Abstract
Apolipoprotein A-IV (apoA-IV) is secreted by the small intestine in response to fat absorption. Here we demonstrate a potential role for apoA-IV in regulating glucose homeostasis. ApoA-IV–treated isolated pancreatic islets had enhanced insulin secretion under conditions of high glucose but not of low glucose, suggesting a direct effect of apoA-IV to enhance glucose-stimulated insulin release. This enhancement involves cAMP at a level distal to Ca2+ influx into the β cells. Knockout of apoA-IV results in compromised insulin secretion and impaired glucose tolerance compared with WT mice. Challenging apoA-IV−/− mice with a high-fat diet led to fasting hyperglycemia and more severe glucose intolerance associated with defective insulin secretion than occurred in WT mice. Administration of exogenous apoA-IV to apoA-IV−/− mice improved glucose tolerance by enhancing insulin secretion in mice fed either chow or a high-fat diet. Finally, we demonstrate that exogenous apoA-IV injection decreases blood glucose levels and stimulates a transient increase in insulin secretion in KKAy diabetic mice. These results suggest that apoA-IV may provide a therapeutic target for the regulation of glucose-stimulated insulin secretion and treatment of diabetes.
Keywords: gastrointestinal physiology, gut–pancreas communication
Apolipoprotein A-IV (apoA-IV) is a component of intestinally derived, triglyceride-rich lipoproteins (1). It is synthesized predominantly by enterocytes of the small intestine and is secreted into the lymph with chylomicrons. During lipolysis of chylomicrons, a significant proportion of apoA-IV dissociates from the chylomicron remnants and circulates in association with HDL or as free apoA-IV in the plasma (2, 3). Numerous functions have been ascribed to apoA-IV, including roles in lipid absorption, reverse cholesterol transport (4), anti-inflammatory response (5), and suppression of food intake (6). Many of these functions are not unique to apoA-IV and are shared with other apolipoproteins (7, 8); however, as the only apolipoprotein whose expression is regulated by fat intake, apoA-IV may well have a unique role in lipid handling or obesity, although its exact biological role has remained elusive. A recent study reported significant increases in plasma apoA-IV in humans after gastric bypass surgery (9), which was coincident with the amelioration of diabetes. Consistent with this finding, population studies have identified a human polymorphism in apoA-IV, apoA-IV360, associated with elevated fasting glucose (10, 11). These results suggest a previously unexplored role for apoA-IV as a modulator of glucose homeostasis and comorbidities related to type 2 diabetes. To explore these possibilities, we used apoA-IV−/− mice and recombinant apoA-IV protein to unravel the effects of apoA-IV on glucose homeostasis.
Results
Effects of Exogenous ApoA-IV Protein on Insulin Secretion from Isolated Islets.
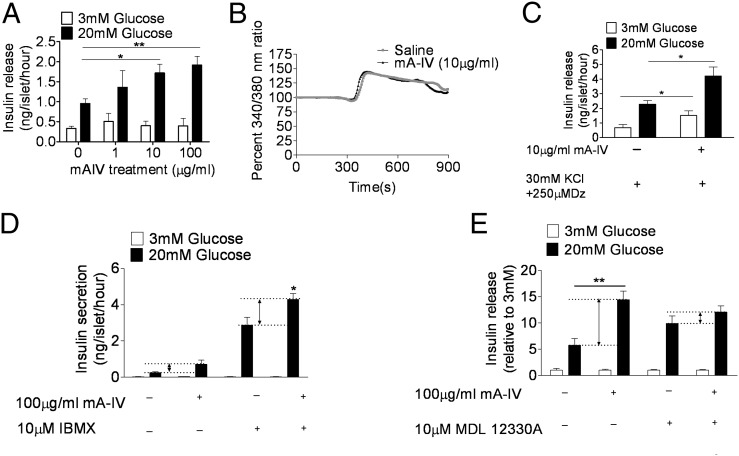
To determine the effect of apoA-IV on pancreatic β cells, we treated isolated mouse islets with recombinant mouse apoA-IV (mA-IV) in the presence of either 3.0 mM or 20 mM glucose. mA-IV augmented insulin secretion in response to the higher level of glucose, but had no effect in the low-glucose condition (Fig. 1A). The mA-IV–dependent enhancement of glucose-induced insulin secretion was completely suppressed by diazoxide, a KATP channel opener, and also by nifedipine, a Ca2+ channel blocker (Fig. S1).
Fig. 1.
Exogenous mA-IV promotes insulin secretion from isolated islets. (A) Islets were incubated with indicated concentrations of mA-IV. Insulin secretion was stimulated with either 3 mM or 20 mM glucose. n = 4. *P < 0.05; **P < 0.01. (B) Ca2+ responses induced by a single-step increase of glucose from 3 to 20 mM in the absence (n = 9) or presence (n = 10) of 10 μg/mL of mA-IV. (C) The effect of 10 μg/mL of mA-IV on insulin secretion in maximally depolarized islets treated by 30 mM KCl plus 250 μM diazoxide (Dz). n = 6. *P < 0.05. (D) Effect of mA-IV on insulin secretion in the presence of 10 μM IBMX. n = 4. *P < 0.05. (E) Effect of mA-IV on insulin secretion in the presence of 10 μM MDL 12330A. n = 4. **P < 0.01.
