Abstract
Human ubiquitin-conjugating enzyme E2, also known as UbcH10, is defined as a cyclin-selective ubiquitin carrier protein and is essential for selective degradation of many short-lived proteins in eukaryotic cells. Recently more and more data show that UbcH10 could be a potential cancer biomarker. In this study, we have developed a monoclonal antibody (MAb) against UbcH10 using an expression recombinant protein. Hybridomas F001, F007, and F008 with high affinities belong to IgG1 subclass with κ light and are highly specific for UbcH10. Further experimentation showed that MAbs F001, F007, and F008 are suitable for the development of immunoassay core agents with sufficient sensitivity and specificity in vitro by Western-blot, immunofluorescence, and immunohistochemistry. These MAbs can be used as a tool for further investigation on functions related to the role of UbcH10 in tumorigenesis and development.
Introduction
Ubiquitin activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3) have the role of transferring ubiquitin to specific substrate proteins in an ubiquitin proteasome pathway (UPP). In a eukaryotic organism, the E2 and E3 are not as highly conserved as E1 and ubiquitin. The various E2 and E3 proteins work in cognate pairs and provide specificity in target protein ubiquitination. The UbcH10 gene, also named cyclin-selective ubiquitin carrier protein, belongs to the E2 gene family, and coded a protein with 179 amino acids. It has been shown that UbcH10 is involved in the mitotic destruction of securin and cyclin B and the formation of anaphase promoting complex or cyclosome (APC/C), which confers the target protein specificity for ubiquitination.(1–3) Therefore, UbcH10 is essential for controlling cell cycle and degrading cyclins.
Recently, the potential role of UbcH10 in tumor initiation, progression, and transformation was found.(4,5) The UbcH10 gene is located at 20q13.1, a genome region known to be amplified in diverse tumors. It has been shown that UbcH10 expression is cancer-associated.(4) The expression level of UbcH10 is extremely low in most normal tissues but prominently high in the majority of cancerous cell lines. In primary tumors derived from the lung, stomach, uterus, breast, ovary, and bladder, UbcH10 is overexpressed compared with their corresponding normal tissues.(4,5) This phenomenon was also found in lymphomas.(6) Inhabiting the expression of UbcH10 by RNA interference in breast carcinoma cell lines can suppress the cell growth of breast carcinoma.(7) Clinical data revealed that elevated expression of UbcH10 is associated with higher histological grade breast tumor.(7,8) Also there are some reports that show abundant UbcH10 levels present in highly invasive, undifferentiated thyroid carcinomas.(9,10) UbcH10 expression significantly correlates with tumor grade, undifferentiated histotype of ovarian carcinomas, and overall survival.(11–13) UbcH10 has also been found overexpressed in some hepatocellular carcinomas,(14) esophageal adenocarcinoma,(15) colon cancer,(16–18) and colon cancer with liver metastases.(19) In 2009, Jiang and associates reported that knockdown of UbcH10 expression by RNA interference could inhibit glioma cell proliferation and enhance cell apoptosis in vitro.(20) Moreover, it is well documented that ubiquitin becomes ubiquitous in cancer and many ubiquitin ligases, and deubiquitinases have a major role in tumorigenesis and could be identified as therapeutic targets.(21) All of these suggest that UbcH10 plays an important role in tumorigenesis and progression and becomes a potential cancer biomarker. In this study, we developed a monoclonal antibody against this potential cancer biomarker, UbcH10, providing a helpful tool for further investigation of the function of UbcH10 in tumorigenesis and development.
