Abstract
P pili are prototypical chaperone–usher pathway-assembled pili used by Gram-negative bacteria to adhere to host tissues. The PapC usher contains five functional domains: a transmembrane β-barrel, a β-sandwich Plug, an N-terminal (periplasmic) domain (NTD), and two C-terminal (periplasmic) domains, CTD1 and CTD2. Here, we delineated usher domain interactions between themselves and with chaperone–subunit complexes and showed that overexpression of individual usher domains inhibits pilus assembly. Prior work revealed that the Plug domain occludes the pore of the transmembrane domain of a solitary usher, but the chaperone–adhesin-bound usher has its Plug displaced from the pore, adjacent to the NTD. We demonstrate an interaction between the NTD and Plug domains that suggests a biophysical basis for usher gating. Furthermore, we found that the NTD exhibits high-affinity binding to the chaperone–adhesin (PapDG) complex and low-affinity binding to the major tip subunit PapE (PapDE). We also demonstrate that CTD2 binds with lower affinity to all tested chaperone–subunit complexes except for the chaperone–terminator subunit (PapDH) and has a catalytic role in dissociating the NTD–PapDG complex, suggesting an interplay between recruitment to the NTD and transfer to CTD2 during pilus initiation. The Plug domain and the NTD–Plug complex bound all of the chaperone–subunit complexes tested including PapDH, suggesting that the Plug actively recruits chaperone–subunit complexes to the usher and is the sole recruiter of PapDH. Overall, our studies reveal the cooperative, active roles played by periplasmic domains of the usher to initiate, grow, and terminate a prototypical chaperone–usher pathway pilus.
Keywords: biolayer interferometry, macromolecular assembly, urinary tract infections, virulence factor, bacterial pathogenesis
The chaperone–usher pathway (CUP) is used by Gram-negative pathogens to assemble hundreds of different adhesive proteinaceous surface fibers called pili or fimbriae (1). Pili are important virulence factors, which in part determine tropism for host tissues of many pathogens. In uropathogenic Escherichia coli (UPEC), type 1 pili facilitate bladder colonization and invasion of the bladder tissue in cystitis (2–5), and P pili mediate pyelonephritis by binding to the globoseries of glycolipids in the human kidney (6–9). Type 1 and P pili are prototypes for understanding assembly by the CUP. Each of these pilus systems are encoded in a separate gene cluster (fim for type 1 and pap for P pili), with each operon encoding regulatory proteins, a tip adhesin, multiple pilin subunits, and a dedicated chaperone and usher. The corresponding proteins in each system share significant homology, and studies have shown that the chaperone and usher proteins function in homologous ways.
The chromosomally encoded pap (pyelonephritis-associated pilus) gene cluster encodes five Ig-like pilin subunits (PapF, PapE, PapK, PapA, PapH) and a two-domain adhesin, PapG, with one Ig-like pilin domain and a ligand-binding domain. Upon their transfer to the periplasm (10), these structural subunits are assembled through the actions of the periplasmic chaperone (PapD) and the integral outer membrane usher (PapC) in a specific order into bipartite P pilus fibers composing an open helical tip fibrillum made up of (from the tip) PapG (11), PapF, multiple PapEs, and PapK (12, 13), connected to a right-handed helical rod made up of thousands of copies of PapA (14–17) and terminated by a single copy of PapH (18, 19). Each pilin Ig fold is missing the seventh C-terminal β-strand of the canonical Ig fold. Due to the lack of the C-terminal strand, pilin subunits are unable to fold independently (12). Thus, folding of pilins in the periplasm is facilitated by the PapD chaperone (13, 20). PapD is a two-domain periplasmic protein, with each domain having a complete Ig fold (21). PapD binds and transiently donates a β-strand from its N-terminal domain to transiently complete the Ig fold of subunits, resulting in a noncanonical parallel interaction between the sixth strand of the subunit and the seventh strand provided by the chaperone (13). This process has been termed donor strand complementation (DSC). The subunits in the resulting complexes are stable. However, subunits in complex with PapD are not completely condensed but instead are in an open conformation that is “primed” for assembly (20). DSC has been demonstrated both for P and type 1 pilus systems, as well as for Sfa, Saf, Caf, Fml, and others (22), demonstrating the prototypical nature of P pili.
Once formed, P pilus chaperone–subunit complexes are targeted to the outer membrane usher PapC (23, 24). PapC is an 809-residue outer membrane protein that forms an assembly platform for pilus biogenesis (23, 25). PapC has five functional domains, all of which are required for pilus biogenesis (23, 25–27). It has a 24-stranded β-barrel transmembrane domain that allows translocation of the polymerized pilus fiber across the outer membrane and four globular domains: a periplasmic N-terminal domain (NTD), two periplasmic C-terminal domains (CTD1 and CTD2), and a plug domain (Plug) (23, 28). Mutations of the NTD or either CTD abolish pilus biogenesis (25, 27). The Plug domain is also required for pilus biogenesis (29, 30). A Plug-deleted PapC folds but does not assemble pili, suggesting that the Plug domain plays a direct role in catalyzing pilus biogenesis (29, 30). Binding studies have suggested that the chaperone–adhesin complex is initially targeted to the NTD of PapC (26), and this is thought to activate the usher protein such that the β-sandwich Plug domain shifts from the channel, where it is located in the apo usher, to the periplasmic space, resulting in an open translocation pore (28). This affinity of the NTD for the chaperone–adhesin complex has also been shown for the type 1 pilus system (31). However, the crystal structure of the type 1 pilus usher (FimD) bound to its cognate chaperone–adhesin complex (FimCH) revealed interactions between the chaperone–adhesin complex and the CTDs, as suggested in an earlier study by copurification after trypsin protection assays (25, 28).
