Abstract
Deformed amphibians have been observed in eutrophic habitats, and some clues point to the retinoic acids (RAs) or RA mimics. However, RAs are generally thought of as vertebrate-specific hormones, and there was no evidence that RAs exist in cyanobacteria or algae blooms. By analyzing RAs and their analogs 4-oxo-RAs in natural cyanobacteria blooms and cultures of cyanobacteria and algae, we showed that cyanobacteria blooms could produce RAs, which were powerful animal teratogens. Intracellular RAs and 4-oxo-RAs with concentrations between 0.4 and 4.2 × 102 ng/L were detected in all bloom materials, and extracellular concentrations measured in water from Taihu Lake, China, were as great as 2.0 × 10 ng/L, which might pose a risk to wildlife through chronic exposure. Further examination of 39 cyanobacteria and algae species revealed that 32 species could produce RAs and 4-oxo-RAs (1.6–1.4 × 103 ng/g dry weight), and the dominant cyanobacteria species in Taihu Lake, Microcystis flos-aquae and Microcystis aeruginosa, produced high amounts of RAs and 4-oxo-RAs with concentrations of 1.4 × 103 and 3.7 × 102 ng/g dry weight, respectively. Most genera of cyanobacteria that could produce RAs and 4-oxo-RAs, such as Microcystis, Anabaena, and Aphanizomenon, often occur dominantly in blooms. Production of RAs and 4-oxo-RAs by cyanobacteria was associated with species, origin location, and growth stage. These results represent a conclusive demonstration of endogenous production of RAs in freshwater cyanobacteria blooms. The observation of teratogenic RAs in cyanobacteria is evolutionarily and ecologically significant because RAs are vertebrate-specific hormones, and cyanobacteria form extensive and highly visible blooms in many aquatic ecosystems.
Keywords: eutrophication, phytoplankton, deformities, Asia
Retinoic acids (RAs) are essential for physiological processes such as reproduction, cell proliferation and differentiation, vision, and embryonic development (1). However, extensive research has proved that RAs are among the most potent known animal teratogens (2). Exposure of embryos to exogenous RAs causes a spectrum of malformations, including defects of the neural tube and central nervous system; skeleton, palate, ear, and other craniofacial malformations; defects in the heart, thymus, and urogenital system; as well as missing or duplicate limbs and digits in offspring of humans, rodents, chickens, the African clawed frog (Xenopus laevis), and fish (3–10). When frogs were exposed to RAs during metamorphosis, a suite of abnormalities, such as reductions and deletions of the hind limb, bony triangles, and eye deformities, could occur (11–15). These abnormalities observed in the laboratory are similar to those found in wild frogs (16, 17).
Deformed amphibians have been observed in eutrophic habitats (18). Although the infections with parasites that were related to eutrophication were thought to be the mechanism of malformation (19, 20), the causes for frog deformities still remain controversial (21–23). Some clues point to RAs, including all-trans-RA (atRA), 13-cis-RA (13cRA), and 9-cis-RA (9cRA), or RA mimics (16, 17). These substances could be the cause of malformations through regulation of RA receptor-mediated signaling if they occurred in the surface waters or algae and vegetation eaten by tadpoles (24). However, because they were one of the first morphogens identified in vertebrates, RAs were generally thought of as vertebrate-specific hormones (25), and there was no evidence that RAs were found in the eutrophic environment.
Under normal physiological conditions, atRA and 13cRA are present in human urine (26). In addition to the endogenous origin, atRA and 13cRA are also synthesized artificially for clinical treatment of dermatitis, such as acne and psoriasis (27). RAs and their metabolites can enter into the aquatic environments through discharge of domestic sewage. Concentrations of RAs and their bioactive metabolites 4-oxo-RAs in treated wastewater have generally been found to be less than 1.0 ng/L (28, 29). Recently, anomalous concentrations of RAs and 4-oxo-RAs (up to 20 ng/L) were measured in some protected drinking water sources (30), which suggested that there might be unidentified sources of RAs in aquatic systems. There are some reports on the existence of retinal, one of the precursors of RAs, in algae, and isomerization of the all-trans-retinal chromophore into 13-cis-retinal activated the photoreceptor channel, whereas RAs were not associated with photoreceptors in algae (31–33). The nonanimal hydroxylase enzyme (CYP120A1) in the cyanobacteria Synechocystis was reported to be capable of converting RAs to corresponding hydroxyl derivatives (34), and a hydroxylated RA, 7-OH-RA, has recently been observed in several species of cyanobacteria (35). These results led us to postulate that cyanobacteria or algae might have the potential to synthesize RAs, and such autogenously produced RAs could cause adverse effects on wildlife in eutrophic environments.
To demonstrate this hypothesis, the production of RAs and 4-oxo-RAs in cyanobacteria bloom materials from a eutrophic lake in China was studied by using a unique analytical method described in this paper. Further examination of 39 representative cyanobacteria and algae species was carried out to determine the specific species that could produce teratogenic RAs and 4-oxo-RAs.
Results and Discussion
RAs and 4-Oxo-RAs in Cyanobacteria Blooms.
