Abstract
Here the first reproductive sterility system for the tephritid fruit fly pest, Anastrepha suspensa, is presented, based on lethality primarily limited to embryos heterozygous for a conditional lethal transgene combination. This tetracycline (Tet)-suppressible system uses a driver construct having the promoter from the newly isolated embryo-specific A. suspensa serendipity α gene linked to the Tet-transactivator. This was used to drive expression of a phosphomutated variant of the pro-apoptotic cell death gene, hid, from A. ludens, that was isolated, based on its identity to A. suspensa hid. The AlhidAla2 variant was shown to have the highest cell death activity in an in vitro A. suspensa cell death assay compared to the orthologous genes Ashid, Dmhid, and the variant DmhidAla5. These cell death assays also allowed a determination of the most-efficient driver-effector cassette combinations for use in A. suspensa transformants, resulting in two hybrid strains exhibiting 100% lethality. One strain was 96% lethal in embryos in the absence of tetracycline, with none surviving past the first larval instar, which is critical for pests that are most damaging in late-larval stages. We demonstrate that the isolation and in vitro validation of species-specific promoters and lethal effector genes can greatly improve the efficiency of creating high-performance conditional lethality strains that may be extended to other insect pest species.
Keywords: apoptosis, insect pest management, sterile insect technique
The Caribbean fruit fly (caribfly), Anastrepha suspensa, and the Mexican fruit fly (mexfly), A. ludens, are established model organisms for the genera Anastrepha, which are economically important insect pests (1–4). An effective biologically based control system for these, and other tephritid pests, is the sterile insect technique (SIT) that relies on the field release en masse of males sterilized by irradiation, resulting in progeny that are zygotic lethal (5, 6). In the process of developing improved methods for SIT programs for the Mediterranean fruit fly (medfly), Ceratitis capitata, two transgenic approaches have been taken based on tetracycline-suppressibilty of lethality in the progeny of released males, using the Tet-off gene expression system (7–9). One system uses self-regulated accumulation of the tetracycline-controlled transactivator (tTA) that is toxic at high concentrations. The limitation of this system, however, is that unlike typical SIT, lethality occurs in late larval or pupal progeny after damage to host plants has been sustained (8). The other system, however, uses embryonic-specific serendipity α (sryα) promoters to drive tTA expression, which then activates expression of the Drosophila hidAla5 pro-apoptotic lethal gene. Significantly, transgenic hybrid strains were created for both Drosophila melanogaster and medfly in which complete embryonic lethality occurred in the progeny of parentals reared on media lacking tetracycline, thereby eliminating pest individuals previous to the damaging larval stages (9, 10).
The ability to conditionally regulate embryonic lethality in insects is important, since many plant pests are most harmful as larvae, including fruit flies and moths, among others, as well as serious animal pests, such as the New World screwworm. It would therefore be highly beneficial to develop similar Tet-off embryonic lethality systems for these insect pests as well, and especially for those species amenable to SIT control and insect transformation. A caveat, however, is that the embryonic lethality systems tested required a species-specific sryα promoter to drive the tTA, and while the phosphomutated Drosophila hidAla5 lethal effector (11) resulted in complete embryonic lethality in both D. melanogaster (10) and in C. capitata (9), the latter study suggested that these systems are most effective using genes for promoters and lethal effectors endogenous to the respective host species. This specificity has the advantage of limiting the lethal system primarily to the host species, which is an important consideration for risk assessment of released transgenic insects. On the other hand, isolating and validating the genes and transgene constructs required can be a lengthy and time-consuming process, as occurred in the previous studies, and would be more so for species difficult to transform and having long reproductive cycles.
With the desire to test the Tet-off embryonic lethality system in another tephritid species and to make the evaluation process for species-specific system components more efficient, we initiated development of a lethality system for Anastrepha species using qPCR and in vitro functional assays. First, homologs to the Drosophila pro-apoptotic cell death genes, hid and reaper (rpr), were previously isolated from A. suspensa (12), and sequence identity to Ashid was used to isolate the hid ortholog from A. ludens (Alhid). To functionally validate the identity of these genes, a cell death assay was developed using embryonic cell lines derived from a homologous (A. suspensa) and heterologous (D. melanogaster) species (12). This assay was used to assess the relative capacity of these genes to induce cell death in A. suspensa cells, in addition to creating and testing a phosphomutated version of Alhid (AlhidAla2). A similar variant of D. melanogaster hid was found to decrease the natural ability of hid down-regulation by phosphorylation (11) and was effective in the lethality systems tested in Drosophila and medfly.
Second, to avoid lengthy in vivo testing of the various embryonic promoter driver and lethal effector components requiring transformant lines for each possible combination, we used cell death assays to test these components to find the optimal combination. Transgenic flies carrying these systems were then evaluated by qPCR, that confirmed in vivo the trend of lethal intensity first observed in in vitro cell death assays. The presented evaluation strategy improves the rapid selection of optimal vectors from a large number of potential candidates and reduces the lengthy and labor-intensive studies required for the generation and functional analysis of transgenic flies. For A. suspensa, this resulted in the development of two effective lethality strains in less than one year, relative to the several years taken to develop effective strains for Drosophila and medfly (both having shorter reproductive cycles). Notably, one of the caribfly strains achieved 100% lethality in the first larval instar, which is essential for larval pests. It is expected that similar protocols will be applicable to many other pest species, allowing the rapid creation of new strains for biologically based population control systems.
Results
Isolation of Early Embryonic and Cell Death Genes.