In the absence of energy from either ATP hydrolysis or the proton-motive force, PapC catalyzes the ordered polymerization and translocation of subunits across the outer membrane to the cell surface (1, 32, 33) via a process called donor strand exchange (DSE). In addition to the incomplete Ig fold, every nonadhesin pilin subunit has a short N-terminal extension (NTE) that is exchanged with the donated chaperone strand bound to the previously incorporated subunit. The A and F strands of the subunit condense around the newly donated NTE, driving folding of the subunit into a canonical Ig domain with the seventh strand provided by the next subunit (20). This polymerization and translocation of the growing pilus fiber through the β-barrel transmembrane pore to the cell surface appears to rely on the energy stored in the incompletely condensed Ig folds of chaperone-bound subunits.
Here, we investigated relevant protein–protein interactions that occur at the usher. We characterized the interactions of the periplasmic PapC domains, NTD, CTD2, and Plug to obtain insight into the molecular basis of how the usher catalyzes pilus biogenesis and how these domains function as a molecular machine. We demonstrate that the NTD is the initial site of recruitment of chaperone–adhesin complexes and that CTD2 allosterically destabilizes this complex, resulting in transfer to the CTD domains. Subsequent chaperone–pilin complexes are recruited to the NTD–Plug complex or the Plug domain alone, rationalizing an active role for this domain in pilus assembly. Finally, CTD2 binds nonselectively to all chaperone–subunit complexes except the terminator complex, reflecting its second role as a transient docking site for chaperone–subunit complexes (28) and suggesting that pilus termination and/or anchoring may in part occur by failure of the terminator to transfer from the Plug domain.
Results
PapC Periplasmic Domains Interact Selectively with Chaperone–Subunit Complexes.
It has been previously shown that ushers bind to chaperone–subunit complexes with differing affinities (34–36). Thus, we used biolayer interferometry to assess the binding affinities of chaperone–subunit complexes with the various usher domains of PapC, hypothesizing that one or more of the periplasmic domains of the usher would recapitulate the discriminatory binding demonstrated for the full-length usher. Chaperone alone (PapD) and chaperone complexes with the adhesin (PapDG), major tip subunit (PapDE), the rod subunit (PapDA), the tip adaptor to the rod (PapDK), and the terminator (PapDH) were purified as previously described (11, 13) (SI Materials and Methods). To prevent homopolymerization of PapA and PapE subunits, we used PapA and PapE constructs in which the PapA NTE was replaced with the PapK NTE (PapAKnte) and the PapE NTE was deleted to create PapEntd. A truncate of PapG containing the galabiose-binding N-terminal domain of PapG (PapGtrunc) was also purified. A total of 50 μg/mL of biotinylated NTD, Plug, or CTD2 was incubated with Super Streptavidin pins (ForteBio Inc.). Super Streptavidin pins coated with biotinylated NTD, CTD2, or Plug were then incubated with each of the purified chaperone–subunit complexes. The NTD bound only to the PapDG and PapDEntd complexes with nanomolar and micromolar affinity, respectively, consistent with their order of assembly at the usher (Table 1). The NTD did not bind to apo-PapD, PapGtrunc, any of the other chaperone–subunit complexes, or bovine serum albumin (BSA). In contrast, CTD2 bound to apo-PapD and all of the chaperone–subunit complexes with micromolar affinities, with the exception of PapDH (Fig. 1). The Plug domain bound to each of the chaperone–subunit complexes with high affinity (∼20–50 nM), including apo-PapD and PapDH (Fig. S1, Table 1). PapGtrunc did not bind to CTD2, Plug, or NTD (Table 1). These data argue that PapDG and PapDEntd are selectively recruited to the usher by the NTD and that recruitment of all other chaperone–subunit complexes involves the Plug domain. The CTD domains are not thought to be involved in subunit recruitment, and the lower affinity of CTD2 for chaperone–subunit complexes likely reflects its role as a docking site for the assembling pilus fiber (28).
Table 1.
