Abstract
Transgenic birds embody one of the most potent and exciting research tools in biotechnology for agriculture, medicine, and model animals. To date, retrovirus- or lentivirus-mediated transgenesis has been established in chickens and quail. However, despite having a valid technique for viral transduction to achieve transgenic birds, many obstacles exist for practical applications because of relatively low and variable rates of germ-line transmission and transgenic offspring showing transgene silencing, as well as safety issues related to viral vector use. Thus, the generation of transgenic poultry by nonviral integration is a prerequisite for the introduction of biotechnology to practical applications. Herein, we show that a germ-line–competent chicken primordial germ-cell (PGC) line was established with high efficiency of transmission to offspring and that piggyBac transposition into PGCs improved the efficiency of transgenic chicken production and led to high-level transgene expression. GFP transgene-expressing donor PGC-transferred recipient chickens produced donor-derived progenies, and the germ-line transmission efficiency of donor PGCs was 95.2% on average. Subsequently, half of the donor-derived offspring (52.2%) were transgenic chicks because GFP-expressing donor PGCs, in which a transgene was inserted into one chromosome 20, were heterozygous. In all of the transgenic chickens, GFP expression was constant and strong, regardless of age. Our results demonstrate that piggyBac transposition into the chicken PGC line could be the surest way to generate transgenic chickens safely for practical applications.
Keywords: germ-line chimera, piggyBac transposon, avian
The primordial germ-cell (PGC), a precursor of the sperm or oocyte, is a unique and invaluable cell type because a germ cell is the only cell that can transfer genetic information to the next generation. Nevertheless, an in vitro long-term culture system for stable PGC lines remains to be established in mammals, including the mouse. Only chicken PGCs have been propagated continuously in vitro without loss of germ-cell properties (1–3). Germ-line–competent PGC lines could be versatile tools for studying germ-cell biology, including the transcriptional regulation of microRNAs (4), and for creating transgenic chickens (1). van de Lavoir et al. (1) first reported the germ-line transmission of genetically modified chicken PGCs by electroporation of a nonviral expression vector. In avian species, however, the frequencies of transgene integration into the genome, as well as the rates of gene transfer into avian germ cells, remain inefficient for generating transgenic birds using virus-independent conventional methods. On the other hand, transgene silencing has hampered the stable expression of drug selection genes when transgenes are transferred by nonviral transfection (1, 5, 6).
DNA transposons are genetic elements that can relocate between different genomic sites, and the first DNA transposon was discovered in maize (7). Because of their tendency to insert into the genome, these elements have been used extensively for the genetic modification of various organisms. Among DNA transposons, a piggyBac transposon isolated from the cabbage looper moth Trichoplusia ni (8) was found to efficiently transpose a transgene in chicken cells as well as in mice and humans (9–11). piggyBac exhibits significantly higher transposition efficiency and also accommodates relatively large transgenes without compromising transposition efficiency (12). Additionally, piggyBac-mediated mutagenesis is very efficient for germ-line transmission in mice (10). This virus-independent gene transfer system has demonstrated that fibroblasts can be successfully reprogrammed to induced pluripotent stem cells in mouse and human (13). This unique property of the piggyBac transposon by a cut-and-paste mechanism rearranges a transgene into the TTAA sequences of the genome regardless of species (11, 14).
In avian species, Lu et al. (11) first demonstrated that a piggyBac transposon usefully mediated the insertion of a transgene into chicken embryos during developmental stages. However, there is no report on the creation of viable transgenic chickens using a piggyBac transposon. In the present study, we developed a germ-line–competent PGC line with high-transmission efficiency, and established drug selection after efficient gene transfer with the piggBac transposon and transposase. This nonviral transgenic poultry is a promising approach that could lead to practical applications in agriculture and biopharmacy, and help advance a comprehensive understanding of avian biology itself.
Results
Establishment of Gonadal PGC Line.
PGCs from 6-d-old gonads of each White Leghorn (WL) embryo were prepared and maintained without loss of germ-cell integrity. In our previous report (2), chicken PGCs were maintained without any feeder layer, but in the present study chicken PGCs were subcultured onto mitomycin-inactivated mouse embryonic fibroblasts (MEFs) in 5- to 6-d intervals. Compared with the feeder-free procedure, the majority of chicken PGCs on MEFs grew as a single cell and could be subpassaged by gentle pipetting without any enzyme treatment.