Taihu Lake, the third-largest freshwater lake in China, is hypereutrophic and has experienced blooms of cyanobacteria that have drawn worldwide attention (36, 37). The northern part of Taihu Lake, which is the most hypertrophic area, was chosen for this study (Fig. S1), and details of the study area are described in SI Materials and Methods. RAs and 4-oxo-RAs were detected in 20 of 24 water samples with an average concentration of 3.9 ± 4.4 ng/L (Table 1, Fig. 1, and Fig. S2), which was greater than those (0.7 ± 0.8 ng/L) reported previously in a river basin, Liaodong Bay, China, where untreated wastewaters are discharged directly into the rivers (29). The greatest total concentration of RAs and 4-oxo-RAs in water from Taihu Lake was 2.0 × 10 ng/L, which was comparable to concentrations in raw influents of sewage treatment plants (28). Taihu Lake is an important drinking water source for local residents, and domestic wastewaters without treatment are not directly discharged into the lake. Thus, such high concentration of RAs and 4-oxo-RAs were hypothesized to come from other sources. Of the RAs and 4-oxo-RAs, atRA/all-trans-4-oxo-RA (at-4-oxo-RA) and 13cRA/13-cis-4-oxo-RA (13c-4-oxo-RA) were the predominant compounds and accounted for 53% ± 26% and 33% ± 28%, respectively, of total concentrations in detected samples (Fig. S2). Compound 9cRA/9-cis-4-oxo-RA (9c-4-oxo-RA) was detected in 12 water samples at concentrations ranging from 0.1 to 3.3 ng/L and accounted for 14% ± 14% of total concentrations in detected samples. This report of 9cRA, a natural retinoid X receptor ligand in animal and human, in the environment is significant.
Table 1.
Concentrations of intracellular and extracellular RAs and 4-oxo-RAs in cyanobacteria blooms collected from Taihu Lake, China
| Intracellular concentrations, ng/L |
Extracellular concentrations, ng/L |
|||||||||||
| Sampling sites | at-4-oxo-RA | 13c-4-oxo-RA | 9c-4-oxo-RA | atRA | 13cRA | 9cRA | at-4-oxo-RA | 13c-4-oxo-RA | 9c-4-oxo-RA | atRA | 13cRA | 9cRA |
| 1 | 0.4 | 0.1 | 0.2 | ND | ND | ND | ND | ND | ND | 1.5 | 3.7 | ND |
| 2 | 0.2 | 0.1 | 0.1 | ND | ND | ND | ND | ND | ND | 1.9 | 1.9 | ND |
| 3 | 0.2 | 0.1 | 0.1 | 1.1 | ND | ND | ND | ND | ND | 1.5 | 5.8 | 3.2 |
| 4 | 0.1 | 0.2 | 0.1 | ND | ND | ND | ND | ND | ND | 1.0 | ND | ND |
| 5 | 0.2 | ND | ND | 0.8 | 0.3 | ND | ND | ND | ND | 1.5 | 0.7 | 1.7 |
| 6 | 2.2 | 0.4 | 0.7 | 7.2 | 3.6 | ND | ND | ND | ND | 1.4 | 4.3 | ND |
| 7 | 2.2 | 0.4 | 0.3 | 2.9 | 1.5 | ND | ND | ND | ND | ND | ND | ND |
| 8 | 2.8 | 0.7 | 0.6 | 6.3 | 1.4 | ND | ND | ND | ND | 2.6 | 4.5 | ND |
| 9 | 9.8 | 1.7 | 2.7 | 4.4 × 10 | 2.1 × 10 | ND | ND | ND | ND | ND | ND | ND |
| 10 | 2.1 | 0.2 | 0.5 | 6.4 | 3.2 | ND | ND | ND | ND | 1.0 | 0.8 | 0.7 |
| 11 | 2.2 × 10 | 4.5 | 4.7 | 9.0 × 10 | 3.7 × 10 | ND | 6.0 | ND | 1.0 | 5.9 | 4.6 | 2.3 |
| 12 | 1.3 | 0.2 | 0.4 | 7.6 | 2.0 | ND | ND | ND | ND | ND | ND | ND |
| 13 | 1.5 | 0.2 | 0.3 | 1.6 × 10 | 9.8 | ND | 2.0 | ND | 0.4 | 2.9 | 1.6 | 0.5 |
| 14 | 1.3 | 0.2 | 0.3 | 1.8 × 10 | 6.4 | ND | ND | ND | ND | 1.0 | 2.2 | ND |
| 15 | 1.7 | 0.2 | 0.2 | 2.5 | 1.4 | ND | ND | ND | ND | 3.3 | 2.0 | 2.3 |
| 16 | 3.6 | 0.5 | 0.9 | 1.9 × 10 | 6.0 | ND | 1.0 | ND | 0.4 | ND | 3.2 | ND |
| 17 | 2.1 | 0.3 | 0.6 | 1.2 × 10 | 6.8 | ND | ND | ND | ND | 0.7 | 1.0 | ND |
| 18 | 0.1 | ND | ND | 0.7 | 0.5 | ND | ND | ND | ND | 0.9 | 0.7 | 0.8 |
| 19 | 0.1 | ND | ND | 2.2 | 0.7 | ND | ND | ND | ND | 2.5 | ND | ND |
| 20 | 2.7 | 0.3 | 0.5 | 3.1 × 10 | 1.9 × 10 | ND | 0.6 | ND | 0.1 | ND | ND | ND |
| 21 | 2.7 × 10 | ND | 4.9 | 3.7 × 10 | 1.3 × 10 | ND | 2.0 | ND | 0.6 | ND | ND | ND |
| 22 | 1.6 × 102 | 2.1 × 10 | 2.1 × 10 | 9.8 × 10 | 1.2 × 102 | ND | 0.8 | ND | 0.2 | ND | ND | ND |
| 23 | 3.7 | 1.9 | 1.6 | 2.6 × 10 | 1.6 × 10 | ND | ND | ND | ND | ND | ND | ND |
| 24 | 6.0 × 10 | 1.5 × 10 | 1.1 × 10 | 2.2 × 102 | 4.1 × 10 | ND | 1.1 | ND | 0.3 | ND | ND | ND |
ND, not detected.
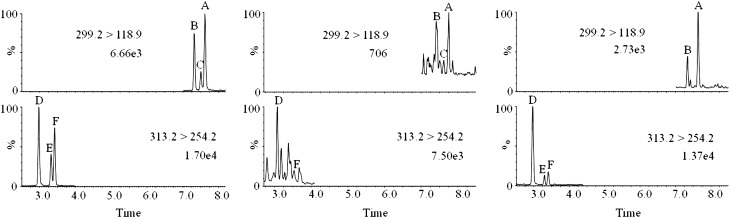
Fig. 1.