Binding affinities of PapC periplasmic domains for chaperone–subunit complexes
| KD (M) | kon [1/(M*s)] | koff (1/s) | R2* | |
| NTD binding | ||||
| PapDG | 3.20 × 10−9 | 2.35 × 10+6 | 6.68 × 10−3 | 0.83 |
| PapG lectin | No binding | |||
| PapDEntd | 1.19 × 10−6 | 4.64 × 10+2 | 6.11 × 10−4 | 0.99 |
| PapDK | No binding | |||
| PapDAKnte | No binding | |||
| PapDH | No binding | |||
| PapD | No binding | |||
| Plug binding | KD (M) | kon [1/(M*s)] | koff (1/s) | R2* |
| PapDG | 5.11 × 10−8 | 2.45 × 10+3 | 1.25 × 10−4 | 0.97 |
| PapG lectin | No binding | |||
| PapDEntd | 3.38 × 10−8 | 2.70 × 10+4 | 1.31 × 10−3 | 0.94 |
| PapDK | 1.79 × 10−8 | 2.23 × 10+4 | 3.19 × 10−4 | 0.99 |
| PapDAKnte | 3.02 × 10−8 | 1.86 × 10+4 | 4.54 × 10−4 | 0.99 |
| PapDH | 1.65 × 10−8 | 1.62 × 10+4 | 2.70 × 10−4 | 0.96 |
| PapD | 2.26 × 10−8 | 2.19 × 10+4 | 5.38 × 10−4 | 0.98 |
| CTD2 binding | KD (M) | kon [1/(M*s)] | koff (1/s) | R2* |
| PapDG | 5.47 × 10−6 | 4.43 × 10+3 | 9.81 × 10−3 | 0.79 |
| PapG lectin | No binding | |||
| PapDEntd | 1.05 × 10−6 | 6.90 × 10+2 | 6.92 × 10−4 | 0.98 |
| PapDK | 1.87 × 10−6 | 3.38 × 10+3 | 7.33 × 10−3 | 0.88 |
| PapDAKnte | 1.10 × 10−6 | 5.95 × 10+3 | 6.24 × 10−3 | 0.91 |
| PapDH | No binding | |||
| PapD | 1.70 × 10−6 | 3.49 × 10+3 | 5.91 × 10−3 | 0.71 |
*R2 is the coefficient of determination estimating the goodness of a curve fit reported by ForteBio Data Analysis software version 6.4.
Fig. 1.
PapC periplasmic domain CTD2 mediates binding with chaperone–subunit complexes. PapC CTD2 mediates concentration-dependent, micromolar affinity interactions with chaperone–subunit complexes PapDG, PapDEntd, PapDAKnte, and apo-PapD, but has no affinity for PapDH. BSA was used as a binding specificity control. The tips of Super Streptavidin pins were coated with 50 μg/mL of biotinylated PapC CTD2 and washed to remove unbound protein. These pins were dipped in increasing concentrations of chaperone–subunit complexes (shown at 2 μM each) to measure binding of chaperone–subunit complexes to CTD2 (left side of graph) and then moved to wells containing HBS to measure dissociation rates (right side of graph).
Intrausher Interactions of PapC Plug Domain, CTD2, and NTD.
Crystallographic studies of the type 1 pilus usher, FimD, in complex with its chaperone–adhesin complex FimC–FimH showed that the Plug domain had relocated to a site in close proximity to the NTD domain of the usher, thus representing the usher’s active state (28). Because the individual domains of FimD do not express well or are unstable, we used the Pap system to directly investigate the interaction that the NTD makes with the Plug domain and/or CTD2 of the PapC usher using biolayer interferometry assays. We were unable to test the CTD1 domain in this assay because this domain is unstable in isolation, likely due to its close association with the transmembrane barrel domain as seen in the FimDCH structure (28).
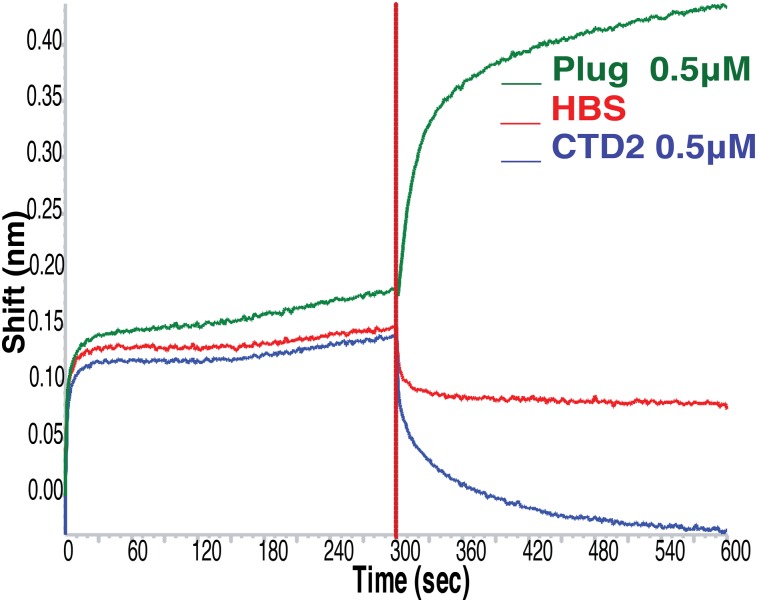
Purified PapC Plug domain was biotinylated and immobilized on Super Streptavidin pins (ForteBio Inc.) at 50 μg/mL, washed, and incubated in wells containing pure PapC NTD in a dilution series ranging from 0.2 to 13.2 μM (Fig. 2A). We found that the Plug domain of PapC binds the NTD with high affinity (KD: 4.12 × 10−10) (Fig. 2A, Table 2), whereas a control protein (BSA) showed no affinity. Despite structural homology between PapC’s Plug and CTD2 domains (37), CTD2 showed no affinity for NTD (Table 2) and no interaction was observed between the Plug and CTD2 domains.