Characterizations After Transfection and G418-Selection of Chicken PGCs.
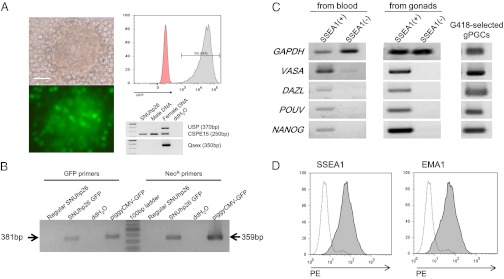
The expression rate of the piggyBac GFP plasmid was 15–20% after 2 d of transfection and GFP-expressing PGC colonies were completely selected after 1–1.5 mo with 300 μg/mL G418 (Fig. 1 A and B). Flow cytometry analysis showed that the selected male PGCs (SNUhp26) strongly expressed GFP without any transgene silencing (Fig. 1A). In addition, these PGCs continuously exhibited germ-cell properties with the expression of germ-cell– and stem-cell–specific genes (VASA, DAZL, PouV, and NANOG) (Fig. 1C) and were positive to germ-cell–specific antibodies (SSEA1 and EMA1 antibodies) (Fig. 1D). Similarly, other independent PGC line (SNUhp1) could be transfected and G418-selected after piggBac transposition (Fig. S1A). After transplantation into recipients, GFP+ PGCs relocalized into the recipient embryonic gonads and constantly expressed the GFP transgene during the development of embryonic gonads (Fig. 2 A and B, and Fig. S1 B and C).
Fig. 1.
G418-selected SNUhp26 PGCs after transfection with a piggyBac CMV-GFP expression vector. (A) G418-selected SNUhp26 PGCs (p48) for 61 d. Flow-cytometry analysis represented that G418-selected PGCs constantly expressed GFP. Genomic PCR results indicated that SNUhp26 was a male-derived PGC line. (Scale bar, 50 μm.) (B) PCR analysis of G418-selected PGCs using GFP-specific primers and NeoR-specific primers. (C) RT-PCR analysis of G418-selected PGCs for germ-cell–specific (vasa and dazl) and stem-cell–specific genes (PouV and nanog). SSEA1-positive intact PGCs from blood vessel of 53-h embryos or 6-d-old embryonic gonads expressed both germ-cell– and stem-cell–specific genes, whereas SSEA1-negative somatic cells did not. (D) Flow cytometry analysis of G418-selected PGCs with SSEA1 and EMA1, which are specific antibodies for chicken germ cells. Phycoerythrin (PE)-conjugated secondary antibody for mouse IgM was used and the results were analyzed by flow cytometry.
Fig. 2.
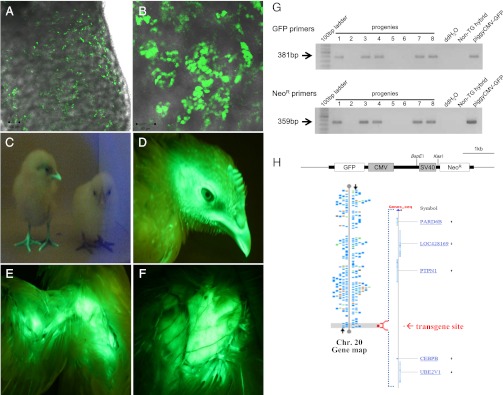
Detection of GFP-expressing PGCs in recipient embryonic gonads and GFP-expressing transgenic chickens. Detection of GFP-expressing donor PGCs in recipient testes of 18-d-old (A) and hatched chicks (B) by confocal laser scanning microscope. Fluorescent illustrations of hatched chicks (C, the left chick is transgenic and the right chick is a normal hybrid), and head (D), wing (E), and breast (F) of a 10-wk-old transgenic chicken. Transgenic chickens showed strong GFP expression regardless of age. (G) Genomic PCR analysis of transgenic offspring. Transgenic progenies (lanes 1, 3, 4, 7, and 8) were positive for both GFP primers and NeoR primers. Nontransgenic hybrids from the test-cross were negative control chicks. (H) Identification of the piggyBac GFP transgene site by DNA walking. A transgene was integrated into the distal end of chromosome 20. No functional gene or transcript was found within ∼0.1 Mb of the transgene integration site. Alignment of the transgene-flanking sequences from DNA walking analysis was conducted using the BLAST Assembled Genome Database (http://blast.ncbi.nlm.nih.gov).