UPLC-MS/MS chromatograms of RAs and 4-oxo-RAs in no. 11 sample from Taihu Lake. (Left) Standards (10 parts per billion). (Center) Water sample. (Right) Bloom sample. A, atRA; B, 13cRA; C, 9cRA; D, at-4-oxo-RA; E, 13c-4-oxo-RA; F, 9c-4-oxo-RA.
To determine potential sources of RAs and 4-oxo-RAs in Taihu Lake, these compounds were further investigated in samples of blooms. The target compounds were detected in all bloom samples from Taihu Lake at concentrations ranging from 0.4 to 4.2 × 102 ng/L (Table 1, Fig. 1, and Fig. S2). The predominance of atRA/at-4-oxo-RA and 13cRA/13c-4-oxo-RA levels observed in bloom samples were similar to those in water (Fig. S2). Because concentrations of RAs and 4-oxo-RAs in blooms were greater than those in water (Fig. S2), the blooms were considered to be the main source of RAs and 4-oxo-RAs in lake water. Although 9cRA was not detected in samples of blooms, its metabolite 9c-4-oxo-RA was detected in 21 of 24 bloom samples at concentrations from 0.1 to 2.1 × 10 ng/L in detected samples, suggesting that 9cRA occurred in blooms at relatively low concentrations during the period of sampling.
The biomass of phytoplankton at each sampling location in Taihu Lake ranged from 6.7 × 106 to 9.7 × 1010 cells per liter. The phytoplankton community in Taihu Lake was dominated by cyanobacteria (Fig. S3), comprising primarily the genera Microcystis, Aphanizomenon, Anabaena, Pseudanabaena, and Planktothricoides (Fig. S4). In most samples, Microcystis accounted for more than 90% of the total abundance of cyanobacteria cells (Fig. S4). Microcystis in blooms consisted primarily of three species: Microcystis aeruginosa, Microcystis wesenbergii, and Microcystis flos-aquae (Fig. S5). A statistically significant correlation was observed between intracellular concentrations of RAs/4-oxo-RAs and numbers of Microcystis cells (P = 0.006, r2 = 0.2938), which further suggested that Microcystis in blooms was capable of producing the observed concentrations of RAs and 4-oxo-RAs.
RAs and 4-Oxo-RAs in Cultures of Cyanobacteria and Algae.
To further determine which cyanobacteria species were mainly responsible for the production of RAs and 4-oxo-RAs by blooms in Taihu Lake, 39 species of freshwater cyanobacteria and algae (Table S1), including those observed in Taihu Lake and some other species commonly observed in aquatic environments, were studied to determine their potential for producing RAs and 4-oxo-RAs. Cultures were purchased from the Freshwater Algae Culture Collection of the Institute of Hydrobiology (FACHB), Chinese Academy of Sciences. Intracellular RAs and 4-oxo-RAs were detected in 32 species studied, including 22 Cyanophyta, 6 Chlorophyta, 3 Bacillariophyta, and 1 Euglenophyta (Table 2, Fig. 2, and Fig. S6). The greatest amount of intracellular RAs and 4-oxo-RAs was found in M. flos-aquae [FACHB-1028; 1.4 × 103 ng/g dry weight (DW)]. Other species, including Anabaena sp. (FACHB-1088; 5.7 × 102 ng/g DW), Chlamydomonas sp. (FACHB-715; 3.8 × 102 ng/g DW), M. aeruginosa (FACHB-912; 3.7 × 102 ng/g DW), M. ichthyoblabe (FACHB-1294; 3.4 × 102 ng/g DW), and Ankistrodesmus sp. (FACHB-1044; 3.2 × 102 ng/g DW), also produced measureable quantities of RAs and 4-oxo-RAs. These compounds were also detected in the culture media of 12 cyanophyta and 1 chlorophyta (Table 2, Fig. 2, and Fig. S6). The greatest extracellular concentrations of RAs and 4-oxo-RAs were detected in the culture medium of Aphanizomenon flos-aquae (FACHB-1039; 2.4 × 103 ng/L), followed by M. flos-aquae (FACHB-1028; 1.2 × 103 ng/L) and Phormidium sp. (FACHB-1099; 3.0 × 102 ng/L). Other than cyanobacteria, only green algae Ankistrodesmus sp. (FACHB-1044) produced detectable extracellular at-4-oxo-RA (2.5 × 10 ng/L) in culture medium. Because all cultures obtained from FACHB were unialgal, an additional analysis of axenic cyanobacterial cultures was conducted. The axenic strains were obtained from the Pasteur Culture Collection of Cyanobacteria (PCC), France. The result demonstrated that the axenic strains of the cyanobacteria M. aeruginosa (PCC 7806) and Synechococcus sp. (PCC 7002), also produced RAs and 4-oxo-RAs (Fig. S7), and the concentrations were 1.8–2.5 ng/g DW.
Table 2.