In a first step, the embryonic cellularization-specifically expressed gene, serendipity α (sryα), was isolated from A. suspensa. The complete ORF and its UTRs were isolated by 3′- and 5′-RACE PCR with gene-specific primers designed on an in-silico isolated putative Assryα fragment from an A. suspensa transcriptome. The gene consists of a 377 bp 5′UTR, a 1,941 bp open reading frame, and a 459 bp 3′UTR. In a BLASTx search against the National Center for Biotechnology Information nr database, the best match was the C. capitata sry α gene, Ccsryα (9). Comparing the medfly and A. suspensa sry α proteins resulted in a pairwise identity of 60.1% with several well-conserved amino acid stretches (Fig. S1). A 2.5 kb upstream maximal promoter/enhancer region of Assryα (including the 5′UTR and the entire 5′ upstream flanking sequence (up to the next identifiable gene) was then isolated by inverse PCR, fused to the tetracycline transactivator (tTA) and subcloned into a piggyBac vector to generate the promoter-driver component of a Tet-off-based lethality system. The upstream promoter showed low conservation when compared to the closest related known sry α upstream region from medfly.
In addition, the hid ortholog from the Mexican fruit fly, A. ludens (Alhid), was isolated by 3′- and 5′-RACE with gene specific primers designed to the recently identified A. suspensa hid gene (Ashid) (12). These two Hid homologs are identical except for a single amino acid substitution (Fig. S2), indicating a very high level of conservation that surpasses the similarity of hid in D. melanogaster and its closest known relative, D. simulans, having eight substitutions between them. Studies in Drosophila, where hid phosphorylation sites were mutated to hidAla3 and hidAla5, resulted in improved cell death activity (11). Therefore, Alhid was analyzed in-silico for possible MAPK phosphorylation sites, according to definitions in Drosophila (11), revealing two potential sites at positions 85 (PASP) and 116 (PRTP) that were subsequently mutated to PAAP and PRAP, respectively, to create the variant AlhidAla2.
Evaluation of AlhidAla2 and the Promoter Region of Assry α in A. suspensa Cell Assays.
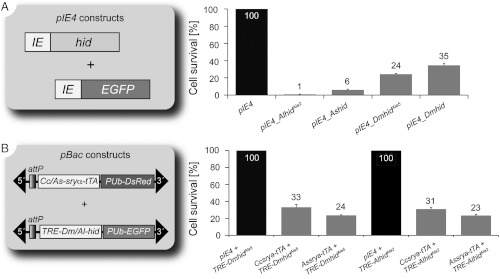
To validate the cell death activity of AlhidAla2, it was first cloned into the overexpression vector pIE-4 and compared to pIE-4 vectors containing Ashid, DmhidAla5, and Dmhid (Fig. 1) (12). All cell death assays were performed in A. suspensa AsE01 embryonic cells, as previously described (12, 13). To determine AlhidAla2 cell death activity relative to its orthologs, pIE-4 vectors expressing the genes and the pIE-EGFP control were co-transfected into AsE01 cells (Fig. 1A). Cell death activity was measured as the percent reduction in the number of surviving green fluorescent cells when pIE-EGFP was co-transfected with a cell death gene vector compared to the control pIE-4. We found that AlhidAla2 has a potent cell death function that was greater than the other hid genes and variant derivatives tested (Fig. 1A). Relative to 100% cell survival in controls, only 1% of AsE01 cells survived when co-transfected with AlhidAla2, which is significantly (P < 0.001) lower than the 6% survival from unaltered Ashid, as supported by one-way ANOVA statistical analysis (Table S1). This is compared to the greater survival after transfection with the Drosophila homologs, DmhidAla5, and Dmhid, yielding survival rates of 24% and 35%, respectively, in the heterologous cell line. The significant differences between the wild type and variant hid gene variants resembles earlier results in Drosophila where the phospho-acceptor mutated DmhidAla5 promoted greater cell death in developing eyes and in insect cell cultures compared to Dmhid (11, 12).
Fig. 1.
Cell death assays using AsE01 cells. (A) Comparison of cell death activity of pIE-4 overexpression vectors carrying hid orthologs or their phosphomutated variants; (B) Cell death activity of a transgene combination of piggyBac-based driver and effector transformation vectors. In (B), TRE-DmhidAla5 and TRE-AlhidAla2 represent the effector constructs TREhs43_DmhidAla5 and TREhs43_AlhidAla2, respectively. Error bars show standard deviations (SD) with the mean percentage of cell survival from three independent experiments shown above.
To determine if piggyBac transformation vectors carrying the driver (D-) and lethal effector (E-) components of the lethality system could be pre-evaluated in cell culture before the more laborious generation of transformants for testing in vivo, AlhidAla2 was subcloned into the piggyBac transformation vector, pBXL_TREhs43_PUbEGFP. The driver constructs D-Ccsryα-tTA (9) and D-Assryα-tTA were then co-transfected into cells with either E-TREhs43-DmhidAla5 (9) or E-TREhs43-AlhidAla2 (Fig. 1B) to identify the most effective driver and effector construct combination. Neither effector construct performed significantly different from the other when co-transfected with either of the driver constructs. However, in combination with either effector construct, D-Ccsryα-tTA reduced cell survival to 31–33% and D-Assryα-tTA to 23–24%. Thus, the endogenous Assryα promoter/enhancer induced cell death at significantly higher levels (P = 0.002–0.008) than the heterologous C. capitata promoter/enhancer in the AsE01 cell line.
Germline Transformation and Quantitative tTA Expression Analysis.