Materials and Methods
Plasmid construction and purification of recombinant proteins
A full-length cDNA of UbcH10 (GenBank accession no. NM_007019) was amplified from RNA of HepG2 cell using PrimeScript RT-PCR Kit (TakaRa Co., Dalian, China) with a pair of gene specific primers (forward: 5′-GTCGAATTCATGGCTTCCCAAAACCG-3′, reverse: 5′-ATTCTCGAGTTAGGGCTCCTGGCTGG-3′) containing the EcoRI and XhoI restriction sites, respectively. The reaction was carried out with the following procedures in a Mastercycler® gradient PCR System (Eppendorf, Hamburg, Germany): initial denaturation was at 94°C for 5 min followed by 30 consecutive cycles of denaturation at 94°C for 30 s, annealing for 30 s at 58°C, extension at 72°C for 1 min, and final extension at 72°C for 5 min. After digestion with EcoRI and XhoI, the amplified UbcH10 product was inserted into the corresponding region of pET32a (+) expression vector with T4 ligase. The correct recombinant prokaryotic expression vector confirmed by restriction analysis and sequencing was named pET32a (+)/UbcH10. The fusion protein with His tag was expressed in Escherichia coli BL21 cells on a large scale. Protein expression was induced by 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 4 h at 37°C. The cultured E. coli BL21 cells were collected by centrifugation at 10,000 rpm for 10 min at 4°C. The suspension from the pellet suspended in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 1 mM PMSF) was repeatedly frozen and thawed three times. Following sonication in an ice bath, the suspension was centrifuged at 12,000 rpm for 15 min. The clear supernatant (soluble fraction) and pellet (insoluble fraction) were collected and analyzed by 12% SDS–PAGE. Recombinant protein with His-tag was purified by Ni-NTA affinity chromatography (GE Healthcare, Buckinghamshire, United Kingdom) according to the manufacturer's protocol. Briefly, the column was equilibrated with five column volumes of binding buffer (20 mM sodium phosphate, 0.5 M NaCl, 30 mM imidazole [pH 7.4]). After being filtered with 0.45 μm filter, the sample was loaded onto the column at a flow rate of 1–2 mL/min, and the bound protein was eluted by elution buffer (20 mM sodium phosphate, 0.5 M NaCl, 500 mM imidazole [pH 7.4] at a flow rate 1–2 mL/min). The eluted protein was carefully collected and analyzed by 12% SDS–PAGE. The purified protein was then identified by Western blot analysis using rabbit anti-His polyclonal antibody (Sigma, St. Louis, MO), and the concentration of the recombinant protein with 6×His-tag was tested by BCA method.
Immunization of mice and establishment of hybridoma
BALB/c female mice (6–8 weeks old) were immunized by subcutaneous injection (s.c.) with 50 μg UbcH10 emulsified with 250 μL Freund's complete adjuvant. After three booster injections were given with 50 μg recombinant protein each in incomplete Freund's adjuvant at 2-week intervals, the sera were collected and assayed antibody titer by ELISA. The splenocytes segregated from the immunized BALB/c mice were fused with SP2/0 myeloma cells. The detailed procedure was as follows: the immunized BALB/c mouse was killed and the spleen was segregated. The splenocytes and the myeloma cells (at ratio of 5:1) were washed twice with 1640 culture medium without calf serum. The final pellet of two kinds of cells were mixed by tapping the tube and 1 mL of 50% (v/v) PEG 1450 (Sigma) in 1640 culture medium without calf serum for fusing was added with gentle shaking. Then the fused cell pellet was resuspended in HAT medium and distributed (100 μL per well) into the 96-well tissue culture plates. After 20% confluence was reached, aliquots of hybridoma supernatants (50 μL) were tested by ELISA to detect anti-UbcH10 antibodies. Selected positive clones were subcloned by limiting dilution. The MAb producing clones of interest were transferred for amplification culture. After 1 week, every mineral oil-primed mouse was injected with 106–107 of MAb hybridoma cells. One week later, ascites were collected and centrifuged at 1500 g for 10 min. IgG was purified from the ascites using ammonium sulfate precipitation and a Protein G Sepharose column (GE Healthcare) and analyzed by 12% SDS–PAGE.
Indirect ELISA assay
The titer of hybridoma supernatants and ascites was determined with indirect ELISA tests using the following procedure: 50 μg recombinant UbcH10 protein diluted with 10 mL coating buffer (0.05 M CBS [pH 9.6]) was pipetted into a 96-well microtiter plate (100 μL per well) and incubated at 4°C overnight. Plates were washed three times with PBST buffer [0.05% (V/V) Tween-20 in PBS] and blocked for 2 h at 4°C with 1% BSA. After 100 μL hybridoma supernatants were added to each well and incubated for 1 h at 37°C, unbound compounds were removed by washing solution. A total of 100 μL goat anti-mouse HRP-IgG conjugate diluted with PBS [1:10000 (V/V)] was added to each well for 1 h at 37°C, then washed three times with washing buffer. Then 100 μL TMB substrate solution was added to each well and the enzymatic reaction was stopped after 15 min incubation at 37°C by the addition of a 2 M H2SO4 solution. Absorbance (OD) values were measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA).
Determination of MAb isotype
MAb isotyping determination was carried out with the selected clone supernatants using ImmunoPure® Monoclonal Antibody Isotyping Kit (HPR/ABTS, Pierce, Rockford, IL) as instructed.