Concentrations of intracellular and extracellular RAs and 4-oxo-RAs in cultured cyanobacteria and algae
| Intracellular concentration, ng/g DW |
Extracellular concentration, ng/L |
||||||||||||
| Sample | Density, g DW/L | at-4-oxo-RA | 13c-4-oxo-RA | 9c-4-oxo-RA | atRA | 13cRA | 9cRA | at-4-oxo-RA | 13c-4-oxo-RA | 9c-4-oxo-RA | atRA | 13cRA | 9cRA |
| FACHB-286 | 1.10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-898 | 1.68 | 5.0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1009 | 1.82 | 2.4 × 10 | 1.1 × 10 | 4.5 | ND | ND | ND | 8.9 | ND | ND | 8.1 × 10 | 3.8 × 10 | ND |
| FACHB-1028 | 1.85 | 1.2 × 102 | 5.1 × 10 | 4.0 × 10 | 2.5 × 102 | 4.3 × 102 | 5.2 × 102 | 1.4 × 102 | 2.7 × 10 | 3.2 × 10 | 4.2 × 102 | 2.0 × 102 | 3.9 × 102 |
| FACHB-905 | 1.90 | 5.6 | 7.9 | 2.7 | 2.5 × 10 | 2.5 × 10 | ND | ND | ND | ND | ND | ND | ND |
| FACHB-912 | 2.06 | 3.8 × 10 | 2.2 × 10 | 1.1 × 10 | 9.0 × 10 | 7.0 × 10 | 1.4 × 102 | 6.7 × 10 | ND | ND | ND | ND | ND |
| FACHB-908 | 1.83 | 3.4 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1284 | 1.90 | 5.6 | 1.5 | 2.0 | 3.6 × 10 | 3.6 × 10 | 1.0 × 102 | ND | ND | ND | ND | ND | ND |
| FACHB-1294 | 1.62 | 2.0 × 10 | 5.8 | 5.5 | 6.1 × 10 | 8.6 × 10 | 1.6 × 102 | ND | ND | ND | ND | ND | ND |
| FACHB-193 | 2.33 | 3.4 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1042 | 2.38 | 4.2 × 10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1043 | 2.60 | 1.2 × 10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1038 | 1.77 | 5.4 × 10 | 3.7 | 7.4 | ND | ND | ND | 1.2 × 10 | ND | ND | ND | ND | ND |
| FACHB-1088 | 1.82 | 1.4 × 102 | 4.8 × 10 | 3.0 × 10 | 1.5 × 102 | 9.0 × 10 | 1.1 × 102 | 9.0 | ND | ND | ND | ND | ND |
| FACHB-1092 | 1.74 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 7.0 × 10 | ND | ND |
| FACHB-1277 | 1.78 | 2.6 | ND | ND | ND | ND | ND | 3.2 | ND | ND | ND | ND | ND |
| FACHB-247 | 2.22 | 8.4 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-708 | 2.00 | 5.6 | 1.2 | 1.4 | 8.0 × 10 | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-881 | 1.79 | 3.8 | ND | ND | ND | ND | ND | ND | ND | ND | 1.1 × 102 | ND | ND |
| FACHB-723 | 2.41 | 2.5 | ND | ND | 1.9 × 10 | 1.4 × 10 | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1099 | 2.02 | 1.6 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.0 × 102 |
| FACHB-1039 | 1.80 | 1.2 × 102 | 2.0 × 10 | 2.2 × 10 | ND | ND | ND | 6.9 × 102 | 1.4 × 102 | 1.6 × 102 | 6.1 × 102 | 2.9 × 102 | 5.0 × 102 |
| FACHB-1171 | 1.78 | 7.5 | 4.9 | 2.3 | ND | ND | ND | 3.4 | ND | ND | ND | ND | ND |
| FACHB-1247 | 2.16 | 6.4 × 10 | 1.2 × 10 | 7.2 | ND | 3.9 × 10 | ND | 1.2 × 10 | 4.3 | 1.9 × 10 | ND | ND | ND |
| FACHB-715 | 1.14 | 3.1 | ND | ND | 1.1 × 102 | 2.7 × 102 | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1067 | 2.15 | 2.1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-704 | 1.53 | 1.0 × 10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-933 | 2.62 | 2.1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-271 | 1.85 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1044 | 2.27 | 2.0 × 10 | 8.0 | 5.0 | 4.3 × 10 | 1.5 × 102 | 9.5 × 10 | 2.5 × 10 | ND | ND | ND | ND | ND |
| FACHB-686 | 1.73 | ND | ND | ND | ND | 1.3 × 102 | ND | ND | ND | ND | ND | ND | ND |
| FACHB-719 | 2.10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1030 | 1.13 | 2.4 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-510 | 1.54 | 1.4 × 10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1054 | 1.42 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1034 | 1.38 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-1131 | 0.90 | 4.8 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-844 | 38.39 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| FACHB-848 | 2.26 | 9.5 | 1.1 × 10 | 3.0 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
ND, not detected.
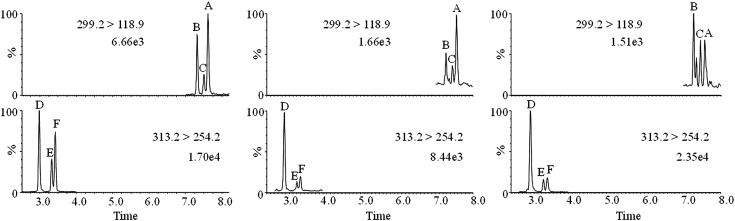
Fig. 2.
UPLC-MS/MS chromatograms of RAs and 4-oxo-RAs in culture of M. flos-aquae (FACHB-1028). (Left) Standards (10 parts per billion). (Center) Extracellular sample. (Right) Intracellular sample. A, atRA; B, 13cRA; C, 9cRA; D, at-4-oxo-RA; E, 13c-4-oxo-RA; F, 9c-4-oxo-RA.