We next asked whether increased cytoplasmic calcium is responsible for the enhanced insulin secretion. A typical cytoplasmic calcium response to glucose in pancreatic islets involves a short silent period coincident with an initial lowering of [Ca2+]i, followed by a sharp rise and then a subsequent decrease. None of these parameters was altered significantly by mA-IV (Fig. 1B). Thus, the data suggest that apoA-IV affects insulin exocytosis at a site downstream of Ca2+ influx. This is supported by the observation that mA-IV further augmented insulin secretion in islets that were maximally depolarized by 30 mM KCl plus 250 μM diazoxide (Fig. 1C).
Because cAMP regulates a late step of Ca2+-dependent exocytosis, we next asked whether cAMP is involved in apoA-IV–related insulin secretion. In the presence of IBMX, a phosphodiesterase inhibitor that raises intracellular cAMP, the effect of mA-IV on insulin secretion was exaggerated (Fig. 1D). In contrast, in the presence of MDL 12330A, an adenylyl cyclase inhibitor that inhibits generation of cAMP, the effect of mA-IV on insulin secretion was largely diminished (Fig. 1E).
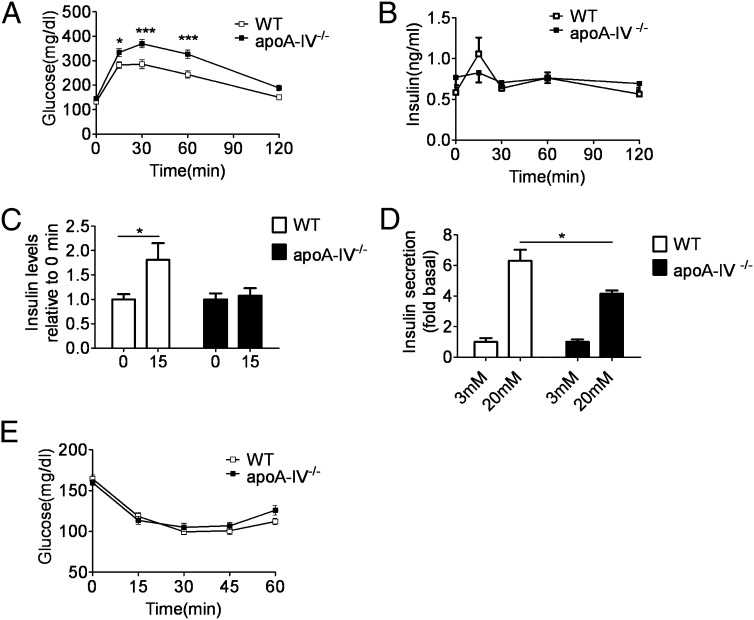
ApoA-IV−/− Mice Have Reduced Insulin Secretion and Impaired Glucose Tolerance.
The absence of apoA-IV did not alter total body weight or body composition in mice fed a chow diet relative to WT controls (Table 1). Likewise, glucose and insulin levels were not different in fed and overnight-fasted apoA-IV−/− and WT mice (Table 1). During an i.p. glucose tolerance test (IPGTT), apoA-IV−/− mice exhibited delayed glucose clearance, characterized by significantly higher glucose levels than WT mice at 15, 30, and 60 min after glucose injection (Fig. 2A). WT mice had significantly increased insulin levels at 15 min after the glucose challenge, which was completely absent in apoA-IV−/− mice (Fig. 2 B and C). Consistent with this finding, isolated islets from apoA-IV−/− mice exhibited a smaller fold increase in insulin secretion from 3 mM to 20 mM glucose compared with WT islets (Fig. 2D). An insulin tolerance test indicated that the glucose-lowering effect of insulin was similar in the apoA-IV−/− and WT mice (Fig. 2E).
Table 1.
Body weight, body composition, and metabolic parameters in chow-fed mice
| Characteristic | WT | apoA-IV−/− |
| Body weight, g | 26.8 ± 0.4 | 27.3 ± 0.4 |
| Body fat, % | 3.6 ± 0.3 | 4.0 ± 0.5 |
| Body lean, % | 88.7 ± 0.7 | 89.1 ± 0.6 |
| Fasting glucose, mg/dL | 94.5 ± 6.2 | 103.6 ± 11.0 |
| Fed glucose, mg/dL | 147.1 ± 7.5 | 145.1 ± 11.2 |
| Fasting insulin, ng/mL | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Fed insulin, ng/mL | 0.7 ± 0.1 | 1.0 ± 0.2 |
| Fasting GLP-1, pM | 6.4 ± 0.3 | 5.9 ± 0.2 |
| Fed GLP-1, pM | 6.3 ± 0.2 | 6.7 ± 0.2 |
| Fasting GIP, pg/mL | 50.3 ± 8.0 | 53 ± 5.2 |
| Fed GIP, pg/mL | 59.6 ± 5.7 | 83.5 ± 8.1* |
Results are expressed as mean ± SEM from at least seven mice per group.
*P < 0.05.
