The physicist Richard Feynman once quipped that the key to solving or making significant progress on a scientific problem was to “find the open channel.” For the cell biological problem of nucleus-to-cytoplasm transport, the literal open channels have long been known: the nuclear pore complexes (NPCs). However, in the metaphorical sense as well, recent work has significantly “opened” the NPC channel as regards the biophysics of the engagement and transit of outward-bound cargo. In this issue of PNAS, Siebrasse et al. (1) track single molecules of a native messenger RNA and provide details on the approach of these transcripts to the NPCs, the probability and persistence of engagement, and the channel transit times. This impressive study was enabled by the use of an iconic system for tracking specific transcripts in the nucleus of a living cell, together with a recently developed innovation in the method of microscopy used.
Although the parts list and supramolecular organization of the NPC are presently understood as well as or better than any component of the nucleus (2, 3), the details of how RNA–protein complexes (mRNP) are exported have remained relatively ill-defined as biophysics and thermodynamics and as studied in vivo with intact cells (reviewed in refs. 4, 5). The intranuclear movement of RNAs from their transcription sites to the nuclear periphery is diffusion-mediated (reviewed in ref. 6) but the subsequent step(s) at which metabolic energy is required is uncertain. Do the nucleoplasm-facing components of the NPC irreversibly snag potential cargo or is the initial encounter more tentative? When it has been positioned within approximately the first nanometer of the NPC central channel, is export irreversibly committed? Is the outward vector of cargo in the transport channel saltatory, with frequent (or infrequent) pauses, or is it perhaps the sum of outbound and inbound translocations with the number and/or single step size of the former eventually exceeding that of the latter? These and other fundamental questions about nuclear export have remained frustratingly refractive to investigation, as their resolution obviously requires the tracking of individual cargoes.
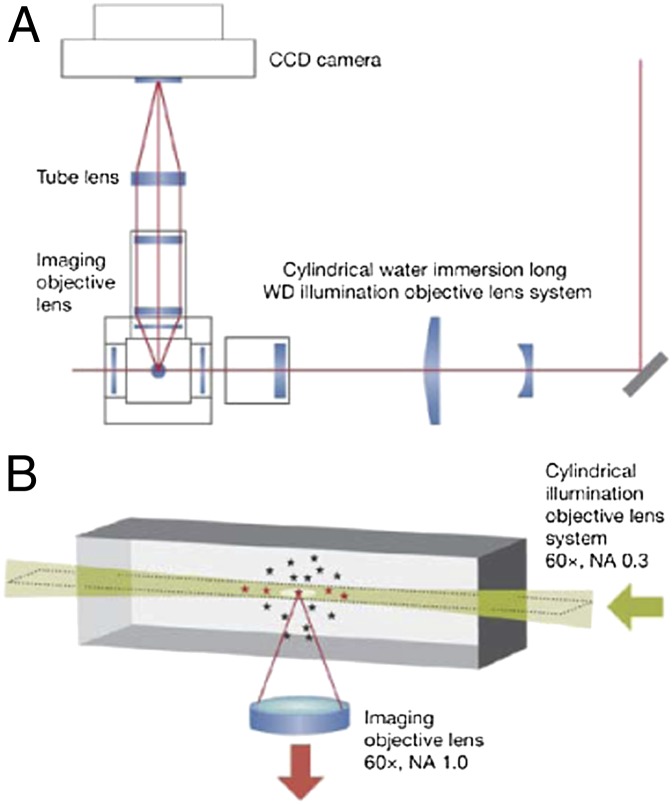
The live cell detection of single fluorescent molecules, or even particles with more than one to more than five copies of labeled components, is severely limited by the relatively low signal-to-noise ratio (SNR) obtained with even the brightest dyes and most sensitive cameras. The goal is to reduce the background autofluorescence and yet introduce sufficient excitation light to activate enough of the molecules under interrogation, ideally sparsely populated to ensure single-molecule (or -particle) spatial resolution. A number of recent advances have yielded significant reductions in the SNR for biological specimens, especially those with a deep z axis. The one used by Siebrasse et al. (1) is termed selective plane illumination microscopy, also known as light sheet fluorescence microscopy (LSFM), the latter term having been adopted recently by most leaders in the microscopy field. LSFM (Fig. 1) combines the speed and sensitivity of wide field detection with the enhanced resolution inherent in a 90° decoupling of the planes of excitation and detection (refs 7–9 and refs. therein), so that only those fluorophores in a narrow z-axis “sheet” are excited, with the relatively low autofluorescence generated in the sheet by this illumination geometry contributing significantly to the enhanced SNR.
Fig. 1.