Fig. 2.
PapC Plug domain mediates a high-affinity, stable interaction with PapC NTD, and this complex is capable of recruiting chaperone–subunit complexes. (A) In a biolayer interferometry assay, Super Streptavidin pins incubated in 50 μg/mL of biotinylated PapC Plug domain were incubated with increasing concentrations of purified PapC NTD for 2 min (0.2, 0.4, 3.2, 6.6, and 13.2 μM) to detect NTD–Plug association. The pins with NTD–Plug complex were then moved to wells containing HBS to measure dissociation for 30 min. A concentration-dependent, stable interaction between NTD and Plug domains of PapC was observed. (B) The stable complex that forms between PapC NTD and Plug domains is active in recruiting all tested chaperone–subunit complexes and apo-PapD. Using biolayer interferometry as in A, a stable complex between NTD and Plug domain was obtained by incubating Super Streptavidin pins coated with 50 μg/mL of Plug domain in 24.6 μM NTD and then washing them. The pins coated with NTD–Plug complex were incubated in chaperone–subunit complexes for 5 min in increasing concentrations of chaperone–subunit complexes or chaperone alone (shown at 1 μM each) and then moved to wells containing HBS for measurement of dissociation rates.
Table 2.
Binding kinetics of intrausher interactions
| Interaction | KD | kon [1/(M*s)] | koff (1/s) | R2* |
| Plug–NTD | 4.12 × 10−10 | 4.10 × 10+4 | 1.34 × 10−5 | 0.985 |
| NTD–CTD2 | No binding | |||
| Plug–CTD2 | No binding |
*R2 is the coefficient of determination estimating the goodness of a curve fit reported by ForteBio Data Analysis software version 6.4.
The interaction between the Plug and NTD may stabilize the open, ungated conformation of the usher and help position the NTD (and possibly the Plug) in a proper orientation to recruit chaperone–subunit complexes and facilitate pilus biogenesis. To investigate the hypothesis that the stable PapC NTD–Plug complex is active in recruiting chaperone–subunit complexes, we carried out an additional biolayer interferometry assay by incubating Super Streptavidin pins in 50 μg/mL of biotinylated Plug, which were then washed and incubated in 24.6 μΜ NTD to establish a stable NTD–Plug complex. This complex was briefly washed and incubated in chaperone–subunit complexes (PapDG, PapDEntd, PapDK, PapDAKnte, PapDH) and apo-PapD to test its ability to recruit chaperone–subunit complexes. NTD–Plug complex was able to bind to all tested chaperone–subunit complexes, even to those that have no affinity for NTD alone such as PapDK, PapDAKnte, PapDH, and apo-PapD (Fig. 2B). CTD2 was used as a binding control as it has no affinity for apo-NTD or Plug domains (Fig. 2B). Binding affinities of apo-PapD and chaperone–subunit complexes for the NTD–Plug complex were similar to that of the Plug domain alone (Table 1). The apparent affinity of the NTD–Plug complex for PapDG was two orders of magnitude lower than that for NTD alone and one order of magnitude lower than that for the Plug domain alone, suggesting that NTD in the context of a closed pore may be the initial targeting site for PapDG, thus initiating pilus formation, and that subsequent recruitment of chaperone–subunit complexes requires the NTD–Plug complex or the Plug domain alone.
CTD2 Mediates Dissociation of the NTD/Chaperone–Adhesin Complex.
The PapC NTD has been implicated as the initial targeting site for chaperone–subunit complexes (26), and in the present study we show that NTD selectively binds to the PapDG chaperone–adhesin complex. However, the FimDCH crystal structure showed the chaperone–adhesin complex (FimCH) binding to the usher’s CTDs (28). Knowing that CTD2 directly interacts with the chaperone–adhesin complex (Fig. 1), we hypothesized that CTD2 is capable of causing the dissociation of the initial PapDG complex from NTD. We set up a biolayer interferometry experiment, conjugating Super Streptavidin pins with 50 μg/mL of biotinylated PapC NTD and incubating these pins in 0.5 μM PapDG complex. Pins containing the NTD–PapDG complex were moved to either buffer only to measure an off rate or to buffer containing 0.5 μM of CTD2 to determine its effect on dissociation of the NTD–PapDG complex. PapC Plug domain (0.5 μM) was used as a control. We observed that when CTD2 was present the dissociation rate of the NTD–PapDG complex (fit in the ForteBio software version 6.4) increased by over an order of magnitude from 2.75 × 10−3 ± 1.25(1/s) to 6.77 × 10−2 ± 5.2 (1/s) compared with buffer alone, suggesting that CTD2 is involved in catalyzing the dissociation of NTD–PapDG complex (Fig. 3). Further analysis of the data by fitting (Origin 8; OriginLab) the curves to the sum of two exponentials revealed that the Hepes buffered saline (HBS) data were best fit as a double exponential with fast (0.057/s ± 0.003) and slow (0.00035/s ± 0.00014) rates, with the slow phase accounting for five times the amplitude of the fast phase, whereas the CTD2-catalyzed reaction was best fit as a single exponential with a fast rate (0.054/s ± 0.004), confirming that CTD2 accelerates the rate of dissociation by over an order of magnitude. Interestingly, however, the Plug domain of PapC associated only with the NTD–PapDG complex. Thus, the catalysis of the dissociation of the NTD–PapDG complex was specific to CTD2 (Fig. 3).