Through DNA walking analysis of G418-selected and GFP-expressing PGCs, a single copy of GFP transgene was identified as a distal insertion of chromosome 20 (Fig. 2). Analysis of the 5′-flaking insertion sequences of transgene on chromosome 20 showed that there was no functional gene or transcript at the insertion site (Fig. 2) and that the piggyBac transposon was integrated into the TTAA sequences similar to a previous report in chicken cells (11).
Production of Germ-Line Chimeras and Transgenic Offspring.
For transplantation into recipients, PGCs (p46–p49) maintained for about 7–8 mo, including the G418-selection period of 45–67 d, were used (Table 1). In the recipient hosts following sexual maturation, the cultured PGCs differentiated into functional gametes and generated viable offspring (Fig. 2, Table 1, and Fig. S2). All of six donor PGC-transplanted roosters produced the white hybrid progenies derived from donor PGCs and the efficiency of germ-line transmission of donor PGCs to the offspring ranged from 90.4–98.9%. A total of 437 of the 459 chicks that hatched from six male founders were donor PGC-derived hybrids and the rate of germ-line transmission was 95.2% on average. Thus, the transmission rates of individual male founders were much higher and uniform in this study (Table 1 and Fig. S2). Only male SNUhp26 PGC-transplanted roosters were test-crossed because the reverse-sexed chimeras failed to produce donor-derived offspring and the efficiency of germ-line transmission was quiet low compared with those of the single-sex chimeras of previous reports (1, 3, 15).
Table 1.
Efficiency of germ-line transmission and transgenic chick production for G418-selected donor PGCs
| ID no. | Period of G418-selection (passage) | No. of hatched chicks | No. of endogenous germ-cell–derived chicks* (%) | No. of donor germ-cell–derived chicks† (%) | No. of transgenic chicks (%)‡ |
| 9154B | 45 d (p46) | 63 | 4 (6.3) | 59 (93.7) | 32 (54.2) |
| 9155B | 45 d (p46) | 76 | 2 (2.6) | 74 (97.4) | 36 (48.6) |
| 9173B | 54 d (p47) | 102 | 5 (4.9) | 97 (95.1) | 51 (52.6) |
| 9176B | 54 d (p47) | 52 | 5 (9.6) | 47 (90.4) | 23 (48.9) |
| 9186B | 67 d (p49) | 89 | 1 (1.1) | 88 (98.9) | 45 (51.1) |
| 9187B | 67 d (p49) | 77 | 5 (6.5) | 72 (93.5) | 41 (56.9) |
| Total | 459 | 22 (4.8) | 437 (95.2) | 228 (52.2) |
*Test-cross analysis was conducted by mating between KO (i/i) and germ-line chimeric KO (i/i); that is, transplanted GFP-expressing donor PGCs of WL (I/I). The phenotype of endogenous recipient germ-cell–derived chick is black KO (i/i).
†The phenotype of offspring derived from donor GFP+ PGCs of WL (I/I) is white hybrid (I/i) between KO (i/i) and WL (I/I).
‡The percentage of GFP-expressing transgenic chicks in donor germ-cell–derived hybrid chicks.
Among the donor PGC-derived progenies, the transgenic chicks could be easily identified with a fluorescent excitation lamp and the detection filters because of strong GFP expression in all transgenic chick tissues, including the beak, feather pulp, feet, and muscle (Fig. 2 C–F and Fig. S2). A total of 52.2% of donor-derived hybrids were GFP-expressing transgenic chicks because the transgene of the donor PGCs was heterozygous. Approximately half of donor-derived offspring were transgenic, and the others were nontransgenic hybrids (52.2% vs. 47.8%) (Table 1). Transgenic chickens constantly expressed GFP in all tissue regardless of age (Fig. 2 C–F and Fig. S3). Additionally, G1 offspring were fertile and able to produce GFP-expressing G2 progenies after sexual maturation.
Identification of Transgenic Chickens.