Western blot analysis
Proteins of purified UbcH10 and lysate of hepatoma carcinoma cells HepG2 and SMMC7721 were separated by SDS-PAGE. Samples were boiled for 5 min in loading buffer before running. Each lane was loaded with about 50 μg of proteins. After 1 h of electrophoresis at 100 V, the gel was immersed in the transfer buffer [48 mM Tris-HCl, 39 mM glycine, and 20% methanol, 0.037%(W/V)SDS], and the fractionated proteins were then transferred onto a 0.22 μM pore PVDF membrane (Bio-Rad) at 40 V for 1.5 h in an ice bath in a mini Trans-Blot electrophoretic transfer cell (Bio-Rad). The blotted membrane was rinsed with PBST and blocked with blocking buffer (5% skimmed milk powder in PBST) overnight at 4°C. The membrane was then cut into several strips according to the sample lane, and these strips were put into purified mouse monoclonal antibody against UbcH10 diluted by PBS (1:800, non-immunized mouse serum replaced UbcH10 antibody as negative control) and incubated at 37°C for 1 h, followed by three washings (10 min each) with TBS containing 0.1% Tween-20. These strips were then incubated with goat-anti-mouse IgG horseradish peroxidase diluted by PBS (1:5000, V/V) conjugate at room temperature for 1 h. The membrane was washed three times with TBS containing 0.1% Tween-20 and then analyzed using the enhanced chemiluminescence detection system (Pierce) and exposed to Fuji Medical X-ray film (Fujifilm, Tokyo, Japan) for 2–5 min.
Cross-reaction analysis of MAbs
The cross-reactivity of ascites antibody to various other similar proteins was tested. Five μg/mL recombinant human glutamate dehydrogenase, dual-specificity protein phosphatase 18 (DUSP 18), dual-specificity protein phosphatase 23 (DUSP 23), and normal rat serum with 50-fold dilution (100 μL per well, n=5) were added, respectively, in place of UbcH10 for coating in a 96-well microtiter plate. The coloration reaction was the same as indirect ELISA.
Immunofluorescence
Hepatoma carcinoma cells SMMC7721 grown on square glass coverslips in a 6-well cell culture plate were washed once with cold PBS and fixed in cold paraform prior to immunostaining. Purified MAbs diluted with PBS (1:500) were applied to the slips, incubated at 37°C for 1 h, and used as negative controls. After three washes with PBS, the slips were stained with goat-anti-mouse IgG conjugated with fluorescein isothiocyanate (Sigma) for 1 h at room temperature. The stained coverslips were rinsed with PBS and examined under a fluorescence microscope (Nikon, Tokyo, Japan).
Immunohistochemistry
Expression of UbcH10 protein was assessed by immunohistochemical analysis. Ten paired HCC paraffin-embedded tissue section samples (aged 23–79, with a mean of 48 years; 2 females and 8 males) for specificity assay of the MAb, containing tumor tissues and adjacent non-cancerous tissues, were collected from Nanfang Hospital (Guangzhou, China). Slides of deparaffinized tissue sections were placed in citrate buffer and treated with microwave heating for 20 min. A tissue immunohistochemistry staining kit (Maixin, Fujian, China) was used according to the manufacturer's protocols. Anti-UbcH10 MAbs (F001, F007, and F008) as primary antibody were used at a dilution of 1:500, and the sections were incubated overnight at 4°C. After washing in PBS, the sections were incubated with HRP-conjugated secondary antibody for 1 h at room temperature. DAB staining was carried out according to protocol.
Results
Expression and purification of recombinant UbcH10 protein
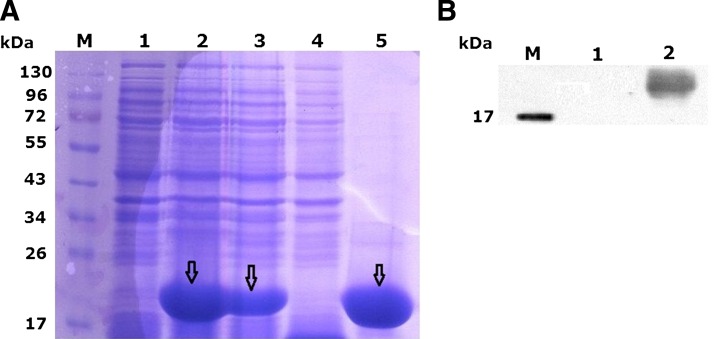
The complete cDNA of UbcH10 was amplified by RT-PCR from HepG2 cells. The recombinant plasmid pET-32a (+)/UbcH10 was identified by restriction analysis and then confirmed by sequencing. The confirmed construct pET-32a (+)/UbcH10 and pET-32a (+) were transformed into E. coli BL21 cells, respectively. Expression of the fusion protein was induced by IPTG and predicted to encode a recombinant protein with a molecular weight of about 20 kDa. Soluble and insoluble fractions from the supernatant and pellet of BL21 carrying pET-32a (+)/UbcH10 after sonication were collected and analyzed by SDS–PAGE and subsequent Coomassie Brilliant Blue staining. Bands of 6×His-UbcH10 with expected molecular weight of about 20 kDa were observed (Fig. 1A). Recombinant protein is mostly in the soluble supernatant fraction. The soluble 6×His-UbcH10 is indicated by an arrow. After purification, the purity of the recombinant protein was up to 90%. The recombinant protein was also analyzed by Western blot analysis using anti-His polyclonal antibody. The predicted bands of about 20 kDa recombinant 6×His-UbcH10 protein were visualized (Fig. 1B).