Fig. 3.
CTD2 mediates dissociation of the PapC NTD/chaperone–adhesin complex. Super Streptavidin pins were incubated in 50 μg/mL of biotinylated NTD, washed, and incubated in 0.5 μM PapDG to measure the NTD–PapDG interaction (left side of graph). The pins containing the NTD–PapDG complex were moved to wells containing HBS only, HBS with 0.5 μM CTD, or 0.5 μM Plug domain (right side of graph). Incubation of the bound NTD–PapDG complex with CTD2 (blue, CTD2) causes faster dissociation of chaperone–adhesin from the NTD compared with buffer alone (red, HBS). Incubating NTD–PapDG complex with the Plug domain causes the Plug domain’s association with the bound complex (green, Plug).
Overexpression of Periplasmic Usher Domains in Trans Inhibits Pilus Assembly.
On the basis of interactions that we observed with the CTD2, Plug, and NTD within the usher and with chaperone–subunit complexes, we hypothesized that, if these interactions were physiological, overexpression of these specific usher domains would interfere with pilus biogenesis. Therefore, the P pilus operon was expressed from its own promoter in the pFJ3 plasmid (38) on Tryptic Soy Agar (TSA) plates, and the CTD2, Plug, or NTD domains were expressed by isopropyl-β-d-thio-galactoside (IPTG) induction of plasmids pKDC3, pKDC5, or pKDC16, respectively. Overexpression of any of these PapC domains interfered with pilus biogenesis as determined by hemagglutination titers of human type A erythrocytes (Fig. 4B). Morphologic investigation of these bacteria via electron microscopy also revealed that overexpression of the CTD2, Plug, or NTD resulted in decreased piliation (Fig. 4A). Overexpression of periplasmic PapC domains did not cause a significant change in type 1 pilus assembly (Fig. S2), suggesting that overexpression of these domains is not cytotoxic and that the effects observed are specific to the P pilus system. Overexpressed PapC domains may titrate the relevant chaperone–subunit complexes away from PapC or block aspects of usher gating and/or transfer of complexes to the CTD2 domain, thus interfering with functional pilus biogenesis and indirectly supporting our in vitro results.
Fig. 4.

In vivo overexpression of usher periplasmic domains interferes with pilus biogenesis. Electron microscopy (A) and hemagglutination assays (B) reveal that overexpression of the PapC domains CTD2, NTD, and Plug in the periplasmic space decreases pilus biogenesis. E. coli C600 cells carrying the pFJ3 plasmid were transformed with pKDC3 (CTD), pKDC5 (Plug), and pKDC16 (NTD). These strains were grown on TSA plates with IPTG for induction of P pili from the pFJ3 plasmid and expression of Plug, NTD, and CTD. A lawn of cells was grown and collected for electron microscopy (A) and hemagglutination assays (HA) (B) to investigate the impact of overexpressed PapC domains on pilus biogenesis. HA titer is the highest dilution of bacteria that still provides agglutination of human type A erythrocytes.