Fig. 2.
Impaired glucose tolerance and insulin secretion, but normal insulin sensitivity, in apoA-IV−/− mice. (A) IPGTT (2 g/kg body weight) in WT and apoA-IV−/− mice after a 5-h fast. n = 17. *P < 0.05; ***P < 0.001. (B) Plasma insulin levels in WT and apoA-IV−/− mice in response to an IPGTT at indicated times. n = 15. (C) Insulin response at 15 min relative to 0 min in WT and apoA-IV−/− mice. n = 15. *P < 0.05. (D) Insulin response in isolated islets from WT and apoA-IV−/− mice. n = 5. *P < 0.05. (E) Blood glucose levels in 5-h fasted WT and apoA-IV−/− mice at indicated times after injection of 0.75 U/kg of insulin. n = 10.
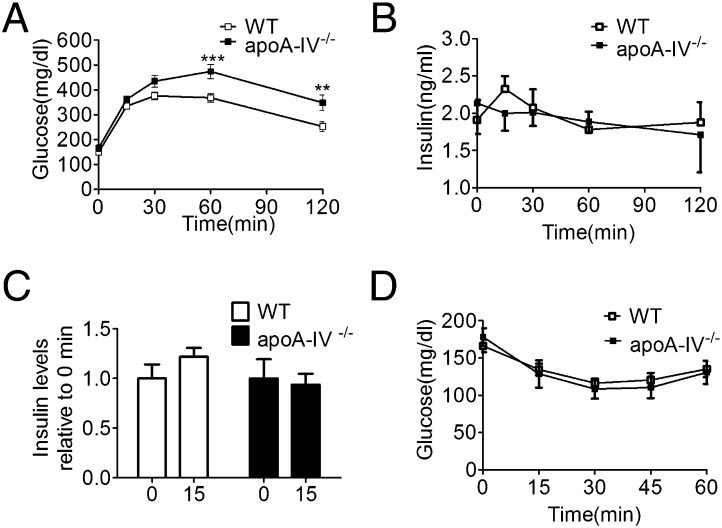
Response to a High-Fat Diet.
After a 10-wk high-fat diet (HFD), weight gain was comparable in the WT and apoA-IV−/− mice, and neither food intake nor body composition differed between the genotypes (Table 2). However, blood glucose was significantly elevated in the apoA-IV−/− mice relative to the WT mice after an overnight fast (Table 2). Chronic consumption of the HFD was associated with the development of impaired glucose tolerance in both WT and apoA-IV−/− mice during the IPGTT. However, apoA-IV−/− mice developed more significant hyperglycemia that persisted for up to 120 min after glucose administration (Fig. 3A). Basal insulin levels were increased in both WT and apoA-IV−/− mice compared with chow-fed controls (Figs. 2B and 3B). Insulin levels in response to a glucose challenge tended toward an increase in WT mice but not in apoA-IV−/− mice (Fig. 3C). After an insulin injection, the WT and apoA-IV−/− mice had a comparable pattern of decreasing plasma glucose levels (Fig. 3D), suggesting that the absence of apoA-IV does not affect the acute action of insulin in these HFD-induced obese mice.
Table 2.
Body weight, body composition, and metabolic parameters after a 10-wk HFD
| Characteristic | WT (n = 10) | ApoA-IV−/− (n = 7) |
| Body weight, g | 34.2 ± 1.2 | 33.3 ± 1.0 |
| Food intake, g/day | 3.2 ± 0.1 | 3.0 ± 0.1 |
| Body fat, % | 16.5 ± 2.0 | 17.7 ± 2.0 |
| Body lean, % | 78.1 ± 1.9 | 77.5 ± 2.0 |
| Fasting glucose, mg/dL | 104.6 ± 5.1 | 125.3 ± 5.6* |
| Fed glucose, mg/dL | 154.7 ± 7.1 | 162.5 ± 5.4 |
| Fasting insulin, ng/mL | 0.39 ± 0.06 | 0.4 ± 0.06 |
| Fed insulin, ng/mL | 1.9 ± 0.4 | 1.5 ± 0.2 |
Results are expressed as mean ± SEM.
*P < 0.05.
Fig. 3.
Glucose tolerance, insulin secretion, and insulin sensitivity after a 10-wk HFD in WT and apoA-IV−/− mice. (A) Blood glucose levels after i.p. glucose (2 g/kg body weight) in WT mice (n = 10) and apoA-IV−/− mice (n = 7) after a 5-h fast. **P < 0.01; ***P < 0.001. (B) Plasma insulin levels in WT mice (n = 10) and apoA-IV−/− mice (n = 7) in response to an IPGTT at the indicated times. (C) Insulin response at 15 min relative to 0 min in WT mice (n = 10) and apoA-IV−/− mice (n = 7). (D) Blood glucose in 5-h fasted WT (n = 10) and apoA-IV−/− mice (n = 7) at the indicated times after injection of 0.75 U/kg of insulin.
Exogenous ApoA-IV Improves Glucose Tolerance by Enhancing Insulin Secretion.