Although transgenic chicks could be identified with the fluorescent excitation lamp and the filters, the piggyBac GFP transgene was confirmed in the transgenic offspring by genomic PCR and Western blotting. Transgenic progenies were positive for both GFP- and NeoR-specific primers by PCR (Fig. 2G). Subsequently, the insertion site in the genome was identified by DNA walking with genomic DNAs of four transgenic chicks from three different male founders. As a result (Fig. S4), the GFP transgene integration site in all transgenic progenies was identical to the donor PGCs located on the distal end of chromosome 20. Surprisingly, all organs of transgenic chicks including intestine, heart, and liver constantly expressed GFP without tissue-specific transgene silencing, and GFP expression was confirmed in the muscle, heart, and liver of transgenic chicks by Western blotting (Fig. S3).
Discussion
The culturing of chicken PGCs could be a versatile tool with applications in industry as well as germ-cell research. Recently, Choi et al. (2) and Macdonald et al. (3) demonstrated that basic FGF (bFGF) plays a critical role in the activation of MEK/ERK signaling and the stimulation of the proliferation of chicken PGCs in vitro. In these previous reports, chicken PGC lines derived from migrating PGCs in embryonic blood vessels were established and characterized for studying cell signaling and generating germ-line chimera (1–3). In the present study, PGCs retrieved from embryonic gonads at 6 d were prepared individually and maintained to develop PGC lines. The advantage of using gonadal PGCs compared with circulating PGCs is that a greater number of PGCs can be retrieved from one embryo; therefore, many PGC lines can be developed easily from each embryo. From gonadal PGCs, the best germ-line–competent PGC line could be efficiently selected to generate transgenic chickens, as well as germ-line chimeras. Basically, cell growth and characteristics were not different between blood and gonadal PGCs, and gonadal PGCs also showed the bFGF-dependent growth rate.
The transgene integration site was only on chromosome 20 without any other insertion, and the piggyBac transposon was integrated into the TTAA sequences similar to a previous report on chicken cells (11). No functional gene or transcript existed in the flanking regions of the transgene. This result suggests that the transgene insertion site in the chicken genome should be a critical factor for the success of transgenic chicken generation, as well as the constant expression of the transgene. PGCs in which any functional gene is disrupted could not survive or proliferate during G418-selection culture, and only PGCs without any destruction of integrity were naturally selected and dominantly populated. Additionally, the picking-up of GFP+ PGCs during the selection led to uniformity of the donor PGC population. The insertion preference of a piggyBac transposon should be further investigated in the chicken genome. After transplantation into recipients, GFP+ PGCs relocalized into the recipient embryonic gonads and showed constant GFP transgene expression during the development of embryonic gonads (Fig. 2 A and B, and Fig. S1).
In the first report on germ-line chimera production using established PGC lines (1), the efficiency of donor PGC-derived chicks in the total hatchlings from germ-line chimeric chickens varied from 0.1 to 86.0%. Choi et al. (2) reported 12.5–82.6% (49.0% on average) germ-line transmission by transferring mixed PGCs, and Macdonald et al. (3) estimated that the frequency of donor-derived sperm was 1–30% in the semen of recipient roosters, even though the endogenous PGCs of recipient embryos were depleted by irradiation. The frequencies in these previous reports were similar to the rates of germ-line transmission obtained from chimeras after the transfer of freshly isolated or short-term cultured PGCs (15, 16). However, in the test-cross analysis of the present study, the efficiency of germ-line transmission of donor PGCs ranged from 90.4 to 98.9% in six donor PGC-transplanted founders (437 donor-derived chicks of 459 total hatched chicks, 95.2%). In contrast with the previous data, these transmission rates were relatively high and uniform. One possible explanation would be the difference between circulating PGCs in blood vessels and gonadal PGCs, suggesting that PGCs prepared from gonads could be more easily adapted and programmed to the environment of gonads in the recipient embryos when they migrate. Although no phenotypic change between the two sources of PGCs was observed and the expression patterns of germ-cell– or stem-cell–specific markers were identical, further understanding is necessary to evaluate the difference between circulating PGCs and gonadal PGCs. Another possibility is that imperceptible differences could exist between each PGC line regardless of their origins. The germ-cell differentiation of donor PGCs in the recipients should be PGC line-dependent and impalpable distinctions for adaptation and differentiation may occur among PGC lines.