In LSFM, an excitation beam, or “sheet” (height × width × thickness, 20 μm × 20 μm × 2 μm), is delivered within the focal plane by using a cylindrical lens (magnification of 60×; NA, 0.3), and the emitted fluorescence is detected by using an objective oriented perpendicular to the illumination path (magnification of 60×; NA, 1.0). (A) Diagram of the overall microscopy system. (B) Schematic of the illumination and detection paths. CCD, cooled CCD; WD, working distance. Reproduced from ref. 16.
Before the work of Siebrasse et al. (1), two studies of mRNA export in mammalian cells used mRNAs genetically engineered to contain a tandem array of fluorescent tags (10, 11), together with a superregistration microscopy innovation in the latter (11). In contrast, Siebrasse et al. (1) used a system in which naturally occurring, unmodified mRNAs, including ones transcribed from the well characterized Balbiani ring (BR) 1 and 2 genes in the polytene larval salivary gland chromosomes of the dipteran insect Chironomus tentans, can be followed. The BR1 and BR2 mRNPs rank as among the most well characterized mRNA transcripts and ribonucleoprotein particles (12–14). Siebrasse et al. (1) labeled the BR mRNPs by injecting into the incubated salivary glands a fluorescent version of an RNA-binding protein, hrp36, with which these transcripts are known to assemble. The authors thus emphasized that the mRNA–ribonucleoprotein complexes they studied are modified only by virtue of a single dye molecule (Alexa 647) attached to the hrp36 protein via a hexapeptide linker. To label the NPCs, they coinjected an Alexa 546-tagged version of the nucleoporin-avid protein NTF-2, permitting simultaneous detection of the mRNP (red) and NPCs (yellow). The authors’ system had an image acquisition power (the image integration time was 20 ms at a 50-Hz frame rate) that, together with their due attention to dual-color signal registration, allowed single mRNPs to be visualized as they approached and engaged NPCs. A possible pitfall in these experiments was the possibility that some of the red signal could be free hrp36 protein, not complexed with mRNA. The authors cleverly ruled this out by showing that a mutant of hrp36 that cannot bind RNA has an intranuclear mobility that is so fast as to not contribute to the focal signals observed.
In movies of the numerous pretransport and engaged transport events, some mRNPs were seen to display a productive sequence of NPC binding and transport, whereas others were observed to more tentatively interact with the NPC. To refine the analysis of mean transit times, the authors arrayed their data for each single mRNP in a given NPC as a kymograph, in which the signal was plotted as a function of time vs. distance traversed toward the cytoplasm. From all their observations, recorded in thousands of movies, distinct patterns of mRNP:NPC behavior were gleaned. One, which they termed “nuclear probing,” was defined as mRNPs making contact with the NPC but then returning to the nucleoplasm. Another was characterized by NPC engagement and transit. In some instances, a cytoplasmic mRNP (having previously undergone nuclear export) was observed to approach an NPC, possibly bind, and then return to the cytoplasm (one of the most fascinating phenomena observed and one worthy of further pursuit, as it hints that the cytoplasmic face of the NPC can recognize an already exported NPC as noncargo as regards the inbound route).
Together with the two recent studies in mammalian cells (10, 11) the study of Siebrasse et al. (1) adds up to very welcome progress in the nuclear export field over a period of only 2 years, a quantum jump in this field. Given the relatively unmodified mRNA used, as well as the exploitation of light sheet microscopy, some of the cognoscenti in the nuclear export field may embrace the new work (1) as the most enabling. This observer would instead admiringly emphasize the remarkable technical achievements embodied in all three studies and would point to the general congruence of the findings.
It remains for us to think about what remains. For example, in none of these studies (1, 10, 11) was it possible to examine how long the cargo was retained by the cytoplasm-facing architecture of the NPC before release. This limitation relates to the z axis resolution and the geometry of the nucleus and cytoplasm in each of the studies. Nonsense-mediated decay of aberrant mRNAs may occur while these transcripts have just emerged from the central channel of the NPC but are still tethered in some way, so this is one reason that knowing the discharge kinetics for normal transcripts themselves would be valuable (as accurate mRNAs would be scanned too by the NMD detection machinery, presumably at this point in the export pathway). Studies with transcripts designed for NMD would be an attractive application of the NPC tracking of mRNAs now at hand. Also in need of resolution is whether and how the penultimate step of cargo release, and indeed the release step itself, manages to occur in the face of incoming traffic (e.g., various nucleus-homing proteins, the small spliceosomal RNAs being imported after their cytoplasmic maturation, returning microRNAs). Do these inbound molecules and particles bounce back from NPCs from which a mRNP is about to emerge? Does the cytoplasm-facing structure of a NPC that has an mRNP in the transport channel or about to be discharged differ from an unoccupied NPC and thus signal this fact to cytoplasmic cargo about to engage for the inbound route? Ensemble studies have suggested that NPCs can promptly switch between export and import modes, but this needs to be confirmed (or conceivably negated) at the level of single NPCs in living cells. The switching between export and import at a single pore could be investigated with dual-color cargo given the advances made by this (1) and related studies.
