Diego, CA). Filamin A monoclonal antibody was purchased from Millipore (#MAB1678; Billerica, MA). M-PER Mammalian Protein Extraction Reagent (# 78501), protein G agarose (#20398), and horseradish peroxidase (HRP)-labeled secondary antibody (goat anti-mouse IgG) were purchased from Thermo Scientific (Waltham, MA). Enhanced chemiluminescence substrate was purchased from PerkinElmer ((#NEL 102001EA; Waltham, MA). Anti-β-actin antibody (#A3854), gelatin, and commonly used chemicals were from Sigma (St. Louis, MO). Fetal bovine serum (FBS) was from PAA Laboratories (Pasching, Austria). RPMI-1640 and DMEM were from HyClone (South Logan, UT). All tumor cell lines were obtained from the ATCC (Manassas, VA).
Preparation and purification of monoclonal antibodies
SZ117 monoclonal antibody was prepared in our own laboratory(11) and purified by protein G agarose beads affinity chromatography as previously described.(8)
Cell culture
Tumor cells were cultured in a humidified incubator with 5% CO2, DMEM, or RPMI 1640 supplemented with 10% FBS and 1x penicillin/streptomycin, as previously described.(8,12)
Western blot analysis
Western blotting was performed as reported previously.(8,13) In brief, tumor cells (107) were incubated with 0.2 mL M-PER protein extraction buffer and 1x protease inhibitor cocktail. The supernatant of cell lysates was mixed with sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The proteins were then resolved on SDS-Tris-glycine acryamide gel and transferred onto nitrocellulose membrane. Immunoblotting was performed using either monoclonal antibody SZ117 or anti-filamin A antibody, followed by the appropriate species-specific horseradish peroxidase-conjugated α-globulin antibodies and SuperSignal chemiluminescence substrate, respectively. The bands were visualized on x-ray film.
Immunoprecipitation
SW480 tumor cells were lysed with 1.0 mL M-PER protein extraction buffer and centrifuged at 12000 rpm for 10 min at 4°C. The supernatant of the cell lysates was pre-cleared by protein G bead-non-immune IgG conjugates. The pre-cleared cell lysates were incubated at 4°C for 2 h with either SZ117 IgG or filamin A antibody, or control non-immune IgG at a concentration of 5 μg/mL, followed by incubation with 30 μL protein G-beads at 4°C for 1 h with agitation. After washing five times, the bound proteins were eluted with 50 μL of reduced SDS-PAGE sample buffer (final 5% SDS, 10 mM DTT) and the sample was heated at 95°C for 3 min. The proteins were separated on SDS-PAGE and visualized after either silver staining or Western blotting.
Mass spectrometry analysis