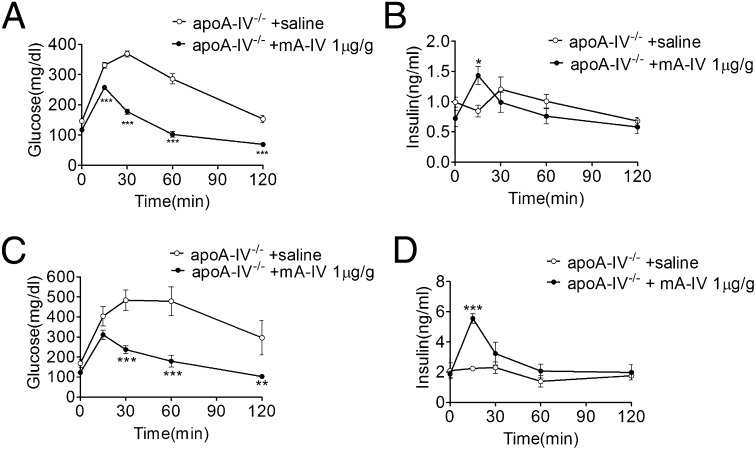
We found that apoA-IV mRNA is not expressed in pancreatic islets (Fig. S2), implying that the effect of apoA-IV on insulin secretion is likely related to circulating apoA-IV. After i.p. administration of mA-IV to apoA-IV−/− mice, mA-IV reached its highest level in plasma at 2 h and disappeared completely by 24 h (Fig. S3). Thus, we performed an IPGTT in apoA-IV−/− mice at 2 h after mA-IV administration, and found a significantly and dose-dependent decrease in blood glucose levels in these mice (Fig. S4). The improved glucose tolerance was associated with the restoration of insulin secretion at 15 min after glucose administration (Fig. 4 A and B). Treating apoA-IV−/− mice with mA-IV restored insulin secretion and improved plasma glucose levels despite the HFD-induced glucose intolerance (Fig. 4 C and D).
Fig. 4.
mA-IV administration stimulates insulin release and improves glucose tolerance in apoA-IV−/− mice. (A) IPGTT in 5-h fasted apoA-IV−/− mice on a chow diet. mA-IV or saline was injected i.p. 2 h before the IPGTT. n = 4. ***P < 0.001. (B) Plasma insulin levels in apoA-IV−/− mice fed chow in response to an IPGTT at the indicated times. mA-IV or saline was injected i.p. 2 h before the IPGTT. n = 7. *P < 0.05. (C) IPGTT in 5-h fasted apoA-IV−/− mice after a 10-wk HFD. mA-IV (n = 4) or saline (n = 3) was injected i.p. 2 h before the IPGTT. **P < 0.01;***P < 0.001. (D) Plasma insulin levels in apoA-IV−/− mice after a 10-wk HFD in response to an IPGTT at the indicated times. mA-IV (n = 4) or saline (n = 3) was injected i.p. 2 h before the IPGTT. *P < 0.001.
Effects of Exogenous ApoA-IV Protein on Blood Glucose and Insulin Levels in KKAy Mice.
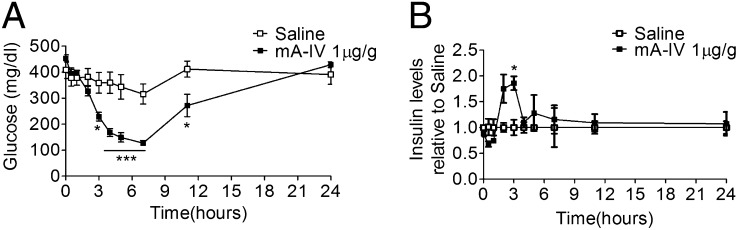
We next asked whether the beneficial effects of exogenous apoA-IV extend to other models of diabetes. Diabetic KKAy mice are characterized by obesity, insulin resistance, and hyperglycemia (12). Blood glucose was significantly decreased by 1 μg/g of mA-IV given i.p.; that is, the maximum decrease in plasma glucose reached about 60% compared with the saline-treated group, and the glucose-lowering effect lasted up to 11 h (Fig. 5A). At the same time, we observed a transient increase of insulin secretion at 2–4 h after mA-IV injection, the period of the most rapid decrease in glucose levels (Fig. 5B).
Fig. 5.
Effect of a single injection of apoA-IV on blood glucose and plasma insulin levels in KKAy mice at age 14 wk. (A) Blood glucose levels at the indicated times after injection of either saline or 1 μg/g of mA-IV. n = 7. *P < 0.05; ***P < 0.001. (B) Plasma insulin levels at indicated times after injection of either saline or 1 μg/g of mA-IV. n = 3. *P < 0.05.
Discussion
These experiments tested the hypothesis that apoA-IV is important to the control of glucose homeostasis. We initially found that apoA-IV augments glucose-induced insulin secretion from isolated pancreatic islets. Because apoA-IV is not synthesized in islets, this implies that apoA-IV derived from the circulation acts directly on islet cells to enhance insulin in response to glucose. We then determined that mice deficient in apoA-IV have impaired glucose tolerance secondary to reduced insulin secretion, and have a tendency to develop diabetes when chronically fed an HFD. Exogenous apoA-IV restored the insulin secretory response to glucose and improved glucose tolerance in apoA-IV−/− mice maintained on either chow or an HFD. Administration of exogenous apoA-IV also significantly reduced glucose levels in KKAy diabetic mice. These findings directly implicate apoA-IV in normal glucose homeostasis.