Discussion
Chaperone–usher pilus assembly requires strict organization and reorganization of usher domains to coordinate the transfer of multiple pilin subunits from chaperone–subunit complexes to various usher domains and their subsequent movement, in a tightly regulated process, through the gated outer membrane usher into pili via a donor strand exchange mechanism. Using PapC as a prototype system, we evaluated interactions between the globular NTD, Plug, and CTD2 domains of the usher with each other and with chaperone–subunit complexes. Each of these usher domains is required for PapC function and, when expressed in excess in trans, inhibit pilus assembly. Using biolayer interferometry, we found that NTD selectively binds the PapDG and PapDEntd complexes with nanomolar and micromolar affinities, respectively, but does not bind to any of the other tested chaperone–subunit complexes (PapDK, PapDAKnte, or PapDH) or to the chaperone alone. Interestingly, CTD2 bound with an affinity in the micromolar range to all of the tested chaperone–subunit complexes, except for the PapDH terminator complex, whereas the Plug domain bound to all of the tested chaperone–subunit complexes, including PapDH as well as the chaperone alone. This argues for direct involvement of the PapC Plug domain in pilus biogenesis beyond being merely a pore gate. Crystallographic studies of the type 1 pilus usher FimD, in complex with its chaperone–adhesin complex FimC-FimH, showed that the Plug domain had relocated to a site adjacent to the NTD domain of the usher in the usher’s active state (28). Using the Pap system, we elucidated an intrausher interaction between the NTD and Plug domains. The interaction of Plug with NTD may be part of a mechanism to open the PapC pore upon PapDG binding. Despite the structural homology between the Plug and the CTD2 (37), NTD showed no affinity for CTD2. Similarly, no interaction between CTD2 and the Plug was observed. Because we found that the Plug–NTD complex was able to bind apo-PapD and PapD subunit complexes, this reorganization of usher domains may serve to recruit subsequent chaperone–subunit complexes. This finding emphasizes that the NTD–Plug complex, in addition to its likely role in retaining an open PapC pore, is also of physiological importance in the essential usher function of chaperone–subunit recruitment and, hence, in the catalysis of pilus biogenesis. Finally, we showed that the affinity of NTD alone for PapDG (KD = 10−9 M) is two orders of magnitude higher than that of the NTD–Plug complex (KD = 10−7 M), suggesting that PapDG is more likely to target the NTD before opening of the pore and formation of an NTD–Plug complex.
CTD1 is another periplasmic domain of PapC that is likely involved in docking of chaperone–subunit complexes along with CTD2 after the complexes are competitively transferred to the CTDs from the NTD by the action of CTD2. This Ig-like domain of PapC is likely involved in extensive interactions with the lectin domain of PapG and the chaperone as this was shown to be the case for the homologous type 1 system (28). CTD1, as a subdomain of the transmembrane β-barrel itself, is likely also important for the overall conformation of PapC.
Our results suggest a mechanism whereby PapDG is targeted to the NTD, triggering the ungating of the usher, likely via allosteric interactions that occur upon PapDG binding. PapC is subsequently stabilized in an open conformation by the NTD–Plug interaction (Fig. 5 A and B). The full-length PapDG is needed for this to occur as the lectin domain of PapG alone shows no binding with any of the usher domains tested. We discovered that the CTD2 was capable of catalyzing the dissociation of PapDG from the NTD in a competitive binding assay. This function of CTD2 in the dissociation of the NTD–PapDG complex is a catalytic activity for this highly conserved domain. This function is likely the result of an allosteric mechanism because CTD2 binds to chaperone–subunit complexes but not to the NTD, ruling out direct competition. Thus, PapDG is transferred to the CTDs via allosteric destabilization by CTD2 (Fig. 5 C and D). This frees the NTD/Plug for the recruitment of subsequent chaperone–subunit complexes. Allosteric handover of the chaperone–adhesin complex to the CTDs is the last step of pilus initiation at the usher because, upon transfer to the CTDs, the adhesin domain likely starts emerging through the usher transmembrane domain as seen in the FimDCH structure (28). During the assembly of the pilus rod, the NTD in its apo state is bypassed, and subunits are instead recruited to the Plug, or NTD–Plug complex, and CTD2 (Fig. 5 E and F). Considering its equal affinity for apo-PapD, the Plug domain may also be involved in catalysis by removing PapD after DSE. Interestingly, Plug is the only domain capable of binding the PapDH terminator complex. Incorporation of PapH is known to terminate pilus assembly due in part to the occluded P5 pocket of PapH (19). The unique interaction of PapDH with Plug implicates the usher’s Plug domain in pilus termination and anchoring, where PapDH is directly targeted to the Plug domain or NTD–Plug complex bypassing CTD2 (Fig. 5G) and resulting in a unique and irreversible (by DSE) interaction that terminates pilus assembly. Overall, our studies provide insight into how ordered assembly of a prototypical chaperone–usher pilus takes place at the usher via coordinated participation of each of its periplasmic domains. We have defined the differential affinities of the usher domains for chaperone–subunit complexes, facilitating subunit ordering. This allows efficient transfer of chaperone–subunit complexes around the usher molecular machine from NTD/Plug to CTD2 (Fig. 5). These studies provide evidence on the collective, direct roles of NTD, CTD2, and Plug domains of PapC in pilus biogenesis. The results afford insight into how the usher catalyzes pilus assembly in the absence of ATP, an important virulence event in many Gram-negative pathogens, which rely on adhesive pili to promote adhesion to host tissues and subsequent infection.
Fig. 5.
Model of pilus biogenesis at the usher. The plug domain resides in the translocation pore in the inactive usher (A). Upon chaperone–adhesin binding to the NTD, the plug domain extends to the periplasm where it stably binds to the NTD (B). CTD2 mediates binding to the chaperone–adhesin complex at the NTD (C) where it catalyzes dissociation of the NTD–PapDG complex and remains bound to PapDG (D). Subsequent interactions of incoming subunits with the CTDs and Plug result in pilus assembly (E–G). Rod and tip components interact with Plug and CTD2, but the terminator complex PapDH is directly targeted to the Plug domain without transfer to the NTD or CTD2 (G).
Materials and Methods
Strains and Constructs.