FIG. 1.
Expression and purification of UbcH10. (A) SDS-PAGE analysis of the recombinant UbcH10 protein. Expressed proteins were analyzed by 12% SDS-PAGE stained with Coomassie Bright Blue. Lanes 1 and 2 represent total cell lysate (TCL) after solubilization under the denatured condition from Escherichia coli BL21, uninduced and induced with 1.0 mM IPTG, respectively. Recombinant UbcH10 protein is indicated by arrow. Lanes 3 and 4 represent soluble supernatant and insoluble pellet extracts from E. coli BL21 cell lysates, respectively. Lane 5 was the soluble recombinant Ubch10 protein purified by Ni-NTA agarose. Purified recombinant UbcH10 protein about 20 kDa is indicated by arrow. M, protein molecular mass standards. (B) Identification by Western blot analysis using anti-His polyclonal antibody. M, protein marker (17 kDa); lane 1, purified recombinant UbcH10 protein; lane 2, supernatant of BL21 cell lysate with pET-32a(+).
MAb characteristics
The characterization of selected clones in terms of titer, affinity, concentration in culture medium, class, and subclass are summarized in Table 1. The antibodies F001, F007, and F008 were of G1 subclass with κ light chain.
Table 1.
Identification and Characterization of Anti-UbcH10 MAbs
| Hybridoma | Class and sub class | Type | Titer in culture medium | Titer in ascites | Content in culture medium (μg/ml) |
|---|---|---|---|---|---|
| F001 | IgG1 | κ | 1:5120 | 1:25600 | 7.20 |
| F007 | IgG1 | κ | 1:5120 | 1:25600 | 8.89 |
| F008 | IgG1 | κ | 1:10240 | 1:102400 | 19.54 |
Assessment of antibody specificity
The study of cross-reaction with protein similar to UbcH10 by ELISA results showed that the MAbs F001, F007, and F008 had a high specificity for UbcH10 and had no detectable reactivity with recombinant human glutamate dehydrogenase, DUSP 18, DUSP 23, and normal rat serum (Table 2), which demonstrated that the MAbs is specific to UbcH10.
Table 2.
Specificity Analysis of Anti-UbcH10 MAbs
| Hybridoma | UbcH10 | Glumate dehydrogenase | DUSP 18 | DUSP 23 | Normal rat serum |
|---|---|---|---|---|---|
| F001 | + | − | − | − | − |
| F007 | + | − | − | − | − |
| F008 | + | − | − | − | − |
Western blot analysis of HCC with MAbs
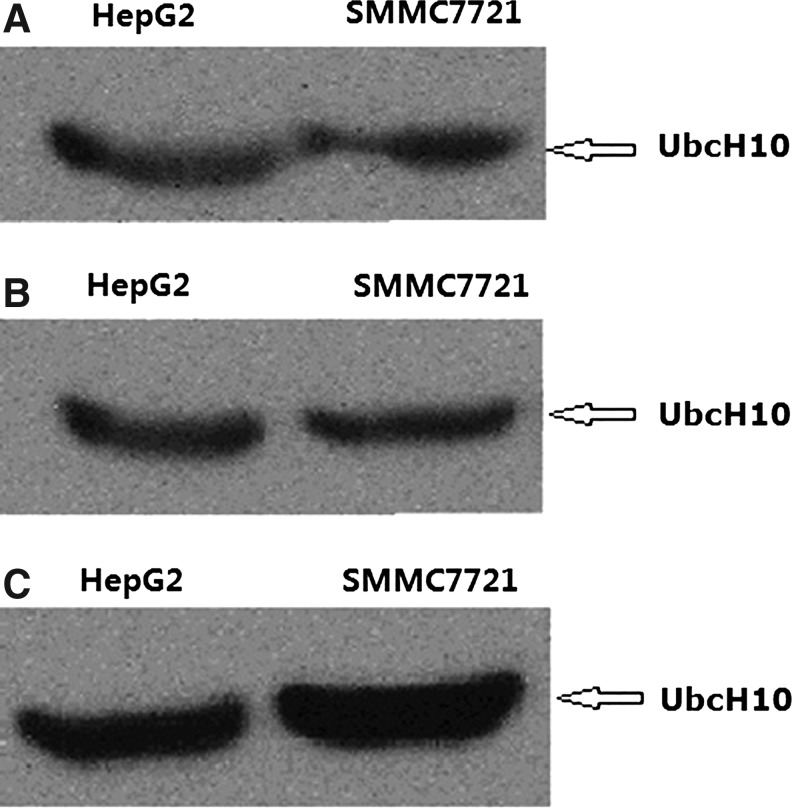
To confirm whether the obtained monoclonal antibodies recognize native UbcH10 protein, the purified MAbs were tested by Western blot analysis using hepatoma carcinoma cells as native antigen. The lysis of HepG2 and SMMC7721 was loaded as the target and the lysis of HepG2, in which PBS replaced MAbs, was used as the negative control. The results of Western blot analysis with the three MAbs are shown in Figure 2. An expected ∼20 kDa band of UbcH10 was detected in whole lysis of hepatoma carcinoma cells HepG2 and SMMC7721, but not in the negative control.