The profiles of RAs and 4-oxo-RAs observed in the cultured cyanobacteria and algae were similar to those in blooms and water samples collected from Taihu Lake. atRA/at-4-oxo-RA were the predominant compounds, accounting for 71% ± 33% of intracellular concentrations of total RAs/4-oxo-RAs in detected samples, followed by 13cRA/13c-4-oxo-RA (19% ± 25%). Of the 39 cyanobacteria and algae species, 9cRA/9c-4-oxo-RA were detected in 14 species with intracellular concentrations from 1.4 to 5.6 × 102 ng/g DW (Table 2). Although the physiological function for the normal existence and role of 9cRA is of great interest in vertebrates (25), 9cRA was only identified in Xenopus embryos (38), in humans, or in mice after excessive amounts of retinol or atRA (39, 40). A recent study determined 9cRA to be a naturally occurring RA with concentrations of 3–12 ng/g (10–40 pmol/g) in mouse pancreas, which confirms that 9cRA contributes to the biological activity of vitamin A in vivo (41). Our findings of relatively frequent detections and relatively great concentrations of 9cRA (up to 5.2 × 102 ng/g DW) and 9c-4-oxo-RA (up to 4.0 × 10 ng/g DW) in cultured cyanobacteria and algae suggested that more studies about RAs should focus on cyanobacteria and algae in the future. Generally, 4-oxo-RAs were considered to be metabolites of RAs formed in vertebrates by cytochrome P450 enzymes such as CYP26A1 and CYP26B1, which have been cloned in human, mouse, rat, and chicken (25). The appearances of RAs and 4-oxo-RAs in natural blooms and cultured cyanobacteria and algae at relatively high concentrations first indicated that cyanobacteria and algae were able to transform RAs into their 4-oxo metabolites. It was inferred that corresponding biotransformation enzymes should exist in cyanobacteria and algae, especially those making up blooms. Based on biotransformation pathways in vertebrates, RAs would also undergo biotransformation to form hydroxylated transformation products (OH-RAs). The OH-RAs are less potent than atRA in binding to RA receptors (42). Recently, 7-OH-RA was identified in cultured cyanobacteria (35), but the estimated concentrations of 7-OH-RA were as much as 35-fold less than those of RAs and 4-oxo-RAs observed in this study.
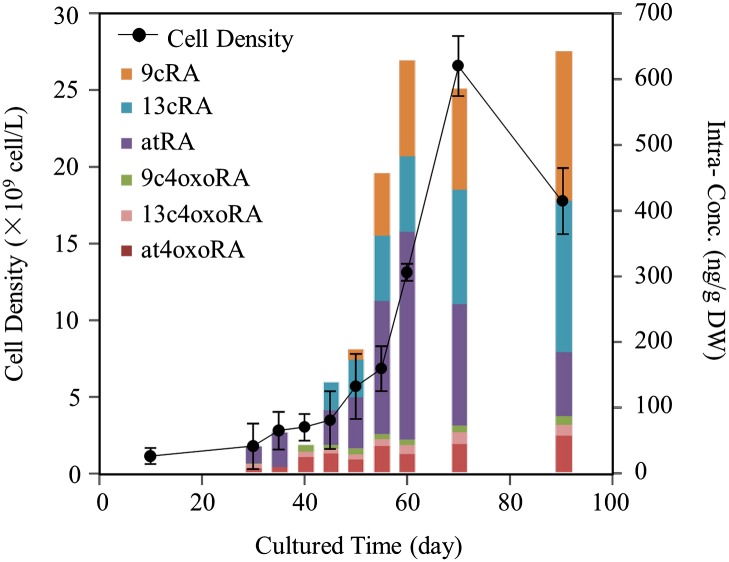
When results of the laboratory and field studies are considered together, it can be concluded that the dominant cyanobacteria species, M. flos-aquae and M. aeruginosa, were primarily responsible for production of RAs and 4-oxo-RAs measured in blooms from Taihu Lake. Absolute and relative concentrations of RAs and 4-oxo-RAs depended on the species, even within the same genus. For instance, concentrations of RAs and 4-oxo-RAs in M. flos-aquae (FACHB-1028) were 3- to 420-fold greater than those in the other six Microcystis species (Table 2). The origin of cyanobacteria was also found to have great effects on the production of RAs and 4-oxo-RAs. For example, FACHB-912, from Taihu Lake, and FACHB-905, from Dianchi Lake, were both M. aeruginosa, but the intracellular concentrations of RAs and 4-oxo-RAs in FACHB-912 (3.7 × 102 ng/g DW) were nearly fivefold greater than those in FACHB-905 (6.6 × 10 ng/g DW). Besides the differences among species and origin locations, the concentrations and profiles of RAs and 4-oxo-RAs were also associated with the phase of cyanobacterial growth. As shown in Fig. 3, whereas concentrations of 4-oxo-RAs in M. flos-aquae (FACHB-1028) remained constant from 40 to 90 d, concentrations of atRA, 13cRA, and 9cRA all increased as a function of time, especially after 50 d, when 9cRA appeared and the concentrations of all RA isomers abruptly increased, then became constant. The bloom season in Taihu Lake is from May to October, and the density of cyanobacteria cells reaches a maximum in July. Based on previously reported (43) and present results, the natural blooms in Taihu Lake were dominated by cyanobacteria (over 90%), and production of RAs and 4-oxo-RAs depended on cyanobacteria cell numbers (Fig. 3); therefore, concentrations of RAs and 4-oxo-RAs in Taihu Lake might increase to their highest in July, and the lowest concentrations would appear in wintertime.
Fig. 3.
Changes in intracellular production of RAs and 4-oxo-RAs with the growth of M. flos-aquae (FACHB-1028).
Blooms, especially cyanobacteria blooms, occur in freshwaters or marine environment all over the world and have been recognized as an important health risk for both humans and ecosystems because of their production of various toxins such as hepatotoxins, neurotoxins, cytotoxins, dermatotoxins, and irritant toxins (44, 45). The results reported here demonstrate that natural cyanobacteria blooms can produce teratogenic RAs and 4-oxo-RAs. On the basis of controlled laboratory studies with individual species, we determined the species responsible for production of RAs and 4-oxo-RAs in Taihu Lake, China. The results also show that some cyanobacteria species, especially those that are common in blooms and sometimes are dominant species, such as M. flos-aquae, M. aeruginosa, Anabaena, and Ap. flos-aquae, can produce significant amounts of RAs and 4-oxo-RAs. Because RAs are generally thought of as vertebrate-specific hormones that signal via nuclear receptors cascades (25), the signal-transduction pathway and function of RAs in cyanobacteria and algae remain to be elucidated. Exposure to RA through both diet and/or directly from water can cause mortality and deformities in tadpoles of X. laevis (14, 15). Extracts of cyanobacteria can cause developmental toxicity, including mortality, growth inhibition, and malformations in X. laevis, and these effects have been shown not to be caused by microcystins (46). Altogether, the results of these studies indicate that RAs and 4-oxo-RAs found in Taihu Lake might pose a risk for animals living in the water and feeding on algae. Because eutrophic lakes and reservoirs are used as sources of drinking water and food, such as freshwater clams and snails, RAs could also pose a risk to the health of humans.