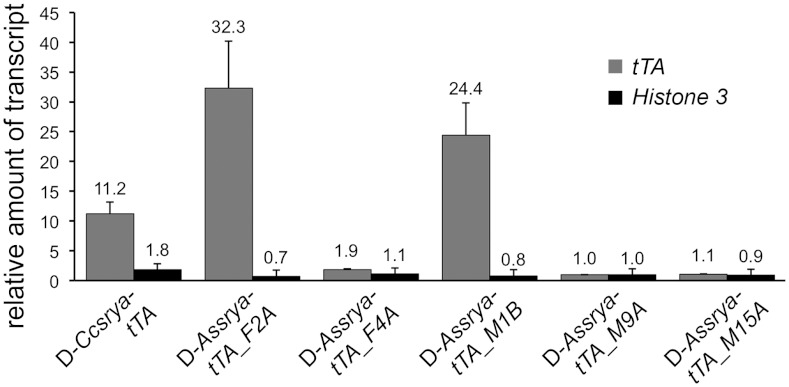
The driver (D-Ccsryα-tTA and D-Assryα-tTA) and effector (E-TREp-DmhidAla5 (10), E-TREhs43-DmhidAla5, and E-TREhs43-AlhidAla2) constructs were then used for germline transformation of A. suspensa. For each construct, a maximum of five independent lines homozygous for the D- or E-transgene were analyzed. First, the expression of tTA from embryos of each driver line was tested by qPCR. Increases of tTA transcript were detected for D-Ccsryα-tTA as well as D-Assryα-tTA, with the highest tTA expression increase (up to 32-fold) occurring with the endogenous Assryα promoter driving the tTA (Fig. 2). Two of the five D-Assryα-tTA lines (D-Assryα-tTA_F2A and D-Assryα-tTA_M1B) showed strong relative tTA expression whereas the remaining three lines showed only a marginal increase over the control Histone 3 gene (Fig. 2). These differences might be due to genomic positional effects as observed previously in medfly (9). The sole D-Ccsryα-tTA line showed a 12-fold tTA increase, significantly lower than the strongest D-Assryα-tTA lines. All driver strains carried a red fluorescent PUbDsRed.T3 marker, which was visibly stronger in adults from the strains D-Assryα-tTA_F2A and D-Assryα-tTA_M1B, compared to the other lines examined under epifluorescence microscopy.
Fig. 2.
Quantitative PCR on cDNA generated from embryos from D-Ccsryα-tTA and D-Assryα-tTA strains. Relative accumulation of tTA transcript (graybars) normalized against AsHis3 transcript using the strain D-Assryα-tTA_M9A as a calibrator is shown. Relative values of AsHis3 (black bars) are also calibrated against D-Assryα-tTA_M9A. Error bars show SD, with the mean fold change from three independent experiments shown above.
Crosses of D-Ccsryα-tTA homozygous flies to eight effector lines (Table S2) yielded only moderately decreased larval hatch rates between 31 and 64%, on Tet-deficient media, compared to the 74% hatch rate of the laboratory-reared A. suspensa wild type (WT) control strain. In contrast, crosses of five homozygous D-Assryα-tTA lines to eight effector lines yielded, in several cases, substantially lower hatch rates. All crosses to the effector line E-TREp-DmhidAla5 resulted in a hatch rate of 20–57%, with the lowest embryonic survival rate in crosses to the strongest tTA-expressing lines D-Assryα-tTA_F2A (32%) and D-Assryα-tTA_M1B (20%) (Table S2). Similarly, in crosses to the effector line E-TREhs43-DmhidAla5, the lines D-Assryα-tTA_F2A and D-Assryα-tTA_M1B performed best and resulted in 0% and 5% survival, respectively (Table 1). Notably, adding tetracycline to the adult diet did not suppress the lethal effect in all combinations. Instead, lethality was irreversible and, after 10 d, females failed to oviposit additional eggs. An attempt to generate flies homozygous for both driver and effector constructs by inbreeding failed, because no double-homozygous flies were observed. One contributing factor was the highly reduced fecundity of all four homozygous E-TREp-DmhidAla5 and E-TREhs43-DmhidAla5 strains (17–24% egg:adult ratio compared to 50–72% of other transgenic strains). Taken together, use of the TRE-linked DmhidAla5 lethal effector constructs appears to be lethal in A. suspensa, which was not observed in medfly (9, 10). Since the effect was observed in several lines, where the DmhidAla5 was driven by TREp or TREhs43, but not when TREhs43 was driving AlhidAla2, an effect of the minimal p or hs43 promoter regions is unlikely.
Table 1.
Survival rate of embryos transheterozygous for a driver (D) and an effector (E) transgene
| E-TREhs43 DmhidAla5_F6A, % | E-TREhs43 DmhidAla5_F7A, % | E-TREhs43 DmhidAla5_F8A, % | E-TREhs43 AlhidAla2_F2A, % | |
| D-Assryα-tTA_F2A | 0* | 11 | 15 | 4 (0 L1 survival) |
| D-Assryα-tTA_M1B | 14 | 5 | 22 | 21 (0 L3 survival) |
*Hatch rates of 300 eggs collected from each cross of a homozygous driver to a homozygous effector line are shown.