FIG. 2.
Western blot analysis of three different positive clones with HCC HepG2 and SMMC7721. 50 μg of supernatant of HCC HepG2 and SMMC7721 lysate were resolved by SDS-PAGE, blotted, and probed with the MAbs of three different positive clones against UbcH10 [staining with F001 (A), F007 (B), and F008 (C)] or non-immunized mouse serum replace Ubch10 antibody as negative control (data not shown), and followed by incubator with goat anti-mouse HRP-conjugated IgG antibody.
Immunofluorescence
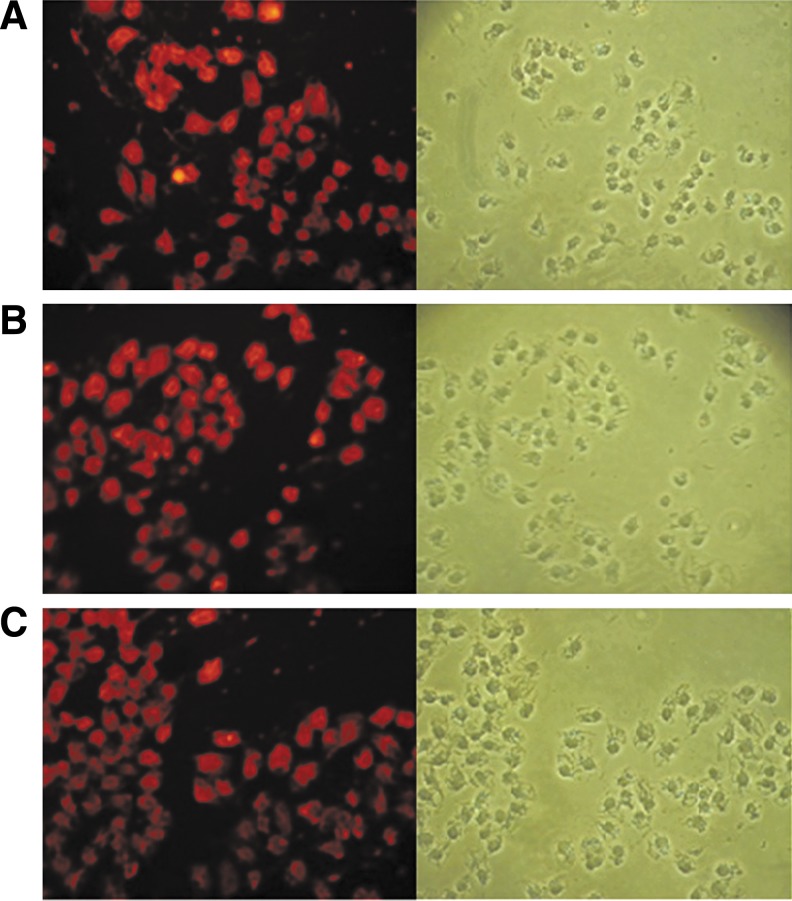
The immunofluorescence staining of hepatoma carcinoma cells with purified anti- UbcH10 MAbs (F001, F007, and F008) are shown in Figure 3. Red fluorescence on HCCs SMMC7721 staining with purified MAbs as primary antibody was observed with fluorescence microscope but not in controls, which showed that purified anti-UbcH10 MAbs (F001, F007, and F008) could specifically react with UbcH10 protein in HCC.
FIG. 3.
HCC immunofluorescence staining results of three different positive clones with HCC SMMC7721,×200. (A) HCC SMMC7721 staining with F001 observed with fluorescence microscope and optical microscope. (B) HCC SMMC7721 staining with F007 observed with fluorescence microscope and optical microscope. (C) HCC SMMC7721 staining with F008 observed with fluorescence microscope and optical microscope.