Materials and Methods
Field Collections.
Twenty-four surface (0–0.5 m) water samples containing blooms of cyanobacteria were collected in October 2010 from Taihu Lake. Samples were stored in amber bottles and were treated immediately after arrival at laboratory. A portion (30–200 mL) of each sample was preserved in Lugol’s solution for algae identification and enumeration.
Cultured Cyanobacteria and Algae.
Thirty-nine unialgal cultures of freshwater cyanobacteria and algae, including 24 Cyanophyta, 8 Chlorophyta, 6 Bacillariophyta, and 1 Euglenophyta (Table S1), were purchased from the FACHB, Chinese Academy of Sciences. Two axenic cultures of cyanobacteria, M. aeruginosa (PCC 7806) and Synechococcus sp. (PCC 7002), were obtained from the PCC, France. The cultures were used for analysis when the cyanobacteria and algae that cultured under axenic condition were in a logarithmic growth phase and the number of cells was >108 cells per liter.
M. flos-aquae (FACHB-1028) was further used to assess the influence of growth stage on the production of RAs and 4-oxo-RAs. The cyanobacteria was grown in BG11 medium at 25 °C on a 14-h light/10-h dark cycle. During the culture time, the culture was shaken three times every day. The cyanobacteria and medium were taken for analysis of RAs and 4-oxo-RAs on days 10, 30, 35, 40, 45, 50, 55, 60, 70, and 90. The cell counting was carried out (n = 6), and the number of cells (cells per liter) was calculated at each sampling time.
Preparation of Samples from Taihu Lake.
Water was vacuum-filtered through GF/C filters (1.2 μm; Whatman) for analysis of extracellular RAs and 4-oxo-RAs, and cyanobacteria cells remaining on filters were used for the measurement of intracellular RAs and 4-oxo-RAs. The details of the chemicals and standards used are provided in SI Materials and Methods. The method used to quantify the extracellular RAs and 4-oxo-RAs was based on previously published methods (29). The methods for analyzing all target RAs and 4-oxo-RAs in cyanobacteria blooms and cultured species were initially developed and validated.
The GF/C filters were placed into a 50-mL centrifuge tube containing 15–20 mL of acetone and sonicated for 10 min. After disruption of cells in an ice bath by use of an ultrasonic cell disrupter system (10- to 20-s pulse, repeat 10 times, 600–800 W) and centrifugation at 3,000 × g for 10 min, the supernatant was transferred to another centrifuge tube and then 15 mL of acetone was added to the residues. The mixture was mechanically shaken for 5 min and subsequently centrifuged (3,000 × g for 10 min). The combined supernatant was concentrated to a final volume of 5 mL by evaporation with nitrogen and then diluted with ultrapure water to 50 mL after surrogate standards (acitretin and atRA-d5) were added. The mixture was loaded onto the preconditioned Oasis HLB cartridge (6 mL, 200 mg; Waters), which was then dried with nitrogen. Analytes were eluted sequentially with 6 mL of ethyl acetate, 3 mL of acetone, and 8 mL of ethyl acetate containing 0.5% formic acid. The combined extracts were dried under nitrogen and then purified by use of previously described methods (29) before injection to the ultraperformance liquid chromatography/electrospray ionization–tandem mass spectrometry (UPLC-ESI-MS/MS) for the analysis of RAs and 4-oxo-RAs.
Preparation of Cultured Cyanobacteria and Algae.
Cultures were centrifuged at 6,000 × g for 10 min. The supernatant was used to analyze the extracellular RAs and 4-oxo-RAs, and the filtered cells were used for the quantification of intracellular RAs and 4-oxo-RAs.
An aliquot (15 mL) of the supernatant was added with 3.75 mL of acetone and spiked with surrogate standards, and then the mixture was loaded under gravity onto the Oasis HLB cartridge, which had been preconditioned with 6 mL of ethyl acetate, 6 mL of methanol and 12 mL of 20% (vol/vol) aqueous acetone. The cartridge was then dried with nitrogen, and analytes were eluted with 6 mL of ethyl acetate followed by 3 mL of acetone. Extracts were evaporated to dryness under a gentle stream of nitrogen and reconstituted with 150 μL of acetonitrile.
For identification and quantification of intracellular RAs and 4-oxo-RAs, cells were suspended in 2–3 mL of phosphate buffer (0.2 M, pH = 6.0) before performing cell disruption as described above. After being spiked with surrogate standards, the sample was extracted three times with 5 mL of hexane/chloroform (3:1; vol/vol) by mechanically shaking for 5 min, and the combined organic layer was evaporated to dryness under a gentle stream of nitrogen. The residue was purified by use of previously described methods (29) and dissolved in 350 μL of acetonitrile before injection to the ultraperformance liquid chromatography-tandem mass spectrometry system for quantification of RAs and 4-oxo-RAs.
Identification and Quantification of RAs and 4-Oxo-RAs.