Crosses of homozygous D-Assryα-tTA flies to the E-TREhs43-AlhidAla2 effector line resulted in hatch rates of 4–87%, with the wide variation possibly related to the transgene genomic integration sites and the level of tTA-expression in the driver lines. The highest lethality in progeny resulted from crosses of homozygous D-Assryα-tTA_F2A and D-Assryα-tTA_M1B drivers to the homozygous effector line E-TREhs43-AlhidAla2_F2A, yielding 96% embryonic lethality (with 100% L1 lethality) and 79% embryonic lethality (100% L3 lethality), respectively (Table S2). Generation of a strain (D-F2A/E-F2A) carrying the driver D-Assryα-tTA_F2A and the effector E-TREhs43-AlhidAla2_F2A transgenes in homozygous condition resulted in 100% embryonic lethality when reared on non-Tet diet, which is consistent with the lethal effect being dosage dependent. In contrast to the DmhidAla5 effector lines, all lines carrying AlhidAla2 showed normal fecundity when reared on Tet diet (52–58% egg to adult survival).
Tet Concentrations for Rearing.
Feeding Tet-containing diet to adult flies is expected to switch off embryonic lethality due to a maternal transfer of tetracycline to their progeny. In Drosophila, this was sufficient to switch off the lethal effect completely (10), whereas in medfly an additional low amount of tetracycline or doxycycline (as low as 0.1 μg/mL) was required in the larval diet (9). Four different adult diet Tet-concentrations (A3, A10, A100, and A300 μg/mL) were fed to D-F2A/E-F2A and WT flies, with the laid eggs transferred onto Tet100 larval diet (L100). While both A10 and A100 resulted in viable progeny comparable to other laboratory-reared transgenic strains (51–56% egg to adult survival), A3 and A300 resulted in a highly reduced egg to adult ratio for D-F2A/E-F2A. The WT control flies showed a relatively high number of descendants for A3, A10, and A100 diets (65–69%), while the A300 diet resulted in a highly reduced number of pupae with no descendants. Thus, A3 was not sufficient to efficiently switch off the lethal system in D-F2A/E-F2A, while A300 had a negative effect on the number of surviving progeny in general.
In a second experiment, D-F2A/E-F2A and WT flies were reared on A10 and A100 diet with eggs collected on larval diet without tetracycline to determine if the maternal Tet contribution was sufficient to suppress lethality in their progeny. As a control, eggs from both groups were also collected on L100 diet. The eggs from both groups hatched, pupated, and eclosed efficiently (Table S3), comparable to controls on L100 diet. Therefore, a maternal source of tetracycline is able to switch off the lethality system effectively in their larval progeny. To assess the persistence of tetracycline in the D-F2A/E-F2A strain, double-homozygous flies were reared on A100 for five days and then placed on adult diet without Tet. Oviposited eggs were then collected over a one-week period beginning on day 5. The egg-to-adult ratio decreased from 51% to 26% over a period of seven days, whereas in medfly a similar experiment resulted in complete lethality after two days (9). This suggests that tetracycline may have enhanced stability in A. suspensa females, possibly due to species differences in gut bacterial populations that metabolize the antibiotic.
In a third experiment, the double-homozygous D-F2A/E-F2A strain was reared on non-Tet diet for 8 d after eclosion resulting in embryonic lethality. Feeding the adults with A10 or A100 diet on day 8 re-established 48% survival on day 9, indicating that lethality in D-F2A/E-F2A was reversible.
Discussion
A major bottleneck in creating the safest and most efficient transgenic insect systems for biologically based control, in a reasonable and cost-effective time period, has been the lack of rapid validation systems between the initial design of system components and the final test crosses of transformed insects. We demonstrate here that a combination of gene finding, functional analysis in in vitro embryonic cell cultures, and qPCR determination of gene expression of final transgene constructs can result in the rapid development of transgenic insect strains potentially useful for effective population control of pest species.
The presented procedure for the development of an embryonic conditional lethality system for an Anastrepha species involved three steps: First, newly isolated components (i.e., promoter drivers and lethal effectors) were tested in an assay that demonstrated that pIE-4 overexpression constructs, as well as expression of transgene constructs within final transformation vectors, can be measured quantitatively in AsE01 cells. The results of these tests reliably provided insights applicable to identifying the most-promising lethality system components for successful in vivo use in the host species. Importantly, direct quantitative gene expression and functional assays could be made in the absence of genomic position effects that might confuse relative function. For example, cell death assays showed that the newly isolated endogenous promoter from Assryα performed better than the medfly Ccsryα promoter, which was consistent with subsequent qPCR analysis in two of the five transgenic lines. The species-specificity of homologous sryα promoter-driver expression is also beneficial in terms of limiting lethality to A. suspensa and closely related species, thereby reducing potential risk to non-target organisms in the rare event of lateral interspecies transfer.
Second, using only the best-performing components from step 1, transgenic driver and effector transformant lines were established, with the driver lines further assessed by qPCR for tTA transcript levels. Lines with the highest tTA transcript levels not only resulted in the highest lethality (when crossed to effector strains), but also consistently expressed the strongest visible red fluorescence from the PUb-DsRed transgenic marker in adults. This might be due to position effects at the vector integration site having a similar positive influence on expression levels of both tTA and DsRed, and, if confirmed by additional tests, selection for the brightest fluorescent driver lines could be used for initial screening of optimal tTA-expressing lines.
In the last step, crosses between pre-validated driver and effector lines provided the final test for embryonic lethality efficiency, whereupon Tet characteristics for the timing and concentrations required for lethality suppression could be evaluated. Use of the sole Ccsryα-tTA driver line with any of the effector lines failed to induce complete lethality. This could be due to the lack of functional sry α promoter conservation between the tephritid species, similar to the differences between Drosophila and medfly sry α (9), or possibly due to genomic positional effects. In contrast, two crosses between endogenous Assryα driver lines to an AlhidAla2 effector line resulted in 100% first instar or third instar larval lethality, with embryonic lethality of 96% and 79%, respectively.