Immunohistochemistry
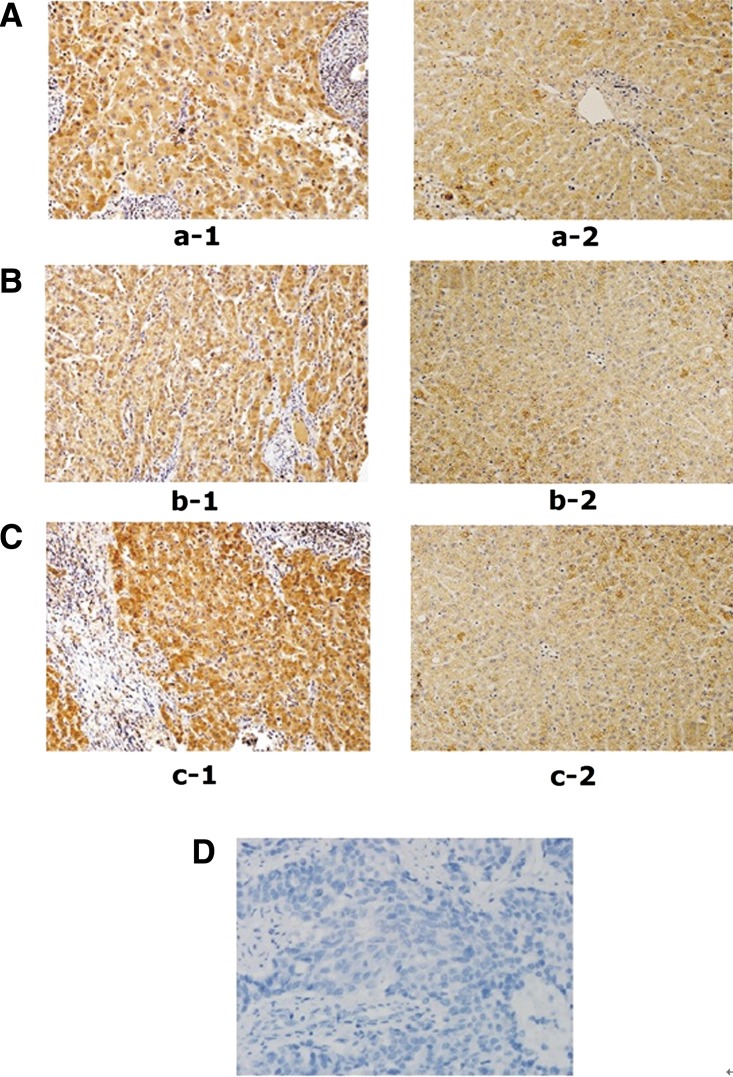
Ten pairs of HCC-including tumor tissues and adjacent non-cancerous tissues of immunohistochemistry staining were performed to examine the expression of UbcH10 in liver tissues and to determine the reactivity of affinity-purified anti-UbcH10 MAbs (F001, F007, and F008). UbcH10 staining was stronger in cancer tissues than in corresponding adjacent non-cancerous tissues, and expression of UbcH10 was detected mostly in the cytoplasm and occasionally in nuclei (Fig. 4). This suggested that UbcH10 expression is up-regulated in HCC tissues compared to adjacent non-cancerous tissues. Moreover, the purified antibodies can recognize the UbcH10 protein with high activity and specificity, and serve as a good tool for further research on the biological functions related to UbcH10.
FIG. 4.
Immunohistochemical analysis of three different positive clones with HCC tissues,×200 (UbcH10-positive staining was brown and mostly located in nucleolus and occasionally in nuclei). (A) HCC tissues staining with F001 (1) and corresponding non-cancerous liver tissues staining with F001 (2). (B) HCC tissues staining with F007 (1) and corresponding non-cancerous liver tissues staining with F007 (2). (C) HCC tissues staining with F008 (1) and corresponding non-cancerous liver tissues staining with F001 (2). (D) Negative control,×400 (non-immunized mouse serum replaced primary antibody as control).
Discussion and Conclusion
In our research, we first developed monoclonal antibodies against UbcH10 using the recombinant expression protein as antigen. The subsequent series assay showed that our developed monoclonal antibodies were suited to indirect ELISA, Western blot, immunofluorescence, and immunohistochemical detection in clinical oncology.
UbcH10 regulates and controls the cell cycle(22) and takes part in initiation, progression, and transformation.(23) To date there is a very little research on the function of UbcH10, partly due to the unavailability of the perfect anti-UbcH10 monoclonal antibody. Therefore, the developed monoclonal antibody is not only a basic tool for further research on the function of the UbcH10 but especially for early diagnosis of cancer. More and more studies on tumor genesis and development demonstrate that the members of the APC directly participate in regulating and controlling cell cycle.(21,22) UbcH10 is a member of the anaphase promoting complex or cyclosome (APC/C) and it not only regulates and controls the cell cycle,(22) but also takes part in initiation, progression, and transformation(23) and significantly increases expression in HCC and other tumor tissues. So detection of the expression of UbcH10 might be a useful indicator in the prognosis of cancer patients, and it could be a potential cancer biomarker.