ApoA-IV stimulated insulin secretion from isolated islets in the presence of 20 mM glucose, but not 3 mM glucose (Fig. 1). This finding suggests that the presence of a stimulatory level of glucose is necessary for the augmenting action of apoA-IV on insulin secretion. An initial step in glucose-induced insulin secretion is the uptake of glucose via the glucose transporter GLUT2, followed by glucose metabolism within the β cell. The subsequent rise in the cellular ATP:ADP ratio causes ATP-sensitive K+ channels to close, leading to depolarization of the plasma membrane and Ca2+ influx, and this process then triggers the exocytosis of insulin-containing granules (13). We found that glucose-induced Ca2+ influx is required for the action of apoA-IV, because apoA-IV had no effect on insulin secretion when ATP-sensitive K+ channels were kept open by diazoxide or when Ca2+ channels were blocked by nifedipine (Fig. S1). Importantly, apoA-IV did not generate a further rise in Ca2+ influx, suggesting that steps distal to Ca2+ influx are involved in mediating the action of apoA-IV (14). In support of this finding, insulin secretion was markedly enhanced by apoA-IV when islets were maximally depolarized using 30 mmol/L KCl plus 250 μmol/L diazoxide (15). cAMP is known to regulate the late step of Ca2+-dependent exocytosis of many neurotransmitters and hormones, including insulin (16). Increasing cAMP by IBMX exaggerated the ability of apoA-IV to augment insulin secretion, whereas inhibiting the generation of cAMP by MDL 12330A diminished the ability of apoA-IV to augment insulin secretion. These findings strongly imply that the cAMP pathway is involved in the mechanism by which apoA-IV potentiates insulin secretion. This is consistent with a report that cAMP mediates other actions of apoA-IV; that is, cAMP-pretreated J774 macrophages exhibited enhanced cholesterol efflux in response to mouse serum obtained from apoA-IV transgenic mice relative to serum from WT mice (17). How apoA-IV interacts with cAMP to affect insulin secretion remains to be determined.
The present study identifies a key effect of apoA-IV on normal glucose tolerance. Specifically, apoA-IV−/− mice exhibited impaired glucose tolerance and a blunted insulin response to glucose challenge compared with WT mice. These differences in glucose tolerance cannot be attributed to altered insulin action, given the similar insulin sensitivity exhibited by WT and apoA-IV−/− mice (Figs. 2E and 3D). These data suggest that insufficient insulin secretion is the key deficit contributing to impaired glucose tolerance in apoA-IV−/− mice. This is also consistent with the idea that the presence of apoA-IV is required to ensure normal glucose-induced insulin secretion. Glucotoxicity is a well-established important cause of reduced insulin secretion (18); however, glucotoxicity cannot explain the impaired insulin secretion seen in apoA-IV−/− mice on a chow diet, given that neither fed nor fasted glucose levels were substantially or significantly elevated (Table 1). After 10 wk of an HFD, although fed glucose levels were still comparable in apoA-IV−/− mice and WT mice, the apoA-IV−/− mice had higher fasting glucose levels (Table 2). Even small increases in fasting glucose levels (115 mg/dL) have been associated with marked reductions in glucose-stimulated insulin secretion (19); thus, under high-fat conditions, glucotoxicity also could contribute to the diminished insulin secretion capacity as well as the deficiency of apoA-IV.
An interesting corollary finding is the significantly higher plasma glucose-dependent insulinotropic peptide (GIP) levels in the fed condition in apoA-IV−/− mice compared with WT mice, which might explain the relatively normal glucose levels in apoA-IV−/− mice (Table 1). It is possible that the increased GIP compensates for the impact of apoA-IV deficiency on insulin secretion. GIP is secreted by K cells in the duodenum (20), a region of high apoA-IV expression (21). Whether a deficiency of apoA-IV directly affects GIP secretion from the duodenum merits further investigation.
In rodents, apoA-IV is expressed predominantly in the small intestine and to a much lesser extent in the liver and hypothalamus (22). We previously reported that apoA-IV does not cross the blood–brain barrier (23), suggesting that apoA-IV’s actions on glucose tolerance occur mainly in the periphery. The lack of apoA-IV mRNA in pancreatic islets (Fig. S2), consistent with a previous report (24), suggests that the effect of apoA-IV on islets likely arises from circulating apoA-IV. To test this hypothesis, we treated apoA-IV −/− mice with i.p. recombinant mouse apoA-IV. Insulin secretion at 15 min after glucose challenge was greatly enhanced by the exogenous apoA-IV treatment, with subsequent improved glucose tolerance (Fig. 4 A and B). In general, diabetes mellitus occurs when β cells are unable to meet insulin demands because of insulin deficiency. Our findings demonstrate that apoA-IV can enhance glucose-induced insulin secretion both in vitro and in vivo, raising the possibility that apoA-IV may have promise in the treatment of diabetes.