In summary, the combination of a tTA driver under endogenous sry α promoter control and an effector construct utilizing the AlhidAla2 cell death gene proved to be the most-efficient system for A. suspensa. The pre-evaluation assays not only indicated that use of such lines could be highly effective; they allowed a rapid quantitative determination of the specific lines that might be most effective. Lines exhibiting relatively low tTA expression or induction of cellular lethality could be eliminated early in the testing process, previous to the generation of transformant individuals for testing in vivo. The rapid screening of many candidate genes/promoters also allows the identification of the most active elements that are also the least phylogenetically conserved. This is likely to increase species-specificity for the lethality system, which should enhance the safety of transgenic releases in terms of potential, though rare, instances of lateral interspecies transfer of transgenes into unintended hosts.
We believe that lethality caused by pro-apoptotic cell death genes (e.g., hid, rpr, or grim), or other well-studied biological mechanisms (10),is preferable to systems for which the lethality mechanism is unknown, including those dependent on a toxic accumulation of the Tet-transcriptional activator (8, 14–17). While tTA toxicity may be highly effective, it is not species-specific nor is its precise mode of action known (8). Thus, it is impossible to fully evaluate or anticipate all potential programmatic or environmental risks related to the field-release of this type of transgenic insect (18); in particular, are the various issues related to potential survival of transgenic individuals due to resistance to tTA toxicity, genetic breakdown of the toxic effect or other inefficiencies of the system.
Another advantage of the system described here is that the transgenic insects can be further altered by site-specific modifications within the vector sequence. This is possible through phiC31 attP recombination sites placed within the driver and effector constructs that have been proven functional in fruit flies (19–22) and mosquitoes (23, 24). This technology could be used for stabilizing the transposon vector, by introducing a tandem duplication of one inverted terminal repeat sequence that allows post-integration deletion of the other terminus, thereby immobilizing remaining transgene sequences. This would enhance stability of the strain and its environmental safety before proceeding from laboratory tests to contained field-trial evaluations. The addition of new marking systems, e.g., for sperm identification (25–27), or other improvements would be possible using already evaluated genomic positions instead of fully re-creating and evaluating strains with new, random genomic integration sites of the transgenes.
A critical consideration for mass rearing Tet-off lethality strains is determining the Tet concentration necessary for optimal survival during rearing, and, in particular, the lowest sufficient Tet concentration necessary to switch the system on or off. In medfly, the amount of Tet was found to be highly dependent on the system used for generating lethality. The Release of Insects Carrying a Dominant Lethal (RIDL) system based on toxic accumulation of tTA required 100 μg/mL Tet to suppress lethality in both adults and larvae (8), whereas the sryα-tTA/TRE-DmhidAla5 embryonic lethality system was efficiently suppressed by a 10-fold lower concentration of Tet fed to adults, and a 100-fold lower concentration fed to larvae (9). In A. suspensa, not only did adults survive on 10 μg/mL Tet, but their progeny survived without any Tet supplementation. The finding that tetracycline is unnecessary in larval diet for survival is critical since several tons of larval diet are typically used daily for mass rearing, and the ability to use a Tet-free larval diet greatly improves efficiency, cost savings, and environmental safety. In particular, Tet-free diet can be disposed of safely without pretreatment, or reused as animal feed (28, 29). It will be important to determine if these Tet-characteristics are similar in species closely related to A. suspensa.
The three-step procedure we have described and tested enabled the generation of an early acting and highly efficient lethality system in A. suspensa in a relatively short time frame of less than one year, compared to a development time in medfly of 2.5–3 years (9), despite caribfly having a 25% longer reproductive cycle. In medfly, 60 test crosses were necessary to obtain lethal lines (9), whereas in caribfly (due to the qPCR evaluation) only four crosses led to successful lethal lines. The savings in time and effort afforded by the pre-evaluation strategy can be largely attributed to the elimination of transgenic line development and the reduction of test crosses. Importantly, this procedure should allow similar conditional lethal strain development in other insects controlled with SIT, where cell cultures are available from the same or closely related species, and genetic transformation is possible. These include other tephritids, the closely related mexfly in particular, and several important mosquito and moth species. Insect species with long generation times and low transformation efficiencies would especially benefit from pre-evaluations and promising component selection at an early stage of strain development.
Materials and Methods
Insect rearing and isolation of the genes Assryα and Alhid are described in SI Materials and Methods. The cDNA sequences are deposited in GenBank [accession numbers: JQ599254 (Alhid) and JQ599256 (Assryα)] All oligonucleotides are shown in Table S4.
Inverse PCR.
Inverse PCR was performed to obtain the 5′ upstream region of Assryα. First, 1 μg of A. suspensa WT genomic DNA was digested with the enzyme Sau3AI for 24 h. Restriction fragments were precipitated and self-ligated in a volume of 500 μL at 16 °C for 24 h. A PCR using BD Advantage 2 PCR polymerase (BD Biosciences) was performed on circularized fragments with the primer pair AH530-AH531, oriented in opposite direction within the 5′UTR/ORF of the gene, using the PCR conditions: 1 min at 95 °C; 6 cycles of 30 s at 94 °C; 45 s at 66 °C (-2 °C each cycle); 6 min at 68 °C; 25 cycles of 30 s at 94 °C; 45 s at 54 °C; 6 min at 68 °C; and 6 min at 68 °C. A resulting 2.5 kb PCR product was cloned into the pCR4 vector (Invitrogen) yielding the clone iAs2600_3 that was sequenced. The upstream sequence of Assryα including the 5´UTR is deposited in GenBank (accession number JQ599257).