For natural samples, the absolute recoveries of target analytes were 54–66% and 51–60% in bloom samples and water samples, respectively (Table S2). Recoveries of surrogate standards were as follows: 45% ± 14% (blooms) and 54% ± 23% (waters) for atRA-d5 and 74% ± 21% (blooms) and 56% ± 16% (waters) for acitretin. Limits of quantification (LOQs) of RAs and 4-oxo-RAs were 0.1–0.8 ng/L in blooms and 0.06–0.4 ng/L in waters, respectively (Table S2). For cultured samples from FACHB, the absolute recoveries of target analytes were 50–70% and 52–76% in intracellular and extracellular samples, respectively (Table S2). Recoveries of surrogate standards in intracellular samples were 44% ± 14% for atRA-d5 and 56% ± 17% for acitretin, and recoveries from extracellular samples were 49% ± 16% for atRA-d5 and 53% ± 18% for acitretin. The limits of quantification for RAs and 4-oxo-RAs ranged from 0.5 to 6.0 ng/g DW in intracellular samples and from 3.0 to 20 ng/L in extracellular samples (Table S2). In this study, atRA-d5 and acitretin served as surrogate standards to quantify RAs (atRA, 13cRA, and 9cRA) and 4-oxo-RAs (at-4-oxo-RA, 13c-4-oxo-RA, and 9c-4-oxo-RA), respectively. Surrogate standards were used to compensate for signal suppression caused by suppression of ionization by cellular matrix, losses of analytes during preparation, and variations in the instrument response from injection to injection in ultraperformance liquid chromatography-tandem mass spectrometry analysis. The relative recoveries for all target analytes (corrected for surrogate standards) were 83% ± 9% to 129% ± 3%. All analyses were quantified within the range of limits of quantification or were reported as not detected.
Statistical Analysis.
Pearson correlation analysis was conducted with SPSS software, version 19 for Windows.
Supplementary Material
Acknowledgments
Financial support from National Natural Science Foundation of China Grants 20977002 and 40632009 is acknowledged. J.P.G. was supported by the Canada Research Chair program; an at-large chair professorship at the Department of Biology and Chemistry and State Key Laboratory in Marine Pollution, City University of Hong Kong; the Einstein Professor Program of the Chinese Academy of Sciences; and the Visiting Professor Program of King Saud University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200062109/-/DCSupplemental.
References
- 1.Gudas LJ, Sporn MB, Roberts AB. Cellular biology and biochemistry of the retinoids. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven Press; 1994. pp. 443–520. [Google Scholar]
- 2.Bryant SV, Gardiner DM. Retinoic acid, local cell-cell interactions, and pattern formation in vertebrate limbs. Dev Biol. 1992;152:1–25. doi: 10.1016/0012-1606(92)90152-7. [DOI] [PubMed] [Google Scholar]
- 3.Lammer EJ, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- 4.Rutledge JC, et al. Limb and lower-body duplications induced by retinoic acid in mice. Proc Natl Acad Sci USA. 1994;91:5436–5440. doi: 10.1073/pnas.91.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- 6.Niederreither K, Ward SJ, Dollé P, Chambon P. Morphological and molecular characterization of retinoic acid-induced limb duplications in mice. Dev Biol. 1996;176:185–198. doi: 10.1006/dbio.1996.0126. [DOI] [PubMed] [Google Scholar]
- 7.Thaller C, Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987;327:625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- 8.Durston AJ, et al. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989;340:140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- 9.Sive HL, Draper BW, Harland RM, Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990;4:932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann K. Teratogenic effects of retinoic acid and related substances on the early development of the zebrafish (Brachydanio rerio) as assessed by a novel scoring system. Toxicol In Vitro. 1995;9:267–283. doi: 10.1016/0887-2333(95)00012-w. [DOI] [PubMed] [Google Scholar]
- 11.Scadding SR, Maden M. Comparison of the effects of vitamin A on limb development and regeneration in Xenopus laevis tadpoles. J Embryol Exp Morphol. 1986;91:35–53. [PubMed] [Google Scholar]
- 12.Scadding SR, Maden M. The effects of local application of retinoic acid on limb development and regeneration in tadpoles of Xenopus laevis. J Embryol Exp Morphol. 1986;91:55–63. [PubMed] [Google Scholar]
- 13.Maden M. The homeotic transformation of tails into limbs in Rana temporaria by retinoids. Dev Biol. 1993;159:379–391. doi: 10.1006/dbio.1993.1249. [DOI] [PubMed] [Google Scholar]
- 14.Degitz SJ, Kosian PA, Makynen EA, Jensen KM, Ankley GT. Stage- and species-specific developmental toxicity of all-trans retinoic acid in four native North American ranids and Xenopus laevis. Toxicol Sci. 2000;57:264–274. doi: 10.1093/toxsci/57.2.264. [DOI] [PubMed] [Google Scholar]
- 15.Alsop DH, Brown SB, van der Kraak GJ. Dietary retinoic acid induces hindlimb and eye deformities in Xenopus laevis. Environ Sci Technol. 2004;38:6290–6299. doi: 10.1021/es049765n. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner DM, Hoppe DM. Environmentally induced limb malformations in mink frogs (Rana septentrionalis) J Exp Zool. 1999;284:207–216. doi: 10.1002/(sici)1097-010x(19990701)284:2<207::aid-jez10>3.3.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Gardiner D, Ndayibagira A, Grun F, Blumberg B. Deformed frogs and environmental retinoids. Pure Appl Chem. 2003;75:2263–2273. [Google Scholar]
- 18.Blaustein AR, Johnson PTJ. The complexity of deformed amphibians. Front Ecol Environ. 2003;1:87–94. [Google Scholar]