All PapC constructs were cloned into the pTrc99a vector with PapD’s signal sequence and a 6His-tag using standard PCR and recombinant techniques and expressed in E. coli strain BL21. Plug domain was expressed from pKDC5, CTD2 was expressed from pKDC3, and NTD was expressed from pKDC16 (Table S1). E. coli strain C600, carrying appropriate plasmids, was used for HA titer and EM assays. The pFJ3 plasmid was used to express WT pap operon under its own promoter on TSA plates (38).
Biolayer Interferometry Assays.
All interaction experiments were conducted at 30 °C in HBS (20 mM Hepes, 150 mM NaCl, pH 7.5) using an Octet Red instrument (ForteBio Inc.). Purified PapC Plug and NTD and CTD2 domains were dialyzed overnight in HBS and biotinylated using NHS-PEO4-biotin at a ratio of 1:1 (Thermo Scientific). A total of 50 μg/mL of CTD2, NTD, or Plug were bound to Super Streptavidin pins (ForteBio Inc.). The purified chaperone–subunit complexes PapDG, PapDEntd, PapDK, PapDAKnte, PapDH, or PapD alone were dialyzed overnight in HBS. The biotinylated PapC domain-bound pins were incubated in wells containing chaperone–subunit complexes (PapDG, PapDEntd, PapDK, PapDAKnte, PapDH) or PapC NTD at concentrations of 0.18, 0.5, 1.66, 2, 5, and 12.5 μM for 5–10 min or 0.2, 0.4, 3.2, 6.6, or 13.2 μM, respectively. They were then incubated in wells containing HBS for measurement of dissociation rates. Inverse experiments where chaperone–subunits were biotinylated and then incubated in PapC domains were also carried out. For NTD–Plug complex-binding experiments, the tips of Super Streptavidin pins were coated with 50 μg/mL of biotinylated Plug domain and incubated in 24.6 μM of NTD. Once a stable interaction is established, the pins were washed and incubated in chaperone–subunit complexes, chaperone alone, or CTD2 (negative control) for 5 min followed by incubation in HBS for 5 min to measure dissociation rates.
PapC Plug, NTD, and CTD2 in Vivo Overexpression Studies.
E. coli C600 cells were transformed with pFJ3 and pKDC5 (Plug), pKDC3 (CTD2), pKDC16 (NTD), or the empty vector pTrc99a. Cells were grown for 8 h in LB broth at 37 °C with shaking and plated on TSA plates containing 1 μM IPTG and the appropriate antibiotics. A lawn of cells was grown at 37 °C overnight, collected, and used for HA titers (39) and negative-stain electron microscopy.
Supplementary Material
Acknowledgments
We gratefully acknowledge the expertise of W. Beatty (Imaging Facility, Washington University School of Medicine). This study was funded by National Institutes of Health RO1 Grant AI029549 and AI049450 (to S.J.H.). D.G.T. is supported by National Institutes of Health Grant GM62987. B.A.F. is supported by National Institutes of Health K08 Grant 1K08DK093707-01.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207085109/-/DCSupplemental.
References
- 1.Thanassi DG, Saulino ET, Hultgren SJ. The chaperone/usher pathway: A major terminal branch of the general secretory pathway. Curr Opin Microbiol. 1998;1:223–231. doi: 10.1016/s1369-5274(98)80015-5. [DOI] [PubMed] [Google Scholar]
- 2.Mulvey MA, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 3.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langermann S, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 5.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuehn MJ, Heuser J, Normark S, Hultgren SJ. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 1992;356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- 7.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JA, et al. The Gal(alpha 1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci USA. 1994;91:11889–11893. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund B, Lindberg FP, Båga M, Normark S. Globoside-specific adhesins of uropathogenic Escherichia coli are encoded by similar trans-complementable gene clusters. J Bacteriol. 1985;162:1293–1301. doi: 10.1128/jb.162.3.1293-1301.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem. 2008;77:643–667. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- 11.Dodson KW, et al. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell. 2001;105:733–743. doi: 10.1016/s0092-8674(01)00388-9. [DOI] [PubMed] [Google Scholar]
- 12.Hultgren SJ, Normark S, Abraham SN. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- 13.Sauer FG, et al. Structural basis of chaperone function and pilus biogenesis. Science. 1999;285:1058–1061. doi: 10.1126/science.285.5430.1058. [DOI] [PubMed] [Google Scholar]
- 14.Båga M, et al. Nucleotide sequence of the papA gene encoding the Pap pilus subunit of human uropathogenic Escherichia coli. J Bacteriol. 1984;157:330–333. doi: 10.1128/jb.157.1.330-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullitt E, et al. Development of pilus organelle subassemblies in vitro depends on chaperone uncapping of a beta zipper. Proc Natl Acad Sci USA. 1996;93:12890–12895. doi: 10.1073/pnas.93.23.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong M, Makowski L. Helical structure of P pili from Escherichia coli. Evidence from X-ray fiber diffraction and scanning transmission electron microscopy. J Mol Biol. 1992;228:735–742. doi: 10.1016/0022-2836(92)90860-m. [DOI] [PubMed] [Google Scholar]