Various transgenic methods have been attempted to manipulate poultry genomes for agricultural and pharmaceutical applications. Viral transduction techniques using retrovirus or lentivirus have been well established for creating transgenic birds. The conventional transgenic protocol was a transduction of blastodermal cells at stage X by injecting replication-deficient virus (17–21). Lillico et al. (21) reported successful generation of transgenic hens by injection of lentivirus containing recombinant human IFN genes controlled by a synthetic tissue-specific promoter, and the expression level of the foreign protein in transgenic hens ranged from 3.5 μg/mL to 426 μg/mL. Using a high-titer retrovirus, Kamihira et al. (19) produced transgenic hens expressing single-chain Fv-Fc fusion protein at the average level of 5.6 mg/mL. However, despite a valid technique of viral transduction for generating transgenic birds, there are lots of obstacles for the practical applications, particularly because of safety issues.
One of the technical problems in transgenic animals is transgene-silencing in vitro or in vivo. van de Lavoir et al. (1) reported that conventional electroporation protocols that were used routinely to introduce genetic modifications into chicken cells failed to yield genetically modified PGCs because of potential transgene silencing. Unexpectedly, various organs, such as intestine, heart, and liver of transgenic chicks from PGCs with a piggyBac GFP transgene constantly expressed GFP without tissue-specific transgene silencing (Fig. 2 C–F and Fig. S3). Lu et al. (11) first adapted piggyBac-mediated gene transfer in chicken embryos by in ovo electroporation and demonstrated that it was a versatile transgene expression technique for chicken embryos. Notwithstanding, no viable transgenic chicken has been generated using the piggyBac system. Thus, in the present study, we report a unique generation of transgenic chickens using piggyBac-mediated gene transfer into chicken PGCs.
The chicken embryo is a classic model that provides an excellent and reproducible system for gaining insights into embryonic developmental processes (22). The efficient transgenic technique described herein ushers in the possibility of using chicks as a system for bridging knowledge among industrial applications in agriculture and biopharmacy, as well as advancing our comprehensive understanding of avian biology itself.
Materials and Methods
Experimental Animals and Animal Care.
The care and experimental use of chickens were approved by the Institute of Laboratory Animal Resources, Seoul National University (SNU-070823-5). Chickens were maintained according to a standard management program at the University Animal Farm, Seoul National University, Korea. The procedures for animal management, reproduction, and embryo manipulation adhered to the standard operating protocols of our laboratory.
Culturing of the Gonadal PGC Line.
PGCs from WL embryonic gonads at day 6 (stage 28) were maintained and subpassaged with knockout DMEM (Invitrogen) supplemented with 20% (vol/vol) FBS (Invitrogen), 2% (vol/vol) chicken serum (Sigma-Aldrich), 1× nucleosides (Millipore), 2 mM l-glutamine, 1× nonessential amino acids, β-mercaptoethanol, 10 mM sodium pyruvate, and 1× antibiotic–antimycotic (Invitrogen). Human bFGF (10 ng/mL; Koma Biotech) was used for PGC self-renewal. Chicken PGCs were cultured in an incubator at 37 °C with an atmosphere of 5% CO2 and 60–70% relative humidity. The cultured PGCs were subcultured onto mitomycin-inactivated MEFs in 5- to 6-d intervals by gentle pipetting without any enzyme treatment. Sex of PGC lines was identified by genomic PCR with W chromosome-specific primers (Table S1).
Construction of the piggyBac Expression Vector.
The plasmids containing CAGG-PBase (pCyL43) and piggyBac transposon (pCyL50), which were used to examine the piggyBac integration site distribution in the mouse genome (10), were donated by the Sanger Institute (http://www.sanger.ac.uk/technology/clonerequests). The GFP gene expressed by CMV immediate-early enhancer/promoter and neomycin-resistance (NeoR) gene with Simian vacuolating virus 40 (SV40) promoter were cloned between the 5′ and 3′ piggyBac transposon elements by PacI digestion and ligation.
Transfection and G418-Selection.
The piggyBac GFP vector and CAGG-PBase (pCyL43) were cointroduced into the established PGC lines using the Amaxa Nucleofector (L buffer and A-020 program) or lipofection with Lipofectamine reagent (Invitrogen). One day after transfection, 300 μg/mL G418 was added to the culture media for selection, and GFP-expressing cells or colonies were picked under a fluorescence microscope during G418 selection.