ApoA-IV−/− mice maintained on an HFD had elevated basal insulin levels, but without a proportional increase in the ability to secrete insulin in response to a glucose challenge (Fig. 3). Thus, insulin secretion was compromised to the extent that it was unable to compensate for the effect of insulin resistance, thus accounting for the reduced glucose tolerance during the IPGTT. Furthermore, a bolus injection of exogenous apoA-IV acutely augmented insulin secretion during the glucose challenge (Fig. 4 C and D), suggesting that the beneficial effect of apoA-IV on insulin secretion is not compromised by an HFD.
It is important to note that in the apoA-IV−/− mice, even though apoA-IV protein was administered 2 h in advance, apoA-IV itself did not stimulate insulin secretion until glucose was administered (Fig. 4). Moreover, in diabetic KKAy mice in the fed condition with high plasma glucose levels, a bolus injection of apoA-IV elicited insulin secretion and reduced blood glucose (Fig. 5). These findings are significant for two reasons. First, circulating apoA-IV protein seems to act directly on islets to enhance glucose-induced insulin secretion. Second, the effect of apoA-IV is particularly evident under conditions of high glucose, which could potentially avoid the unwanted side effect of hypoglycemia with therapeutic administration of apoA-IV. This is important given the current tendency to treat hyperglycemia by targeting the KATP channels of pancreatic β cells to increase insulin secretion, independent of the ambient glucose concentration; however, such treatments carry the risk of dangerous hypoglycemia (25).
A recent report indicated that apoA-I stimulates insulin secretion from a MIN6 mouse insulinoma cell line under both 2.8 mM and 25 mM glucose conditions (26). This differs from our finding that apoA-IV specifically augments glucose-induced insulin secretion only at higher glucose concentrations. Although apoA-IV and apoA-I are similar in structure and function (27), they differ in some important ways. ApoA-IV is a satiation factor that reduces meal size, a function not shared by apoA-I (28). ApoA-IV lacks the strong C-terminal lipid-binding domain that is present in apoA-I, and instead contains a unique C terminus that attenuates lipid binding (29). Moreover, the majority of amphipathic alpha helices in apoA-IV are more hydrophilic compared with those in apoA-I, and consequently, apoA-IV has been predicted to penetrate less deeply into lipid than apoA-1 (30, 31). Whether these structural differences account for the discrepancy in the effects of apoA-IV and apoA-I on insulin secretion requires further investigation. Importantly, in the present study, we found that plasma apoA-I was unaffected in apoA-IV−/− mice with or without exogenous apoA-IV treatment (Fig. S5A). Therefore, the insufficient insulin secretion observed in apoA-IV−/− mice is not likely induced by altered apoA-I. Furthermore, treatment of apoA-IV−/− mice with exogenous apoA-I resulted in only a slight reduction in glucose levels at 60 min during the IPGTT (Fig. S5B), indicating that the effect of apoA-IV on glucose homeostasis is unique and cannot be duplicated by apoA-I.
Compared with intact WT mice, 1 μg/g of exogenous apoA-IV administered to apoA-IV−/− mice in the present study represents approximately 10% of the endogenous apoA-IV plasma level (Fig. S6). A previous study reported an 11–27% increase in plasma apoA-IV over basal levels at 15–60 min after a gastric bolus of 0.1 g of triglyceride (32). Consequently, we suggest that the increase in apoA-IV protein after a meal rich in fat might be sufficient to enhance insulin secretion. Given that apoA-IV is stimulated by dietary fat, we propose that apoA-IV is a gut factor released by the enterocytes to liaison between intestinal lipid absorption and whole-body glucose metabolism. In agreement with this idea, increases in plasma apoA-IV levels were correlated with a decreased incidence of diabetes after gastric bypass surgery (9). In future studies, it will be important to investigate whether the up-regulation of apoA-IV by either dietary fat or bypass surgery physiologically regulates glucose metabolism.
In conclusion, we have identified a unique function of apoA-IV as a potent endogenous regulator of pancreatic insulin secretion and whole-body glucose homeostasis. We suggest that increased plasma apoA-IV may provide a useful therapeutic approach to treat type 2 diabetes mellitus.
Materials and Methods
Isolation of Islets and Measurement of Insulin Secretion.
Pancreatic islets were isolated by the collagenase P digestion method (33) and cultured in RPMI 1640 containing 10% (vol/vol) FBS and 11 mM glucose at 37 °C in a humidified atmosphere of 95% air and 5% CO2 for 48 h. Islets (five in each tube) were preincubated at 37 °C for 1 h in Kreb-Ringer bicarbonate Hepes buffer (KRB; 129 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 5 mM NaHCO3, 10 mM Hepes, and 0.2% BSA) containing 3.0 mM glucose. The islets were then incubated in KRB with 3 mM glucose for 1 h in the presence or absence of mA-IV and other indicated compounds and stimulated with 20 mM glucose for an additional hour in the presence or absence of mA-IV and indicated compounds.
[Ca2+]i Imaging.