- 19.Johnson PTJ, Chase JM. Parasites in the food web: Linking amphibian malformations and aquatic eutrophication. Ecol Lett. 2004;7:521–526. [Google Scholar]
- 20.Johnson PTJ, et al. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc Natl Acad Sci USA. 2007;104:15781–15786. doi: 10.1073/pnas.0707763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor B, et al. Proximity to pollution sources and risk of amphibian limb malformation. Environ Health Perspect. 2005;113:1497–1501. doi: 10.1289/ehp.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souder W. A Plague of Frogs. New York: Hyperion; 2000. [Google Scholar]
- 23.Skelly DK, Benard MF. Mystery unsolved: Missing limbs in deformed amphibians. J Exp Zoolog B Mol Dev Evol. 2010;314:179–181. doi: 10.1002/jez.b.21330. [DOI] [PubMed] [Google Scholar]
- 24.Stocum DL. Frog limb deformities: An “eco-devo” riddle wrapped in multiple hypotheses surrounded by insufficient data. Teratology. 2000;62:147–150. doi: 10.1002/1096-9926(200009)62:3<147::AID-TERA2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 26.Lambert WE, De Leenheer AP. Demonstration of retinoic acid isomers in human urine under physiological conditions. Experientia. 1985;41:359–360. doi: 10.1007/BF02004504. [DOI] [PubMed] [Google Scholar]
- 27.Peck GL, DiGiovanna JJ. Synthetic retinoids in dermatology. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven Press; 1994. pp. 631–658. [Google Scholar]
- 28.Zhen HJ, et al. Identification of retinoic acid receptor agonists in sewage treatment plants. Environ Sci Technol. 2009;43:6611–6616. doi: 10.1021/es9000328. [DOI] [PubMed] [Google Scholar]
- 29.Wu XQ, et al. Determination and occurrence of retinoic acids and their 4-oxo metabolites in Liaodong Bay, China, and its adjacent rivers. Environ Toxicol Chem. 2010;29:2491–2497. doi: 10.1002/etc.322. [DOI] [PubMed] [Google Scholar]
- 30.Wu XQ. Pollution characteristics and sources of retinoids in the aquatic environment. Beijing, China: Peking Univ; 2011. PhD thesis. [Google Scholar]
- 31.Kreimer G, Marner FJ, Brohsonn U, Melkonian M. Identification of 11-cis and all-trans-retinal in the photoreceptive organelle of a flagellate green alga. FEBS Lett. 1991;293:49–52. doi: 10.1016/0014-5793(91)81150-7. [DOI] [PubMed] [Google Scholar]
- 32.Robinson KR, Lorenzi R, Ceccarelli N, Gualtieri P. Retinal identification in Pelvetia fastigiata. Biochem Biophys Res Commun. 1998;243:776–778. doi: 10.1006/bbrc.1998.8176. [DOI] [PubMed] [Google Scholar]
- 33.Hegemann P. Vision in microalgae. Planta. 1997;203:265–274. doi: 10.1007/s004250050191. [DOI] [PubMed] [Google Scholar]
- 34.Alder A, Bigler P, Werck-Reichhart D, Al-Babili S. In vitro characterization of Synechocystis CYP120A1 revealed the first nonanimal retinoic acid hydroxylase. FEBS J. 2009;276:5416–5431. doi: 10.1111/j.1742-4658.2009.07224.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaya K, Shiraishi F, Uchida H, Sano T. A novel retinoic acid analogue, 7-hydroxy retinoic acid, isolated from cyanobacteria. Biochim Biophys Acta. 2011;1810:414–419. doi: 10.1016/j.bbagen.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Guo L. Ecology. Doing battle with the green monster of Taihu Lake. Science. 2007;317:1166. doi: 10.1126/science.317.5842.1166. [DOI] [PubMed] [Google Scholar]
- 37.Yang M, et al. Taihu Lake not to blame for Wuxi’s woes. Science. 2008;319:158. doi: 10.1126/science.319.5860.158a. [DOI] [PubMed] [Google Scholar]
- 38.Kraft JC, Schuh T, Juchau M, Kimelman D. The retinoid X receptor ligand, 9-cis-retinoic acid, is a potential regulator of early Xenopus development. Proc Natl Acad Sci USA. 1994;91:3067–3071. doi: 10.1073/pnas.91.8.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnhold T, Tzimas G, Wittfoht W, Plonait S, Nau H. Identification of 9-cis-retinoic acid, 9,13-dis-cis-retinoic acid, and 14-hydroxy-4,14-retro-retinol in human plasma after liver consumption. Life Sci. 1996;59:169–177. doi: 10.1016/0024-3205(96)00408-0. [DOI] [PubMed] [Google Scholar]
- 40.Ulven SM, Gundersen TE, Sakhi AK, Glover JC, Blomhoff R. Quantitative axial profiles of retinoic acid in the embryonic mouse spinal cord: 9-cis retinoic acid only detected after all-trans-retinoic acid levels are super-elevated experimentally. Dev Dyn. 2001;222:341–353. doi: 10.1002/dvdy.1184. [DOI] [PubMed] [Google Scholar]
- 41.Kane MA, et al. Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. 2010;107:21884–21889. doi: 10.1073/pnas.1008859107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Idres N, Marill J, Flexor MA, Chabot GG. Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J Biol Chem. 2002;277:31491–31498. doi: 10.1074/jbc.M205016200. [DOI] [PubMed] [Google Scholar]
- 43.Song LR, et al. Distribution and bioaccumulation of microcystins in water columns: A systematic investigation into the environmental fate and the risks associated with microcystins in Meiliang Bay, Lake Taihu. Water Res. 2007;41:2853–2864. doi: 10.1016/j.watres.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Žegura B, Štraser A, Filipič M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—a review. Mutat Res. 2011;727:16–41. doi: 10.1016/j.mrrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Anderson DM. Turning back the harmful red tide. Nature. 1997;388:513–514. [Google Scholar]
- 46.Burýsková B, et al. Toxicity of complex cyanobacterial samples and their fractions in Xenopus laevis embryos and the role of microcystins. Aquat Toxicol. 2006;80:346–354. doi: 10.1016/j.aquatox.2006.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.