Proteins from the above immunoprecipitation were resolved in SDS-PAGE and stained with Coomassie blue. The distinct band at 280 kDa was excised. The gel slice was reduced, alkylated, and digested with Trypsin. The digested protein was then run by Nano-LC/MS/MS at the Center for Mass Spectrometer, School of Biological Sciences, Nan Yang Technological University (Singapore). The data were searched against the NCBInr human database using MASCOT.
Results
SZ117 recognizes a 280 kDa protein in Matrigel and tumor cells
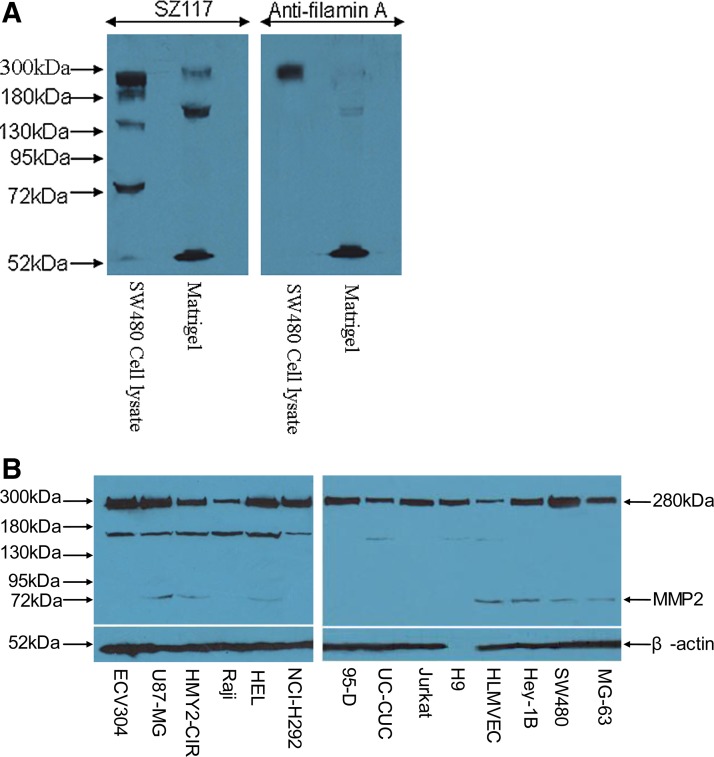
Our recent study showed that MMP-2 monoclonal antibody SZ117 executed an anti-tumor angiogenic effect(8); however, the inhibitory mechanism of the antibody is unsolved. Although SZ117 diminished tumor endothelial cell 3B11-mediated angiogenesis, the cells did not express MMP2 and had no detectable MMP2 activity, suggesting that another mechanism, other than blockage of MMP-2, might contribute to the inhibitory effect of the antibody.(8) In a tumor cell-mediated capillary-like tube formation system, the matrix proteins in Matrigel enhance tumor cell migration and formation of capillary-like tubes. Hence, we performed Western blot analysis to investigate whether SZ117 recognized MMP2 in the Matrigel. As evident in Figure 1, SZ117 recognized 280 kDa, 150 kDa, and 53 kDa protein bands in Matrigel, while a 72 kDa MMP2 was detected in the tumor cell line SW480 by Western blotting, but not detected in the Matrigel (Fig. 1A). Whether the Matrigel is absent in MMP2 or the MMP2 in the Matrigel is degraded is still a question marker, and whether the 53 kDa band is derived from a degradation of 72 kDa MMP2 or 280 kDa filamin A remains to be identified (Fig. 1A). In addition, Western blotting showed several high molecular protein bands in the tumor cells (Fig. 1A). This result prompted us to investigate the SZ-117-recognized proteins in other tumor cell lines. To our surprise, SZ117 recognized a 280 kDa major protein band in a variety of tumor cell lines, including ECV304, U87-MG, HMY2-CIR, Raji, HEL, NCI-H292, 95-D, UC-CUC, HLMVEC, H9, Jurkat, Hey1B, SW480, and MG63 (Fig. 1B); whereas the identity of the 280 kDa protein derived from these tumor cells is unknown.
FIG. 1.
SZ117 recognizes a 280 kDa protein in Matrigel and tumor cells. The 280, 250, 150, and 53 kDa bands in tumor cell SW480 and Matrigel were detected by Western blotting using SZ117 (A, left panel) or commercial filamin A monoclonal antibody (A, right panel). The expression of 280 kDa, 150 kDa, and 53 kDa proteins were also observed in various tumor cell lines, including ECV304, U87-MG, HMY2-CIR, Raji, HEL, NCI-H292, 95-D, UC-CUC, HLMVEC, H9, Jurkat, Hey1B, SW480, and MG63 (B). Of note, β-actin was not detected in H9 cells by the antibody.