[Ca2+]i was measured using the radiometric [Ca2+]i indicator Fura 2-AM as described previously (33). Islets were dye-loaded and recorded in KRB. Islets were loaded for ∼40 min with 1 μM Fura 2-AM, washed, and then transferred to a small-volume chamber (Warner Instruments) mounted on the stage of an Olympus BX51W1 fluorescence microscope. Islets were perfused with a peristaltic pump (Gilson) at ∼35 °C by an in-line heater (Warner Instruments). Images were obtained sequentially with 340-nm and then 380-nm excitation to produce each [Ca2+]i ratio from emitted light at 510 nm using a Hamamatsu ORCA-ER camera. Excitation light from a xenon burner was supplied to the preparation via a light pipe and filter wheel (Sutter Instrument). Islets were recorded in 3 mM (low) glucose for 3 min and then exposed to 20 mM glucose stimulation in the presence or absence of mA-IV. Data were analyzed with IP Lab version 4.0 (Scanalytics), and [Ca2+]i was reported as percent 340/380 ratio.
Animals.
Male ApoA-IV−/− mice were originally obtained from J. L. Breslow (The Rockefeller University, New York, NY) (34) and backcrossed onto the parent C57BL/6J strain for more than 10 generations. C57BL/6J mice were bred and housed in our animal facility. Male KKAy mice were purchased from the Jackson Laboratory. All mice were housed on a 12-h light/dark cycle with ad libitum access to water and food. All animal protocols were approved by the University of Cincinnati’s Institutional Animal Care and Use Committee. For the high-fat feeding study, male WT and apoA-IV−/− mice were fed a HFD (20% fat) for 10 wk, as described previously (35).
Physiological Measurements.
Body composition of age-matched apoA-IV−/− and WT mice was assessed with the EchoMRI-100 system (EchoMRI). Glucose levels were measured with a glucometer (Abbott), and insulin, GIP, and GLP-1 levels were measured by ELISA (Millipore).
IPGTT.
Tail blood samples were collected from mice that had been fasted for 5 h, before and at 15, 30, 60, and 120 min after i.p. injection of glucose (2 g/kg). Glucose and insulin levels were measured at prespecified times.
Insulin Tolerance Test.
Mice were injected with 0.75 U/kg of Humulin R (Lilly) after a 5-h fast. Glucose levels were measured before and at 15, 30, 45, and 60 min after insulin injection.
Treatment with Recombinant mA-IV.
mA-IV constructs were expressed in Escherichia coli, purified by affinity column chromatography, and dialyzed as described previously (36). mA-IV was initially administrated i.p. to apoA-IV−/− mice, and plasma mAIV level was measured by Western blot analysis at different time points up to 24 h. Plasma mA-IV levels were highest at 2 h after administration. To assess the effect of mA-IV on glucose tolerance, apoA-IV−/− mice received mA-IV injections 2 h before IPGTT. As in KKAy mice with ad libitum access to water and food, a single injection of mA-IV was given during the light phase. Blood glucose and plasma insulin levels were measured at prespecified times up to 24 h.
Statistics.
Data were analyzed with the unpaired two-tailed Student t test or ANOVA using PRISM 5.0 as appropriate. All data are presented as mean ± SEM. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank M. Liu, L. Woollett, and R. Jandacek for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health Grants DK056863, DK059630, and DK076928.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201433109/-/DCSupplemental.
References
- 1.Swaney JB, Braithwaite F, Eder HA. Characterization of the apolipoproteins of rat plasma lipoproteins. Biochemistry. 1977;16:271–278. doi: 10.1021/bi00621a018. [DOI] [PubMed] [Google Scholar]
- 2.Ghiselli G, Krishnan S, Beigel Y, Gotto AM., Jr Plasma metabolism of apolipoprotein A-IV in humans. J Lipid Res. 1986;27:813–827. [PubMed] [Google Scholar]
- 3.Lefevre M, Chuang MY, Roheim PS. ApoA-IV metabolism in the rat: Role of lipoprotein lipase and apolipoprotein transfer. J Lipid Res. 1986;27:1163–1173. [PubMed] [Google Scholar]
- 4.Stan S, Delvin E, Lambert M, Seidman E, Levy E. Apo A-IV: An update on regulation and physiologic functions. Biochim Biophys Acta. 2003;1631:177–187. doi: 10.1016/s1388-1981(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 5.Vowinkel T, et al. Apolipoprotein A-IV inhibits experimental colitis. J Clin Invest. 2004;114:260–269. doi: 10.1172/JCI21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tso P, Liu M. Apolipoprotein A-IV, food intake, and obesity. Physiol Behav. 2004;83:631–643. doi: 10.1016/j.physbeh.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Palmer AM, Murphy N, Graham A. Triglyceride-rich lipoproteins inhibit cholesterol efflux to apolipoprotein (apo) A1 from human macrophage foam cells. Atherosclerosis. 2004;173:27–38. doi: 10.1016/j.atherosclerosis.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Van Lenten BJ, et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res. 2008;49:2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culnan DM, Cooney RN, Stanley B, Lynch CJ. Apolipoprotein A-IV, a putative satiety/antiatherogenic factor, rises after gastric bypass. Obesity (Silver Spring) 2009;17:46–52. doi: 10.1038/oby.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson IA, et al. Effects of apolipoprotein A-IV genotype on glucose and plasma lipoprotein levels. Clin Genet. 2002;61:430–436. doi: 10.1034/j.1399-0004.2002.610606.x. [DOI] [PubMed] [Google Scholar]