Our immunohistochemistry results from 10 pairs of clinical samples showed that UbcH10 has a higher expression in HCC tissues than in normal liver tissues, which is consistent with a previous report by Ieta and associates.(14) To date there have been commercial antibody products of UbcH10, but they were mostly polyclonal antibodies. It also proved that the developed monoclonal antibodies can work dependably compared with merchandized polyclonal antibodies.(24)
In conclusion, highly specific and sensitive monoclonal antibodies against UbcH10 were produced for the potential application of clinical cancer pathological detection as a new diagnostic agent for cancer. They could also be a useful tool in the exploration of the function of UbcH10 in tumor genesis and development and the mechanism of the pathogenesis and progression of cancer cells.
Acknowledgment
This work was supported by a grant (no. 20060102A3006 and no. 2012AA020205) awarded by the national “863” High-Tech Development Program (China).
Author Disclosure Statement
The authors have no financial or other conflicts of interest to disclose. In addition, all authors have seen and approved the final version of this manuscript for submission.
References
- 1.Townsley FM. Aristarkhov A. Beck S. Hershko A. Ruderman JV. Dominant-negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase. Cell Biol. 1997;94:2362–2367. doi: 10.1073/pnas.94.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganoth D. Leshinsky E. Eytan E. Hershko A. A multicomponent system that degrades proteins conjugated to ubiquitin. Resolution of factors and evidence for ATP-dependent complex formation. J Biol Chem. 1988;25:12412–12419. [PubMed] [Google Scholar]
- 3.Yamanaka A. Hatakeyama S. Kominami K. Kitagawa M. Matsumoto M. Nakayama K. Cell cycle-dependent expression of mammalian e2-c regulated by the anaphase-promoting complex/cyclosome. Mol Biol Cell. 2000;11:2821–2831. doi: 10.1091/mbc.11.8.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto Y. Ozaki T. Miyazaki K. Aoyama M. Miyazaki M. Nakagawara A. UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 2003;63:4167–4173. [PubMed] [Google Scholar]
- 5.Wagner KW. Sapinoso LM. El-Rifai W. Frierson HF. Butz N. Mestan J. Hofmann F. Deveraux QL. Hampton GM. Overexpression, genomic amplification and therapeutic potential of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of diverse anatomic origin. Oncogene. 2004;23:6621–6629. doi: 10.1038/sj.onc.1207861. [DOI] [PubMed] [Google Scholar]
- 6.Troncone G. Guerriero E. Pallante P. Berlingieri MT. Ferraro A. Del Vecchio L. Gorrese M. Mariotti E. Iaccarino A. Palmieri EA. Zeppa P. Palombini L. Fusco A. UbcH10 expression in human lymphomas. Histopathology. 2009;54:731–740. doi: 10.1111/j.1365-2559.2009.03296.x. [DOI] [PubMed] [Google Scholar]
- 7.Berlingieri MT. Pallante P. Sboner A. Barbareschi M. Bianco M. Ferraro A. Mansueto G. Borbone E. Guerriero E. Troncone G. Fusco A. UbcH10 is overexpressed in malignant breast carcinomas. Eur J Cancer. 2007;43:2729–2735. doi: 10.1016/j.ejca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Chen CC. Chang TW. Chen FM. Hou MF. Hung SY. Chong IW. Lee SC. Zhou TH. Lin SR. Combination of multiple mrna markers (pttg1, survivin, ubch10 and tk1) in the diagnosis of Taiwanese patients with breast cancer by membrane array. Oncology. 2006;70:438–446. doi: 10.1159/000098557. [DOI] [PubMed] [Google Scholar]
- 9.Pallante P. Berlingieri MT. Troncone G. Kruhoffer M. Orntoft TF. Viglietto G. Caleo A. Migliaccio I. Decaussin-Petrucci M. Santoro M. Palombini L. Fusco A. UbcH10 overexpression may represent a marker of anaplastic thyroid carcinomas. Br J Cancer. 2005;93:464–471. doi: 10.1038/sj.bjc.6602721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JJ. Au AY. Foukakis T. Barbaro M. Kiss N. Clifton-Bligh R. Staaf J. Borg A. Delbridge L. Robinson BG. Wallin G. Höög A. Larsson C. Array-CGH identifies cyclin D1 and UBCH10 amplicons in anaplastic thyroid carcinoma. Endo Rel Cancer. 2008;15:801–815. doi: 10.1677/ERC-08-0018. [DOI] [PubMed] [Google Scholar]