Identification of SZ117 recognizes a 280 kDa protein as filamin A
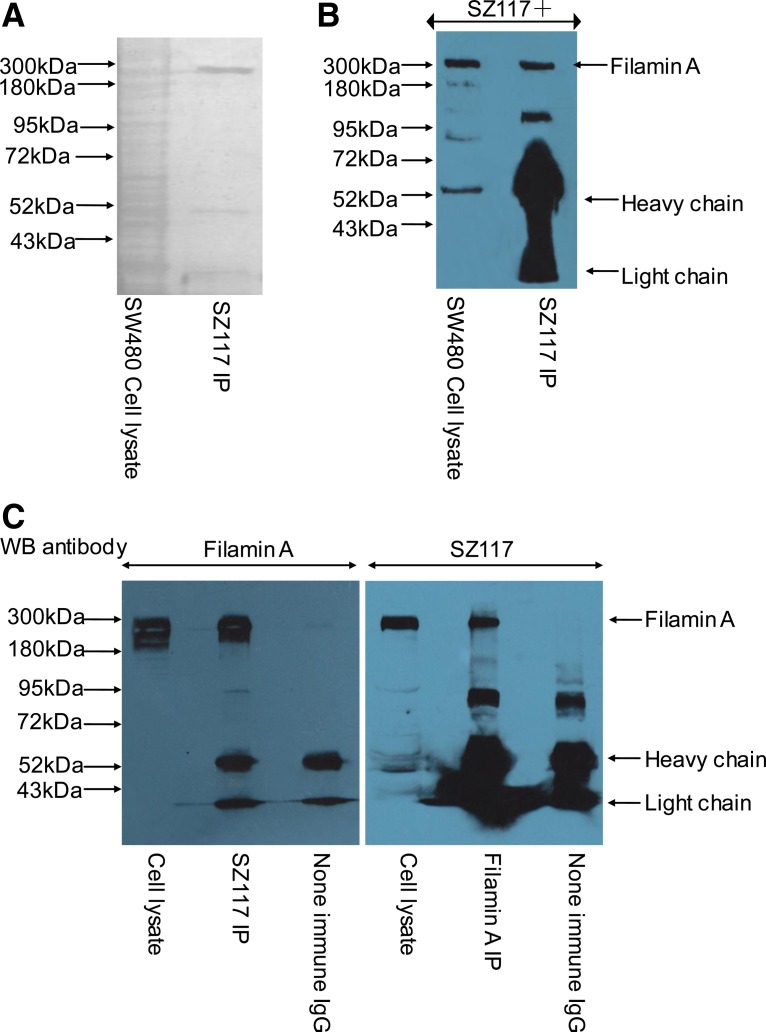
We next carried out immunoprecipitation (IP) and mass spectrometry analyses to identify SZ117-recognized 280 kDa protein. The proteins in SW480 cell lysates were immunoprecipitated by purified SZ117 IgG and resolved on two SDS-PAGE gels, one gel for silver staining (Fig. 2A) and the other for Western blotting using SZ117 (Fig. 2B). The silver staining (SZ117 positive) 280 kDa band from IP was excised and analyzed by mass spectrometry. The result indicated that the 280 kDa protein recognized by SZ117 was human filamin A (Table 1). In addition, SZ117-immunoprecipitated 280 kDa protein was recognized by human filamin A monoclonal antibody (Fig. 2C, left panel). Similarly, filamin A antibody-immunoprecipitated protein was recognized by SZ117 (Fig. 2C, right panel), corroborating the fact that the 280 kDa protein recognized by SZ117 is human filamin A.
FIG. 2.
Identification of SZ117-recognized 280 kDa protein as filamin A. SW480 cell lysates were immunoprecipitated and IP products were resolved in SDS-PAGE. The proteins on the gel were stained with sliver stain (A) or detected by Western blotting using SZ117 (B). Then the 280 kDa protein band was excised for mass spectrometry analysis as mentioned in above method section and shown in the results in Table 1. To further confirm the 280 kDa protein as filamin A, SW480 cell lysates were immunoprecipitated with SZ117 and filamin A antibody, respectively, and SZ117 IP products were detected with filamin A monoclonal antibody (FC, left panel), while filamin A antibody IP products detected using SZ117 (C, right panel).
Table 1.
Identification of SZ117-Recognizes A 280 kDa Protein As Filamin A by Mass Spectrometry
| Mass | Lon score | Peptides (filamin A) | Start amino acid |
|---|---|---|---|
| 368.1956 | 57 | VKCSGPGLER | 1163 |
| 438.2071 | 65 | CSGPGLER | 104 |
| 441.7754 | 68 | AGVAPLQVK | 163 |
| 494.7765 | 70 | KGEITGEVR | 1786 |
| 544.7884 | 63 | TPCEEILVK | 1276 |
| 550.2927 | 59 | GTVEPQLEAR | 428 |
| 605.6609 | 50 | VAQPTITDNKDGTVTVR | 1818 |
| 613.2906 | 75 | EATTEFSVDAR | 1273 |
| 715.3646 | 84 | AFGPGLQGGSAGSPAR | 1072 |
| 721.8564 | 79 | AGQSAAGAAPGGGVDTR | 8 |
| 772.0414 | 50 | SAGQGEVLVYVEDPAGHQEEA | 310 |
| 785.9909 | 69 | GAGTGGLGLAVEGPSEAK | 67 |
| 892.9494 | 69 | VQVQDNEGCPVEALVK | 709 |
Release of filamin A and its fragments from various tumor cells
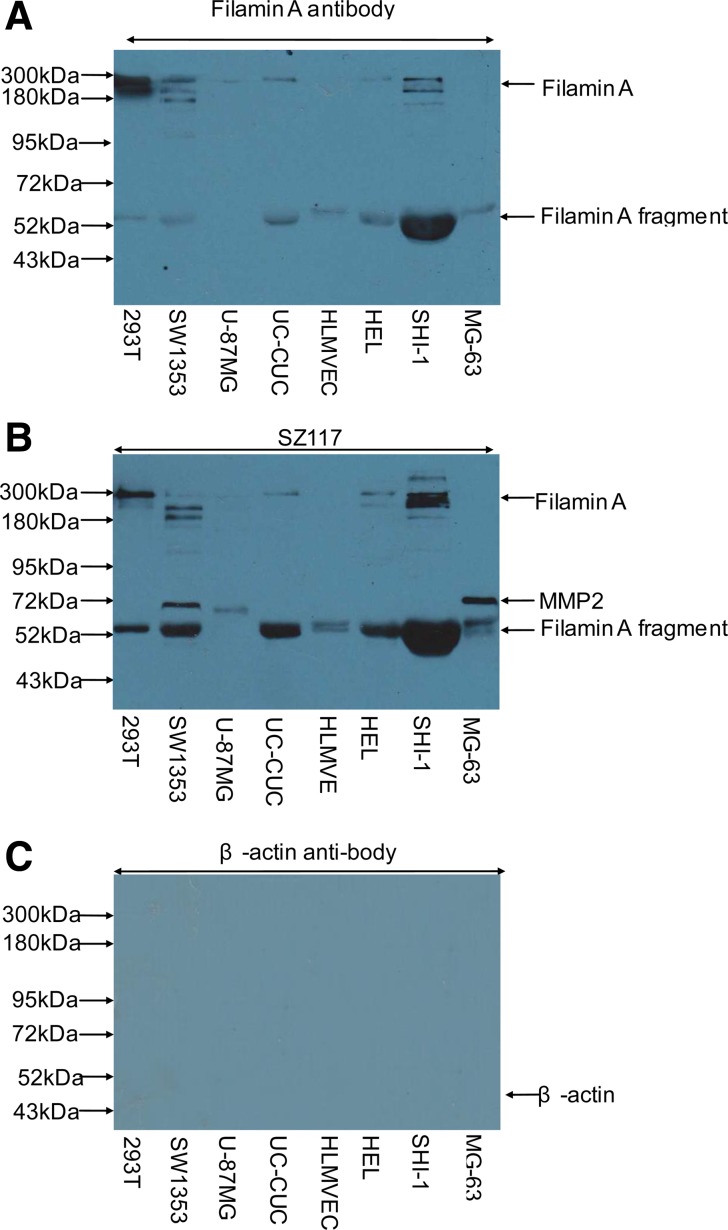