- 11.Visvikis S, et al. Frequency and effects of the apolipoprotein A-IV polymorphism. Clin Genet. 1990;37:435–441. doi: 10.1111/j.1399-0004.1990.tb03527.x. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, et al. Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets. Diabetes. 2008;57:2382–2392. doi: 10.2337/db06-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henquin JC. Regulation of insulin secretion: A matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–751. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 14.Bernal-Mizrachi E, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner MB, Gromada J, Efanov AM, Bokvist K, Mest HJ. Restoration of first-phase insulin secretion by the imidazoline compound LY374284 in pancreatic islets of diabetic db/db mice. Ann N Y Acad Sci. 2003;1009:332–340. doi: 10.1196/annals.1304.042. [DOI] [PubMed] [Google Scholar]
- 16.Skelin M, Rupnik M. cAMP increases the sensitivity of exocytosis to Ca²+ primarily through protein kinase A in mouse pancreatic beta cells. Cell Calcium. 2011;49:89–99. doi: 10.1016/j.ceca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Fournier N, et al. Human ApoA-IV overexpression in transgenic mice induces cAMP-stimulated cholesterol efflux from J774 macrophages to whole serum. Arterioscler Thromb Vasc Biol. 2000;20:1283–1292. doi: 10.1161/01.atv.20.5.1283. [DOI] [PubMed] [Google Scholar]
- 18.Dubois M, et al. Glucotoxicity inhibits late steps of insulin exocytosis. Endocrinology. 2007;148:1605–1614. doi: 10.1210/en.2006-1022. [DOI] [PubMed] [Google Scholar]
- 19.Brunzell JD, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976;42:222–229. doi: 10.1210/jcem-42-2-222. [DOI] [PubMed] [Google Scholar]
- 20.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalogeris TJ, Fukagawa K, Tso P. Synthesis and lymphatic transport of intestinal apolipoprotein A-IV in response to graded doses of triglyceride. J Lipid Res. 1994;35:1141–1151. [PubMed] [Google Scholar]
- 22.Liu M, Doi T, Tso P. Regulation of intestinal and hypothalamic apolipoprotein A-IV. Exp Biol Med (Maywood) 2003;228:1181–1189. doi: 10.1177/153537020322801013. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, et al. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav. 2008;95:161–167. doi: 10.1016/j.physbeh.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenich C, Brecher P, Makrides S, Chobanian A, Zannis VI. Apolipoprotein gene expression in the rabbit: Abundance, size, and distribution of apolipoprotein mRNA species in different tissues. J Lipid Res. 1988;29:755–764. [PubMed] [Google Scholar]
- 25.Ashcroft FM. Mechanisms of the glycaemic effects of sulfonylureas. Horm Metab Res. 1996;28:456–463. doi: 10.1055/s-2007-979837. [DOI] [PubMed] [Google Scholar]
- 26.Fryirs MA, et al. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler Thromb Vasc Biol. 2010;30:1642–1648. doi: 10.1161/ATVBAHA.110.207373. [DOI] [PubMed] [Google Scholar]
- 27.Boguski MS, Elshourbagy N, Taylor JM, Gordon JI. Comparative analysis of repeated sequences in rat apolipoproteins A-I, A-IV, and E. Proc Natl Acad Sci USA. 1985;82:992–996. doi: 10.1073/pnas.82.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262:G1002–G1006. doi: 10.1152/ajpgi.1992.262.6.G1002. [DOI] [PubMed] [Google Scholar]
- 29.Pearson K, et al. Structure of human apolipoprotein A-IV: A distinct domain architecture among exchangeable apolipoproteins with potential functional implications. Biochemistry. 2004;43:10719–10729. doi: 10.1021/bi048978m. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg RB, Ibdah JA, Phillips MC. Adsorption of apolipoprotein A-IV to phospholipid monolayers spread at the air/water interface: A model for its labile binding to high-density lipoproteins. J Biol Chem. 1992;267:8977–8983. [PubMed] [Google Scholar]
- 31.Pearson K, et al. Specific sequences in the N and C termini of apolipoprotein A-IV modulate its conformation and lipid association. J Biol Chem. 2005;280:38576–38582. doi: 10.1074/jbc.M506802200. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez MD, Kalogeris TJ, Wang XL, Wolf R, Tso P. Rapid synthesis and secretion of intestinal apolipoprotein A-IV after gastric fat loading in rats. Am J Physiol. 1997;272:R1170–R1177. doi: 10.1152/ajpregu.1997.272.4.R1170. [DOI] [PubMed] [Google Scholar]
- 33.Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology. 2009;150:607–615. doi: 10.1210/en.2008-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstock PH, et al. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J Lipid Res. 1997;38:1782–1794. [PubMed] [Google Scholar]
- 35.Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, et al. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol Behav. 2003;78:149–155. doi: 10.1016/s0031-9384(02)00959-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.