Detection and Characterization Following G418-Selection.
The GFP transgene in G418-selected PGCs was detected by genomic PCR using GFP-specific primers (forward 5′-gaa gtt cat ctg cac cac cg-3′ and reverse 5′-atg ttg tgg cgg atc ttg aa-3′) or NeoR primers (forward 5′-tgt gct cga cgt tgt cac tg-3′ and reverse 5′-cca cca tga tat tcg gca ag-3′). In addition, RT-PCR and flow-cytometry analyses were conducted to examine whether G418-selected PGCs preserved the germ-cell properties. RT-PCR primer sequences for germ-cell–specific (vasa and dazl) or stem-cell–specific (PouV and nanog) genes are presented in Table S1. For flow cytometry, G418-selected PGCs were resuspended in PBS containing 1% BSA and strained through a cell strainer (40 μm, BD Falcon; Becton Dickinson). Aliquots of cells were incubated in 500 μL 1% BSA in PBS containing primary antibodies (SSEA1, stage-specific embryonic antigen1 or EMA1) on ice for 30 min. After washing with PBS, cells were incubated in phycoerythrin (PE)-conjugated secondary antibodies on ice for another 20 min. Flow cytometry was performed on a FACS Calibur (Becton Dickinson). All subsequent was analyses were performed using FlowJo software (Tree Star).
Identification of the Transgene Integration Site by DNA Walking.
The transgene insertion site was identified using the DNA Walking SpeedUp Premix Kit-II (Seegene) according to the manufacturer’s protocol. The products of the third round of DNA walking PCR were cut out of the agarose gel and purified using the Power Gel Extraction Kit (Takara Bio) and then cloned directly into the pGEM-T Easy Vector (Promega). The cloned plasmids were sequenced using an ABI Prism 3730 XL DNA Analyzer (Applied Biosystems). The sequences of the 5′-flanking region were analyzed using the BLAST Assembled Genome Database (http://blast.ncbi.nlm.nih.gov) and the University of California at Santa Cruz Genome Browser (http://www.genome.ucsc.edu).
Microinjection and Detection of GFP-Expressing PGCs in Recipients.
For PGC transplantation into recipient embryos, a small window was made on the pointed end of the recipient Korean Oge (KO) egg and a 2-μL aliquot containing more than 1,000 PGCs was microinjected with a micropipette into the dorsal aorta of the recipient embryo. The egg window of the recipient embryo was sealed with paraffin film and the egg was incubated with the pointed end down until further screening and hatching. Following transfer into the recipient embryos, GFP-expressing PGCs were detected in the testes of the recipients at 18 d of incubation and hatching. The gonads or testes were dissected at different stages and live images of GFP+ transplanted PGCs were observed using a confocal laser scanning microscope (LSM 700; Carl Zeiss).
Test-Cross Analysis and Detection of GFP Transgenic Chickens.
WLs with a dominant pigmentation inhibitor gene (I/I) and KO with a recessive pigmentation inhibitor gene (i/i) were used for the donor PGCs and the recipient embryos, respectively. Through test-cross analysis by mating with KO females (i/i), the germ-line chimeras were identified by the phenotype of their offspring. Endogenous germ cells in the KO recipient male chickens (i/i) produce only black KO because of the recessive pigmentation inhibitor gene (i/i), whereas WL donor-derived germ cells (I/I) produce white hybrids with I/i. The hatched green transgenic offspring could be identified easily using a fluorescent excitation lamp with detection filters (BLS Ltd.).
Western Blotting.
Aliquots (16 μg) of protein from the heart, liver and muscle were electrophoresed on 12.5% SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Millipore). GFP was detected by blotting with rabbit anti-GFP IgG (Santa Cruz Biotechnology) and goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology) secondary antibody.
Supplementary Material
Acknowledgments
This work was supported by Grant PJ008142 from the Next-Generation BioGreen 21 Program, Rural Development Administration, and by World Class University program Grant R31-10056 through the National Research Foundation funded by the Ministry of Education, Science and Technology, Korea.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203823109/-/DCSupplemental.
