Significant progress in identifying cellular sources of ECM in experimental and human liver injury has led to a clearer understanding of fibrogenic cell dynamics during progressive fibrosis. Hepatic stellate cells, the resident perisinusoidal cell type that stores vitamin A, are the major source of ECM during diseases that injure hepatocytes (e.g., CCl4 in rodents and viral hepatitis in humans) through their activation into contractile myofibroblasts (1), and a similar transition to myofibroblasts from portal fibroblasts drives the fibrogenic response when biliary cells rather than hepatocytes are injured (2). The liver, with its unique regenerative capacity, has a remarkable ability to resorb scar when either hepatocellular or biliary injury is halted (3, 4). Reduced numbers of fibrogenic cells during fibrosis regression have been ascribed primarily to clearance of myofibroblasts through apoptosis (5), but the question has lingered of whether stellate cells can also revert to a quiescent state and persist as the liver’s normal architecture is restored. In the report by Kisseleva et al. (6) in PNAS, reversion is definitively established in two models of experimental liver injury using complementary genetic lineage tracing methods (Fig. 1).
Fig. 1.
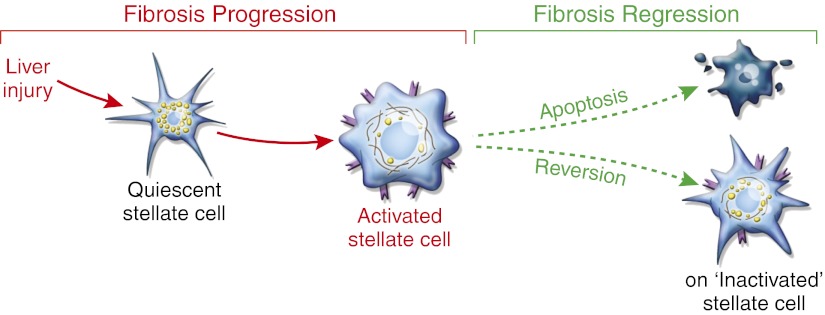
Fates of activated hepatic stellate cells during fibrosis regression. After injury to the liver, stellate cell activation leads to fibrosis progression. Activation reflects the transdifferentiation from a quiescent vitamin A-rich cell to an activated stellate cell/myofibroblast, which expresses mitogenic receptors and is contractile with reduced vitamin A content. If injury is attenuated, fibrosis regression can occur, leading to either apoptosis of stellate cells or reversion to an inactivated state with restored features of quiescence. However, inactivated stellate cells still retain an intermediate phenotype, with enhanced capacity to respond to fibrogenic signals and reactivate on further injury.
The work by Kisseleva et al. (6) generates mice with activation of stellate cells into myofibroblasts that could be permanently marked through a type I collagen promoter-driven Cre-mediated excision of a stop cassette in a reporter mouse. Because only activated stellate cells express type I collagen within injured liver, this leads to expression of YFP that is restricted to this cell type during fibrosis progression. Consistent with earlier studies, short-term CCl4 administration (a standard hepatocellular toxin that provokes fibrosis) leads to marked activation of almost all stellate cells; however, after 1 mo of CCl4 exposure followed by 4 wk of recovery, substantial reduction of activated stellate cell numbers is apparent (7). At the peak of resolution, 2.6% of activated stellate cells bear markers of apoptosis, whereas YFP is detectable in 56% of cells that also contained vitamin A, indicative of a return to quiescence. Because apoptosis is evanescent, this finding does not mean that only a small fraction of cells undergo cell death, but the findings establish the larger point that a significant number of stellate cells reacquire features of quiescence. This conclusion is replicated in another model of injury caused by intragastric ethanol administration, indicating that the findings should be generalizable to all forms of hepatocellular injury.
Although stellate cells reacquired markers of quiescence during fibrosis resolution, they were not identical to cells that had never activated. Instead, these inactivated stellate cells adopted an intermediate phenotype, with a heightened capacity to reactivate when treated with TGF-β1, a classic fibrogenic signal. Whether these primed stellate cells remain in this state indefinitely or slowly return to full quiescence over a longer interval is not known. Regardless, the data reinforce the known heterogeneity of stellate cells (8, 9), and now, this heterogeneity extends to their variable capacity to quiesce and then reactivate.
These findings substantially refine our understanding of stellate cell dynamics and are the most comprehensive characterization of stellate cell turnover to date. The work also raises very intriguing questions. Most importantly, what are the molecular mechanisms underlying the intermediate phenotype of inactivated stellate cells? A very appealing explanation is that epigenetic events biologically mark this subpopulation. A recent study (10) reports a sequence of events during stellate cell activation in which expression of PPARγ, a gene that sustains quiescence, is repressed through methylation conferred by the methyl CpG (-C-phosphate-G-) binding protein 2, which in turn, is regulated by microRNA 132. One might predict, therefore, that PPARγ (peroxisome proliferator-activated receptor alpha) methylation persists in inactivated stellate cells, which is an eminently testable prospect. Moreover, an even broader unbiased characterization of epigenetic changes to detect other methylation events as well as microRNA alterations could yield additional clues to understanding stellate cell activation and reversion, and in doing so, it could unearth new therapeutic targets.