Finally, what really goes on in the NPC central channel? Like others (the translocon, the K+ channel), we know the interior of the NPC transport channel quite well as regards which domains of which proteins line it or extend into it. However, we want to know what a Maxwell demon would see if swimming through. Are there protein spurs (side-chains or, at greater reach, N- or C-termini) that emanate inward acting as a rheological barrier or are they active players (i.e., transport as a “spaghetti opera”)? This remains a controversial aspect of the nuclear export field, and a recent study has suggested a very different biophysical structure of the NPC central channel (15). Single-particle tracking could play a role in the further resolution of this issue, combined with the appropriate experimental variables. For one thing, even answering the simple question of whether the central channel transit time has a temperature dependence (i.e., Q10) indicative of a physical process or one involving enzymatic activity is now easily within reach in a living cell for the first time, especially in the Chironomus system in which the cells have a broader temperature range of physiological integrity than do mammalian cells.
In his metaphor, Feynman, of course, did not specify what would be found on the other side once the “open channel” was identified, pursued, and successfully navigated, regarding “progress” as a generic concept. However, we all know this state: Vannevar Bush famously called it the Endless Frontier. It is why our profession comes from the Latin present participle sciens and not the verb scio (i.e., “knowing” as a constant endeavor). The recent progress in the nuclear export field has all of the hallmarks Bush had in mind: results that unify a problem and wonderfully create new questions and opportunities.
Acknowledgments
My work is supported by National Science Foundation Grant MCB-1051398.
Footnotes
The author declares no conflict of interest.
See companion article on page 9426.
References
- 1.Siebrasse JP, Kaminski T, Kubitscheck U. Nuclear export of single native mRNA molecules observed by light sheet fluorescence microscopy. Proc Natl Acad Sci USA. 2012;109:9426–9431. doi: 10.1073/pnas.1201781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alber F, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. doi: 10.1038/nature06405. [DOI] [PubMed] [Google Scholar]
- 3.Solmaz SR, Chauhan R, Blobel G, Melčák I. Molecular architecture of the transport channel of the nuclear pore complex. Cell. 2011;147:590–602. doi: 10.1016/j.cell.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wente SR, Rout MP. The nuclear pore complex and nuclear transport. In: Misteli T, Spector DL, editors. The Nucleus. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2011. pp. 73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitchison JD, Rout MP. The yeast nuclear pore complex and transport through it. Genetics. 2012;190:855–883. doi: 10.1534/genetics.111.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pederson T. The nucleus introduced. In: Misteli T, Spector DL, editors. The Nucleus. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2011. pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynaud EG, Krzic U, Greger K, Stelzer EH. Light sheet-based fluorescence microscopy: More dimensions, more photons, and less photodamage. HFSP J. 2008;2:266–275. doi: 10.2976/1.2974980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber M, Huisken J. Light sheet microscopy for real-time developmental biology. Curr Opin Genet Dev. 2011;21:566–572. doi: 10.1016/j.gde.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Ritter JG, Veith R, Veenendaal A, Siebrasse JP, Kubitscheck U. Light sheet microscopy for single molecule tracking in living tissue. PLoS ONE. 2010;5:e11639. doi: 10.1371/journal.pone.0011639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mor A, et al. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat Cell Biol. 2010;12:543–552. doi: 10.1038/ncb2056. [DOI] [PubMed] [Google Scholar]
- 11.Grünwald D, Singer RH. In vivo imaging of labelled endogenous β-actin mRNA during nucleocytoplasmic transport. Nature. 2010;467:604–607. doi: 10.1038/nature09438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehlin H, Daneholt B, Skoglund U. Translocation of a specific premessenger ribonucleoprotein particle through the nuclear pore studied with electron microscope tomography. Cell. 1992;69:605–613. doi: 10.1016/0092-8674(92)90224-z. [DOI] [PubMed] [Google Scholar]
- 13.Mehlin H, Daneholt B, Skoglund U. Structural interaction between the nuclear pore complex and a specific translocating RNP particle. J Cell Biol. 1995;129:1205–1216. doi: 10.1083/jcb.129.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneholt B. Assembly and transport of a premessenger RNP particle. Proc Natl Acad Sci USA. 2001;98:7012–7017. doi: 10.1073/pnas.111145498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Goryaynov A, Sarma A, Yang W. Self-regulated viscous channel in the nuclear pore complex. Proc Natl Acad Sci USA. 2012;109:7326–7331. doi: 10.1073/pnas.1201724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pederson T. Diffraction at the Brandenburg G(r)ate. Symposium on the optical analysis of biomolecular machines. EMBO rep. 2006;7:1202–1205. doi: 10.1038/sj.embor.7400841. [DOI] [PMC free article] [PubMed] [Google Scholar]