- 11.Berlingieri MT. Pallante P. Guida M. Nappi C. Masciullo V. Scambia G. Ferraro A. Leone V. Sboner A. Barbareschi M. Ferro A. Troncone G. Fusco A. UbcH10 expression may be a useful tool in the prognosis of ovarian carcinomas. Oncogene. 2007;26:2136–2140. doi: 10.1038/sj.onc.1210010. [DOI] [PubMed] [Google Scholar]
- 12.Walker G. MacLeod K. Williams AR. Cameron DA. Smyth JF. Langdon SP. Estrogen-regulated gene expression predicts response to endocrine therapy in patients with ovarian cancer. Gynecol Oncol. 2007;106:461–468. doi: 10.1016/j.ygyno.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Israeli O. Goldring-Aviram A. Rienstein S. Ben-Baruch G. Korach J. Goldman B. Friedman E. In silico chromosomal clustering of genes displaying altered expression patterns in ovarian cancer. Cancer Genetics Cytogenetics. 2005;160:35–42. doi: 10.1016/j.cancergencyto.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Ieta K. Ojima E. Tanaka F. Nakamura Y. Haraguchi N. Mimori K. Inoue H. Kuwano H. Mori M. Identification of overexpressed genes in hepatocellular carcinoma, with special reference to ubiquitin-conjugating enzyme E2C gene expression. Int J Cancer. 2007;121:33–38. doi: 10.1002/ijc.22605. [DOI] [PubMed] [Google Scholar]
- 15.Lin J. Raoof DA. Wang Z. Lin MY. Thomas DG. Greenson JK. Giordano TJ. Orringer MB. Chang AC. Beer DG. Lin L. Expression and effect of inhibition of the ubiquitin-conjugating enzyme e2c on esophageal adenocarcinoma. Neoplasia. 2006;8:1062–1071. doi: 10.1593/neo.05832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita T. Ikeda H. Taira N. Hatoh S. Naito M. Doihara H. Overexpression of UbcH10 alternates the cell cycle profile and accelerate the tumor proliferation in colon cancer. BMC Cancer. 2009;87:1–10. doi: 10.1186/1471-2407-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S. Chen Y. Hu C. Jing H. Cao Y. Liu X. Association of clinicopathological features with UbcH10 expression in colorectal cancer. J Cancer Res Clin Oncol. 2010;136:419–426. doi: 10.1007/s00432-009-0672-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen SM. Jiang CY. Wu JY. Liu B. Chen YJ. Hu CJ. Liu XX. RNA interference-mediated silencing of UBCH10 gene inhibits colorectal cancer cell growth in vitro and in vivo. Clin Exper Pharmacol Physiol. 2010;37:525–529. doi: 10.1111/j.1440-1681.2009.05348.x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y. Ishii Y. Nishida Y. Ikarashi M. Nagata T. Nakamura T. Yamamori S. Asai S. Detection of aberrations of ubiquitin-conjugating enzyme E2C gene (UBE2C) in advanced colon cancer with liver metastases by DNA microarray and two-color FISH. Cancer Genetics Cytogenetics. 2006;168:30–35. doi: 10.1016/j.cancergencyto.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Jiang L. Bao Y. Luo C. Hu G. Huang C. Ding X. Sun K. Lu Y. Knockdown of ubiquitin-conjugating enzyme E2C/UbcH10 expression by RNA interference inhibits glioma cell proliferation and enhances cell apoptosis in vitro. J Cancer Res Clin Oncol. 2009;136:211–217. doi: 10.1007/s00432-009-0651-z. [DOI] [PubMed] [Google Scholar]
- 21.Shi D. Grossman SR. Ubiquitin becomes ubiquitous in cancer: emerging roles of ubiquitin ligases and deubiquitinases in tumorigenesis and as therapeutic targets. Cancer Biol Ther. 2010;8:737–747. doi: 10.4161/cbt.10.8.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y. Hwang WC. Basavappa R. Structural and functional analysis of the human mitotic-specific ubiquitin-conjugating enzyme, UbcH10. J Biol Chem. 2002;24:21913–21921. doi: 10.1074/jbc.M109398200. [DOI] [PubMed] [Google Scholar]
- 23.van Ree JH. Jeganathan KB. Malureanu L. van Deursen JM. Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J Cell Biol. 2010;188:83–100. doi: 10.1083/jcb.200906147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen ST. Winter JN. Epstein AL. Application of monoclonal antibodies to tumor diagnosis and therapy. Ann Clin Lab Sci. 1983;13:173–184. [PubMed] [Google Scholar]