As mentioned above (Fig. 1), filamin A and its fragments were found in the human tumor cell line SW480 and in Matrigel, which is a gelatinous protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells. For interest, we investigated whether filamin A can be released from other tumor cells. As shown in Figure 3, Western blotting using monoclonal antibody to filamin A showed that 280 kDa full length of filamin A and its degraded products were present in serum free cell-cultured medium of various tumor cells (Fig. 3A); the same protein bands were also recognized by SZ117, notably a major 53 kDa band, which was not reported in the literature (Fig. 3B). Obviously, SZ117 was more sensitive to detect the 53 kDa protein as compared to filamin A antibody, while β-actin, a cytoplasm protein as a negative protein release control, was absent in tumor cell-cultured medium (Fig. 3C). These data indicated that filamin A and its fragments could be released from tumor cells and may become one of the extracellular matrix proteins and a component in the Matrigel.
FIG. 3.
Filamin A and its degraded fragments were released from tumor cells. Filamin A and its fragments in serum free cell-cultured medium of tumor cells and immortalized cells were detected with Western blotting using both filamin A antibody (A) and SZ117 (B). β-actin as a negative release control was also checked by Western blotting using β-actin monoclonal antibody (C).
Discussion
Filamin A is a 280 kDa protein containing an N-terminal actin-binding domain and a rod-like domain of 24 repeats, interrupted by 30-amino-acid flexible loops, H1 (between 15 and 16) and H2 (between 23 and 24), which are highly susceptible to proteolysis. Besides, the secreted variants of filamin A were detected in plasma.(9) Filamin A plays a vital role in tumor cell proliferation, migration, invasion, as well as tumor vascular remodeling, further complicating breast cancer, prostatic carcinoma, melanoma, carcinoma of urinary bladder, and neuroblast carcinoma.(9,10) The mechanisms of extracellular matrix filamin A and its fragments in tumor angiogenesis and metastasis are largely unknown. In this study, we found that SZ117 bound to filamin A in Matrigel and tumor cells and that filamin A as well as its fragments were released from various tumor cells. The role of filamin A and its fragments in tumor angiogenesis remains to be investigated. We speculated that filamin A and its 53 kDa fragment might act as matrix proteins to contribute to tumor angiogenesis, and that the binding of monoclonal antibody SZ117 to filamin A and its fragments in Matrigel might be one of the inhibitory mechanisms of SZ117 in the blockage of tumor cell-mediated angiogenesis, on which we previously reported.(8) Nevertheless, our experimental results warrant a further investigation of tumor cell-derived filamin A and its fragments in tumor angiogenesis and metastasis.