Shuttle Vectors.
We composed our constructs in the cloning shuttle vector pSLfa1180fa (30). From the shuttle vectors, the constructs can be easily placed in transformation vectors, which carry FseI and AscI sites (fa-sites). pSLaf_Assryα-tTA_af (#1400) was created by recombining the 2.5 kb PCR Assryα fragment, amplified from iAs2600_3 using primer pair AH562-AH563 into XbaI cut pSLaf_tTA_af (#1215) (9), using the In-Fusion PCR cloning Kit (Clontech).
To generate a mutated version of Alhid lacking two potential MAPK phosphorylation sites (AlhidAla2), the clone M100 containing the complete Alhid ORF in a pCR4 vector was used to generate three fragments by PCR (1 min at 95 °C; 30 cycles of 30 s at 94 °C; 30 s at 55 °C; 30 s at 68 °C; and 1 min at 68 °C): A 220 bp fragment using primer pair AH664-AH665, a 105 bp fragment using AH666-AH667, and a 650 bp fragment using AH668-AH669. These fragments were then recombined into the BglII-NotI digested vector pSL_TREhs43-SV40_attP220 (#1403), using the In-Fusion PCR Cloning Kit (Clontech) to create pSL_TREhs43-AlhidAla2-SV40_attP220 (M158). #1403 was created by cloning the HindIII-NruI digested TREhs43 fragment, amplified with primer pair AH627-AH628 on vector TREhs43-hidAla5 (9) in the HindIII-NruI digested vector #1405. #1405 was generated by cloning a SalI-NcoI digested SV40 and an NcoI-EcoRI digested attP220 fragment, amplified with primer pair AH635-AH636 on vector #1215 and AH637-638 on vector pTA-attP (31) into a SalI-EcoRI digested pSLfa1180fa vector (30), respectively. The AlhidAla2 sequence is deposited in GenBank (JQ599255).
Transformation Vectors.
The driver construct pBXLII_attP220_PUbDsRed.T3_tTA-Assryα (Assryα-tTA; #419) was generated by ligating the FseI/AscI digested Assryα-tTA fragment from #1400 in opposite orientation to PUbDsRed.T3 in the FseI/AscI site of pBXLII_attP220_PUbDsRed.T3_fa (#1425). The vector #1425 was created by ligating the hybridized primers AH619-AH620 in the BglII site of pBXLII_attP220_PUbDsRed.T3 (#1421). The vector #1421 was created by cloning the BsaI cut attP220 fragment, amplified with primer pair AH617-AH618 on pTA-attP (31), in XmaI digested pBXLII_PUbDsRed.T3 (32).
The effector construct pBXLII_PUbEGFP_attP220_SV40-AlhidAla2-hs43-TRE (TREhs43-AlhidAla2; #1430) was generated by ligating the FseI/AscI digested TREhs43-AlhidAla2-SV40_attP220 fragment from M158 in the FseI/AscI site of pBXLII_PUbEGFP_fa (#1419). The vector #1419 was created by ligating the hybridized primers AH619-AH620 in the BglII site of pBXLII_PUbEGFP.T3 (25).
Vectors pBac{f_attP-sryα2-tTA_a_PUbDsRed} (Ccsrya-tTA), pBac {fa_attP_f_TREp-hidAla5_a_PUbEGFP} (TREp-DmhidAla5), and pBac {fa_attP_f_TREhs43-hidAla5_a_PUbEGFP} (TREhs43-DmhidAla5) are as described (9).
Cell Culture Assays.
The overexpression vector pIE4-AlhidAla2 was created by ligating the NotI-BglII cut AlhidAla2 fragment from M158 into the pIE4 vector (Novagen). Cell death assays were then performed in the A. suspensa cell line UFENY-AsE01 as described (12), with the following modifications: For each well in a 24-well plate, a total of 2 μg (1 μg plasmid A and 1 μg plasmid B) was mixed with 4 μL lipofectin (Invitrogen) in serum-free media and added to 4.5 × 105 AsE01 cells. The vectors pIE4-Ashid, pIE4-Dmhid, and pIE4-DmhidAla5 are as described (12).
Germline Transformation.
Germline transformation experiments were performed by microinjection of piggyBac constructs (500 ng/μL) together with the phspBac transposase helper plasmid (200 ng/μL) into WT embryos (33) as described (34). G1 offspring were selected by DsRed and EGFP epifluorescence using a Leica MZ FLIII microscope with a Texas Red (ex: 560/40; em: 610 LP) and a GFP2 filter set (ex: 480/40; em: 510 LP). Independent homozygous strains were established by single pair inbreeding for successive generations with testing by segregation analysis of transformants outcrossed to wild type flies.
To generate conditional lethality lines, six homozygous driver lines were crossed to eight homozygous effector lines in almost all possible combinations on Tet-containing larval and adult diet. Heterozygous combinations were inbred with progeny visually screened by fluorescent intensity for homozygous individuals that were subsequently inbred to generate lethality lines homozygous for the driver and effector transgene constructs (all crosses performed on Tet-containing larval and adult diet). For screening of double homozygous lines, the Texas Red and YFP filter set (ex: 500/20; em: 535/30) were used.
Quantitative Real-Time PCR.