- 17.Verger D, Bullitt E, Hultgren SJ, Waksman G. Crystal structure of the P pilus rod subunit PapA. PLoS Pathog. 2007;3:e73. doi: 10.1371/journal.ppat.0030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Båga M, Norgren M, Normark S. Biogenesis of E. coli Pap pili: papH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987;49:241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- 19.Verger D, Miller E, Remaut H, Waksman G, Hultgren S. Molecular mechanism of P pilus termination in uropathogenic Escherichia coli. EMBO Rep. 2006;7:1228–1232. doi: 10.1038/sj.embor.7400833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauer FG, Pinkner JS, Waksman G, Hultgren SJ. Chaperone priming of pilus subunits facilitates a topological transition that drives fiber formation. Cell. 2002;111:543–551. doi: 10.1016/s0092-8674(02)01050-4. [DOI] [PubMed] [Google Scholar]
- 21.Hung DL, Knight SD, Woods RM, Pinkner JS, Hultgren SJ. Molecular basis of two subfamilies of immunoglobulin-like chaperones. EMBO J. 1996;15:3792–3805. [PMC free article] [PubMed] [Google Scholar]
- 22.Nuccio SP, Bäumler AJ. Evolution of the chaperone/usher assembly pathway: Fimbrial classification goes Greek. Microbiol Mol Biol Rev. 2007;71:551–575. doi: 10.1128/MMBR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remaut H, et al. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell. 2008;133:640–652. doi: 10.1016/j.cell.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norgren M, Båga M, Tennent JM, Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 25.Thanassi DG, Stathopoulos C, Dodson K, Geiger D, Hultgren SJ. Bacterial outer membrane ushers contain distinct targeting and assembly domains for pilus biogenesis. J Bacteriol. 2002;184:6260–6269. doi: 10.1128/JB.184.22.6260-6269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng TW, Akman L, Osisami M, Thanassi DG. The usher N terminus is the initial targeting site for chaperone-subunit complexes and participates in subsequent pilus biogenesis events. J Bacteriol. 2004;186:5321–5331. doi: 10.1128/JB.186.16.5321-5331.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson NS, Ng TW, Talukder I, Thanassi DG. Function of the usher N-terminus in catalysing pilus assembly. Mol Microbiol. 2011;79:954–967. doi: 10.1111/j.1365-2958.2010.07505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan G, et al. Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate. Nature. 2011;474(7349):49–53. doi: 10.1038/nature10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mapingire OS, Henderson NS, Duret G, Thanassi DG, Delcour AH. Modulating effects of the plug, helix, and N- and C-terminal domains on channel properties of the PapC usher. J Biol Chem. 2009;284:36324–36333. doi: 10.1074/jbc.M109.055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Smith BS, Chen LX, Baxter RH, Deisenhofer J. Insights into pilus assembly and secretion from the structure and functional characterization of usher PapC. Proc Natl Acad Sci USA. 2009;106:7403–7407. doi: 10.1073/pnas.0902789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiyama M, et al. Structural basis of chaperone-subunit complex recognition by the type 1 pilus assembly platform FimD. EMBO J. 2005;24:2075–2086. doi: 10.1038/sj.emboj.7600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waksman G, Hultgren SJ. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat Rev Microbiol. 2009;7:765–774. doi: 10.1038/nrmicro2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob-Dubuisson F, Striker R, Hultgren SJ. Chaperone-assisted self-assembly of pili independent of cellular energy. J Biol Chem. 1994;269:12447–12455. [PubMed] [Google Scholar]
- 34.Dodson KW, Jacob-Dubuisson F, Striker RT, Hultgren SJ. Outer-membrane PapC molecular usher discriminately recognizes periplasmic chaperone-pilus subunit complexes. Proc Natl Acad Sci USA. 1993;90:3670–3674. doi: 10.1073/pnas.90.8.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saulino ET, Thanassi DG, Pinkner JS, Hultgren SJ. Ramifications of kinetic partitioning on usher-mediated pilus biogenesis. EMBO J. 1998;17:2177–2185. doi: 10.1093/emboj/17.8.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, et al. The differential affinity of the usher for chaperone-subunit complexes is required for assembly of complete pili. Mol Microbiol. 2010;76(1):159–172. doi: 10.1111/j.1365-2958.2010.07089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford B, et al. Structural homology between the C-terminal domain of the PapC usher and its plug. J Bacteriol. 2010;192:1824–1831. doi: 10.1128/JB.01677-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob-Dubuisson F, Heuser J, Dodson K, Normark S, Hultgren S. Initiation of assembly and association of the structural elements of a bacterial pilus depend on two specialized tip proteins. EMBO J. 1993;12:837–847. doi: 10.1002/j.1460-2075.1993.tb05724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slonim LN, Pinkner JS, Brändén CI, Hultgren SJ. Interactive surface in the PapD chaperone cleft is conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO J. 1992;11:4747–4756. doi: 10.1002/j.1460-2075.1992.tb05580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.