References
- 1.van de Lavoir MC, et al. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–769. doi: 10.1038/nature04831. [DOI] [PubMed] [Google Scholar]
- 2.Choi JW, et al. Basic fibroblast growth factor activates MEK/ERK cell signaling pathway and stimulates the proliferation of chicken primordial germ cells. PLoS ONE. 2010;5:e12968. doi: 10.1371/journal.pone.0012968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdonald J, Glover JD, Taylor L, Sang HM, McGrew MJ. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS ONE. 2010;5:e15518. doi: 10.1371/journal.pone.0015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SI, et al. MicroRNA-mediated posttranscriptional regulation is required for maintaining undifferentiated properties of blastoderm and primordial germ cells in chickens. Proc Natl Acad Sci USA. 2011;108:10426–10431. doi: 10.1073/pnas.1106141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leighton PA, van de Lavoir MC, Diamond JH, Xia C, Etches RJ. Genetic modification of primordial germ cells by gene trapping, gene targeting, and phiC31 integrase. Mol Reprod Dev. 2008;75:1163–1175. doi: 10.1002/mrd.20859. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, et al. CpG methylation modulates tissue-specific expression of a transgene in chickens. Theriogenology. 2010;74:805–816, e1. doi: 10.1016/j.theriogenology.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 7.McClintock B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci USA. 1950;36:344–355. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cary LC, et al. Transposon mutagenesis of baculoviruses: Analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology. 1989;172:156–169. doi: 10.1016/0042-6822(89)90117-7. [DOI] [PubMed] [Google Scholar]
- 9.Ding S, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, et al. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:9290–9295. doi: 10.1073/pnas.0801017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y, Lin C, Wang X. PiggyBac transgenic strategies in the developing chicken spinal cord. Nucleic Acids Res. 2009;37:e141. doi: 10.1093/nar/gkp686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S, Ying G, Wu Q, Capecchi MR. Toward simpler and faster genome-wide mutagenesis in mice. Nat Genet. 2007;39:922–930. doi: 10.1038/ng2060. [DOI] [PubMed] [Google Scholar]
- 13.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauser CA, Elick TA, Fraser MJ. Proteins from nuclear extracts of two lepidopteran cell lines recognize the ends of TTAA-specific transposons piggyBac and tagalong. Insect Mol Biol. 1999;8:223–230. doi: 10.1046/j.1365-2583.1999.820223.x. [DOI] [PubMed] [Google Scholar]
- 15.Naito M, et al. Differentiation of donor primordial germ cells into functional gametes in the gonads of mixed-sex germline chimaeric chickens produced by transfer of primordial germ cells isolated from embryonic blood. J Reprod Fertil. 1999;117:291–298. doi: 10.1530/jrf.0.1170291. [DOI] [PubMed] [Google Scholar]
- 16.Park TS, et al. Improved germline transmission in chicken chimeras produced by transplantation of gonadal primordial germ cells into recipient embryos. Biol Reprod. 2003;68:1657–1662. doi: 10.1095/biolreprod.102.006825. [DOI] [PubMed] [Google Scholar]
- 17.Harvey AJ, Speksnijder G, Baugh LR, Morris JA, Ivarie R. Expression of exogenous protein in the egg white of transgenic chickens. Nat Biotechnol. 2002;20:396–399. doi: 10.1038/nbt0402-396. [DOI] [PubMed] [Google Scholar]
- 18.McGrew MJ, et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 2004;5:728–733. doi: 10.1038/sj.embor.7400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamihira M, et al. High-level expression of single-chain Fv-Fc fusion protein in serum and egg white of genetically manipulated chickens by using a retroviral vector. J Virol. 2005;79:10864–10874. doi: 10.1128/JVI.79.17.10864-10874.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott BB, Lois C. Generation of transgenic birds with replication-deficient lentiviruses. Nat Protoc. 2006;1:1406–1411. doi: 10.1038/nprot.2006.187. [DOI] [PubMed] [Google Scholar]
- 21.Lillico SG, et al. Oviduct-specific expression of two therapeutic proteins in transgenic hens. Proc Natl Acad Sci USA. 2007;104:1771–1776. doi: 10.1073/pnas.0610401104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han JY. Germ cells and transgenesis in chickens. Comp Immunol Microbiol Infect Dis. 2009;32:61–80. doi: 10.1016/j.cimid.2007.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.