Total RNA was isolated from all embryonic samples (0–24 h collection, including the cellularization stage in A. suspensa), using TRIreagent (Molecular Research Center). The iScriptTM cDNA synthesis kit (BioRad) and 1 μg RNA were used for cDNA synthesis. Quantitative real-time PCR (qPCR) was performed on approximately 100 ng cDNA using the iQ SYBR Green Supermix (BioRad) in a Chromo4™ real-time PCR detector (BioRad). PCR cycling conditions were: 95 °C for 5 min; 45 cycles of 95 °C for 15 s; 60 °C for 10 s; and 72 °C for 10 s with a plate read at the end of each cycle. All reactions were performed on three biological replicates. Gene specific primers for tTA (AH621/AH622) and A. suspensa Histone 3 (AsHis3) (AH439F/AH440R) were designed using Geneious software (Biomatters). Amplified products from randomly selected samples were analyzed on a 2% agarose gel, subsequently cloned into pCR4-TOPO vector, and sequenced to confirm specificity of the tTA and Histone 3 amplification.
Relative accumulation of tTA normalized against AsHis3 was calculated from the formula 2-ΔΔCt (35), where 2 is the reaction efficiency and ΔΔCt is the difference in AsHis3 Ct values between the calibrator (lowest tTA expressing line D-Assryα-tTA_M9A) and the other samples, subtracted from the difference in tTA Ct values between the calibrator (lowest tTA expressing line D-Assryα-tTA_M9A) and the other samples.
Tetracycline Tests.
Backcrosses of transgenic males or females to WT females or males were first performed to verify a transgenic/WT progeny ratio of 1∶1, and the same fluorescent marker tissue specificity consistent with single vector integrations. Homozygous transgenic lines were then generated by inbreeding and selecting for the strongest red fluorescent flies (for driver lines) or green fluorescent flies (for effector lines). To generate an optimal lethality system, homozygous driver lines were crossed to homozygous effector lines, with 300 eggs per cross collected and hatch rate recorded (Table S2). Two crosses (D-Assryα-tTA_F2A x E-TREhs43-AlhidAla2_F2A and D-Assryα-tTA_M1B x E-TREhs43-AlhidAla2_F2A) having a reduced hatch rate, larval and pupal data were recorded in addition. To determine dosage effects for the lethality system, a double homozygous strain of D-Assryα-tTA_F2A x E-TREhs43-AlhidAla2_F2A was generated by inbreeding on A100/L100 diet and selecting for double homozygous red/green fluorescent flies. These flies were then reared on non-Tet diet with the hatch rate of 300 eggs was recorded.
Starting from larval and adult diet Tet-concentrations of 100 μg/mL, the minimal Tet concentrations for the line D-F2A/E-F2A was tested with WT as a control. First, flies were reared on four different adult diet Tet concentrations (A3, A10, A100, A300 μg/mL), eggs were collected on larval medium containing 100 μg/mL (L100) and adult eclosion rates were recorded. Second, freshly eclosed flies from the line D-F2A/E-F2A were placed on adult diet containing 10 or 100 μg/mL Tet. Eight days post-eclosion eggs were collected and reared on Tet-free larval medium. Eclosion rates were recorded and compared with data from WT A. suspensa flies reared on Tet-free adult and larval diet.
Statistical Analysis.
The program SigmaPlot 11 (Systat Software, Inc.) was used to perform one-way ANOVA on the different samples. Within these tests, a normality test was done after Shapiro-Wilk and all pairwise multiple comparison procedures after Holm-Sidak (Table S1).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Shelley Olson (funded by United States Department of Agriculture (USDA)–Animal and Plant Health Inspection Service–Plant Protection and Quarantine) for excellent technical assistance, Seth Britch for assistance with statistical analysis, and Drs. Nirmala Xavier and Irina Häcker for comments on the manuscript. The project benefitted from discussions at International Atomic Energy Agency funded meetings for the Coordinated Research Project: The Use of Molecular Tools to Improve the Effectiveness of SIT. Funding is gratefully acknowledged from the USDA–National Institute of Food and Agriculture–Agriculture and Food Research Initiative.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JQ599254–JQ599257).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203352109/-/DCSupplemental.