Increasing data have shown that tumor cell-derived matrix proteins play important roles in tumor angiogenesis, progression, and metastasis.(1–4) Hence, tumor cell-produced matrix proteins are potential targets for novel anti-angiogenic drug discovery. Our data have shown that SZ117 may block both filamin A in this study and inhibit MMP-2 activity as previously reported,(8) suggesting that monoclonal SZ117 may be useful in anti-angiogenic and anti-cancerous therapies. In addition, since SZ117 recognizes tumor cell-released filamin A and its fragments, the monoclonal antibody is useful in the study of filamin A-mediated tumor angiogenesis, progression, and metastasis.
Acknowledgments
We thank Dr. Jiang Juxiang and Mr. He Cong for their work on the isolation and characterization of filamin A. This study was supported by grants from National Natural Science Foundation of China (Grant No. 30971138), Suzhou City Scientific Research Funds (Nos. SWG0904, SS201004, and SS201138), and a project funded by the priority academic program development of Jiangsu Higher Education Institutions (PAPD).
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Kucera T. Lammert E. Ancestral vascular tube formation, its adoption by tumors. Biol Chem. 2009;390:985–994. doi: 10.1515/BC.2009.115. [DOI] [PubMed] [Google Scholar]
- 2.Furuya M. Yonemitsu Y. Aoki I. Angiogenesis: complexity of tumor vasculature, microenvironment. Curr Pharm Des. 2009;15:1854–1867. doi: 10.2174/138161209788453275. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza M. Khanna C. Revisiting the seed, soil in cancer metastasis. Int J Biochem Cell Biol. 2009;41:1452–1462. doi: 10.1016/j.biocel.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyberg P. Salo T. Kalluri R. Tumor microenvironment, angiogenesis. Front Biosci. 2008;13:6537–6553. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- 5.Ribatti D. The discovery of antiangiogenic molecules: a historical review. Curr Pharm Des. 2009;15:345–352. doi: 10.2174/138161209787315855. [DOI] [PubMed] [Google Scholar]
- 6.Presta LG. Chen H. O'Connor SJ. Chisholm V. Meng YG. Krummen L. Winkler M. Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors, other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 7.Heath VL. Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 8.Bu Z. Pan Y. Shang B. Cao Z. Zhou Q. Ruan C. SZ-117 a monoclonal antibody against matrix metalloproteinase-2 inhibits tumor cell-mediated angiogenesis. Hybridoma. 2012;31(1) doi: 10.1089/hyb.2011.0088. [DOI] [PubMed] [Google Scholar]
- 9.Alper Ö. Stetler-Stevenson WG. Harris LN. Leitner WW. Özdemirli M. Hartmann D. Raffeld M. Abu-Asab M. Byers S. Novel anti-filamin-A antibody detects a secreted variant of filamin-A in plasma from patients with breast carcinoma, high-grade astrocytoma. Cancer Sci. 2009;100:1748–1756. doi: 10.1111/j.1349-7006.2009.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedolla RG. Wang Y. Asuncion A. Chamie K. Siddiqui S. Mudryj M. Prihoda TJ. Siddiqui J. Chinnaiyan AM. Mehra R. de Vere White RW. Ghosh PM. Nuclear versus cytoplasmic localization of filamin A in prostate cancer: immunohistochemical correlation with metastases. Clin Cancer Res. 2009;15:788–796. doi: 10.1158/1078-0432.CCR-08-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X. Shen F. Dong N. Li P. Ruan C. Preparation, study of monoclonal antibody to Matrix metalloproteinase-2. Chin J Path. 2005;5:876–881. [Google Scholar]
- 12.Zhou Q. Zhao J. Wildmer T. Sims PJ. Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood. 2002;99:4030–4038. doi: 10.1182/blood-2001-12-0271. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q. Ben-Efraim I. Bigcas JL. Junqueira D. Wiedmer T. Sims PJ. Phospholipid scramblase 1 binds to the promoter region of the inositol 1,4,5-triphosphate receptor type 1 gene to enhance its expression. J Biol Chem. 2005;280:35062–35068. doi: 10.1074/jbc.M504821200. [DOI] [PubMed] [Google Scholar]