What determines whether an activated stellate cell undergoes apoptosis or regresses to an inactivated state when liver injury resolves? Based on the data by Kisseleva et al. (6), an important rheostat is the relative activity of proapoptotic and antiapoptotic signaling. Specifically, gene expression profiling identifies the antiapoptotic Hspa1a/b genes as strongly but transiently induced during stellate cell activation but down-regulated during recovery. Moreover, stellate cells isolated from Hspa1a/b−/− mice are much more sensitive to apoptosis, and fibrosis resolution in these animals after CCl4 in vivo is greatly accelerated. It would seem likely that layers of pro- and antiapoptotic signals, combined with epigenetic changes, define a continuous spectrum of stellate cell subpopulations with variable capacity to revert, undergo apoptosis, and/or reactivate. A systems biology approach might integrate these converging inputs, much like in a recent study of renal injury that uncovered a novel kinase regulating fibrogenic cell activation (11).
In addition to intracellular signals, stellate cell activation is also influenced by extracellular cues that include matrix composition and stiffness, the inflammatory microenvironment, and the relative activity of metallproteinases and their inhibitors, among others. How do these cues influence stellate cell reversion? For example, matrix stiffness in fibrotic liver activates stellate cells and may increase the risk of hepatocellular carcinoma (12, 13). Does softening of the ECM promote stellate cell apoptosis, reversion, or both? Does the relative expression of tissue inhibitors of metalloproteinases, which are determinants of stellate cell survival (14), also influence stellate cell reversion? By showing the capacity of stellate cells to engraft in liver and contribute to fibrogenesis after adoptive transfer to recipient animals, the model by Kisseleva et al. (6) could enable future studies to assess the impact of the extracellular milieu on reversion of stellate cells. This research could be achieved by assessing cellular behavior after adoptive transfer of stellate cells into livers with different genetic alterations or biochemical properties.
These findings substantially refine our understanding of stellate cell dynamics.
Finally, what are the translational and clinical implications of the study by Kisseleva et al. (6)? Because the findings are restricted to models of hepatocellular rather than biliary injury, they are most relevant to human liver diseases with similar patterns of injury, including viral hepatitis and fatty liver disease associated with either obesity or alcohol abuse. In contrast, it is uncertain whether, in biliary injury, myofibroblasts derived from portal fibroblasts rather than stellate cells have a similar capacity to revert. This question is particularly relevant to fibrosing biliary diseases that include primary biliary cirrhosis and sclerosing cholangitis. Because many fibrogenic pathways are conserved across tissues, the findings could also be extended to studies of fibrosis in lung, kidney, heart, and other organs.
An especially intriguing question is whether accumulated reversion of inactivated but primed stellate cells could contribute to the accelerating rate of fibrosis accumulation known to occur in humans with advancing age (15). Perhaps, because the liver is populated increasingly by inactivated rather than quiescent stellate cells during repetitive cycles of injury and repair (for example, as typically occurs in alcoholic liver disease), a growing fraction of cells is more rapidly and extensively reactivated. This explanation is not likely to be the only one to account for accelerating fibrosis with age, but the question is experimentally tractable using the models developed in the work by Kisseleva et al. (6). Finally, the demonstration that stellate cells can revert introduces a point of therapeutic attack in which interventions that favor apoptosis rather than reversion might yield a more rapid restoration of normal liver architecture as fibrosis regresses.
Acknowledgments
Work in the author's laboratory is supported by National Institutes of Health Grants RO1DK56621 and R01AA020709.
Footnotes
The author declares no conflict of interest.
See companion article on page 9448.
References
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallet V, et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399–403. doi: 10.7326/0003-4819-149-6-200809160-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hammel P, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418–423. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 5.Iredale JP. Models of liver fibrosis: Exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisseleva T, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iredale JP, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Ambrosio DN, et al. Distinct populations of hepatic stellate cells in the mouse liver have different capacities for retinoid and lipid storage. PLoS One. 2011;6:e24993. doi: 10.1371/journal.pone.0024993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 10.Mann J, et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138:705–714. doi: 10.1053/j.gastro.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Y, et al. A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat Med. 2012;18:580–588. doi: 10.1038/nm.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georges PC, et al. Increased stiffness of the rat liver precedes matrix deposition: Implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 13.Schrader J, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy FR, et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 15.Poynard T, et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34:730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]