References
- 1.Hendrichs J, Robinson AS, Cayol JP, Enkerlin W. Medfly areawide sterile insect technique programmes for prevention, suppression or eradication: the importance of mating behavior studies. Fla Entomol. 2002;85:1–13. [Google Scholar]
- 2.Handler AM, Harrell RA. Transformation of the Caribbean fruit fly, Anastrepha suspensa, with a piggyBac vector marked with polyubiquitin-regulated GFP. Insect Biochem Mol Biol. 2001;31:199–205. doi: 10.1016/s0965-1748(00)00119-3. [DOI] [PubMed] [Google Scholar]
- 3.Condon KC, et al. Germ-line transformation of the Mexican fruit fly. Insect Mol Biol. 2007;16:573–580. doi: 10.1111/j.1365-2583.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Martinez V, et al. Mitotic and polytene chromosome analysis in the Mexican fruit fly, Anastrepha ludens (Loew) (Diptera: Tephritidae) Genome. 2009;52:20–30. doi: 10.1139/G08-099. [DOI] [PubMed] [Google Scholar]
- 5.Knipling EF. Possibilities of insect control or eradication through the use of sexually sterile males. J Econ Entomol. 1955;48:459–462. [Google Scholar]
- 6.Dyck VA, Hendrichs J, Robinson AS. Sterile Insect Technique—Principles and Practice in Area-Wide Integrated Pest Management. Dordrecht, NL: Springer; 2005. [Google Scholar]
- 7.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong P, et al. A dominant lethal genetic system for autocidal control of the Mediterranean fruitfly. Nat Biotechnol. 2005;23:453–456. doi: 10.1038/nbt1071. [DOI] [PubMed] [Google Scholar]
- 9.Schetelig MF, Caceres C, Zacharopoulou A, Franz G, Wimmer EA. Conditional embryonic lethality to improve the sterile insect technique in Ceratitis capitata (Diptera: Tephritidae) BMC Biol. 2009;7:4. doi: 10.1186/1741-7007-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn C, Wimmer EA. A transgene-based, embryo-specific lethality system for insect pest management. Nat Biotechnol. 2003;21:64–70. doi: 10.1038/nbt769. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 12.Schetelig MF, Nirmala X, Handler AM. Pro-apoptotic cell death genes, hid and reaper, from the tephritid pest species, Anastrepha suspensa. Apoptosis. 2011;16:759–768. doi: 10.1007/s10495-011-0610-4. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Lawrence PO. An embryonic cell line from the Caribbean fruit fly, Anastrepha suspensa (Diptera: Tephritidae) In Vitro Cell Dev Biol Anim. 1999;35:12–14. doi: 10.1007/s11626-999-0036-2. [DOI] [PubMed] [Google Scholar]
- 14.Phuc HK, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris AF, et al. Field performance of engineered male mosquitoes. Nat Biotechnol. 2011;29:1034–1037. doi: 10.1038/nbt.2019. [DOI] [PubMed] [Google Scholar]
- 16.Fu G, et al. Female-specific insect lethality engineered using alternative splicing. Nat Biotechnol. 2007;25:353–357. doi: 10.1038/nbt1283. [DOI] [PubMed] [Google Scholar]
- 17.Subbaraman N. Science snipes at oxitec transgenic-mosquito trial. Nat Biotechnol. 2011;29:9–11. doi: 10.1038/nbt0111-9a. [DOI] [PubMed] [Google Scholar]
- 18.Reeves RG, Denton JA, Santucci F, Bryk J, Reed FA. Scientific standards and the regulation of genetically modified insects. PLoS Negl Trop Dis. 2012;6:e1502. doi: 10.1371/journal.pntd.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schetelig MF, et al. Site-specific recombination for the modification of transgenic strains of the Mediterranean fruit fly Ceratitis capitata. Proc Natl Acad Sci USA. 2009;106:18171–18176. doi: 10.1073/pnas.0907264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venken KJ, Bellen HJ. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet. 2005;6:167–178. doi: 10.1038/nrg1553. [DOI] [PubMed] [Google Scholar]
- 21.Schetelig MF, Götschel F, Viktorinova I, Handler AM, Wimmer EA. Recombination technologies for enhanced transgene stability in bioengineered insects. Genetica. 2011;139:71–78. doi: 10.1007/s10709-010-9494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handler AM, Zimowska GJ, Horn C. Post-integration stabilization of a transposon vector by terminal sequence deletion in Drosophila melanogaster. Nat Biotechnol. 2004;22:1150–1154. doi: 10.1038/nbt1002. [DOI] [PubMed] [Google Scholar]
- 23.Nimmo DD, Alphey L, Meredith JM, Eggleston P. High-efficiency site-specific genetic engineering of the mosquito genome. Insect Mol Biol. 2006;15:129–136. doi: 10.1111/j.1365-2583.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labbe GM, Nimmo DD, Alphey L. piggybac-and PhiC31-mediated genetic transformation of the Asian tiger mosquito, Aedes albopictus (Skuse) PLoS Negl Trop Dis. 2010;4:e788. doi: 10.1371/journal.pntd.0000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimowska GJ, Nirmala X, Handler AM. The beta2-tubulin gene from three tephritid fruit fly species and use of its promoter for sperm marking. Insect Biochem Mol Biol. 2009;39:508–515. doi: 10.1016/j.ibmb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Scolari F, et al. Fluorescent sperm marking to improve the fight against the pest insect Ceratitis capitata (Wiedemann; Diptera: Tephritidae) N Biotechnol. 2008;25:76–84. doi: 10.1016/j.nbt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Catteruccia F, Benton JP, Crisanti A. An Anopheles transgenic sexing strain for vector control. Nat Biotechnol. 2005;23:1414–1417. doi: 10.1038/nbt1152. [DOI] [PubMed] [Google Scholar]
- 28.Caceres C. Mass rearing of temperature sensitive genetic sexing strains in the Mediterranean fruit fly (Ceratitis capitata) Genetica. 2002;116:107–116. doi: 10.1023/a:1020967810703. [DOI] [PubMed] [Google Scholar]
- 29.Mastrangelo T, Silva J, Abdalla A, Peçanha M, Walder J. Potential use of larval diet disposal from medfly mass-rearing as alternative livestock feed. Livest Res Rural Dev. 2010;22 [Google Scholar]
- 30.Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Dev Genes Evol. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- 31.Groth AC, Olivares EC, Thyagarajan B, Calos MP. A phage integrase directs efficient site-specific integration in human cells. Proc Natl Acad Sci USA. 2000;97:5995–6000. doi: 10.1073/pnas.090527097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nirmala X, Zimowska GJ, Handler AM. Characterization of the proteasome β2 subunit gene and its mutant allele in the tephritid fruit fly pest, Anastrepha suspensa. Insect Mol Biol. 2009;18:333–340. doi: 10.1111/j.1365-2583.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- 33.Handler AM, Harrell RA. Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol Biol. 1999;8:449–457. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- 34.Handler AM, James AA. Insect Transgenesis: Methods and Applications. Boca Raton, FL: CRC Press LLC; 2000. pp. 3–26. [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.