Abstract
The importance of high-incidence “hotspots” to population-level tuberculosis (TB) incidence remains poorly understood. TB incidence varies widely across countries, but within smaller geographic areas (e.g., cities), TB transmission may be more homogeneous than other infectious diseases. We constructed a steady-state compartmental model of TB in Rio de Janeiro, replicating nine epidemiological variables (e.g., TB incidence) within 1% of their observed values. We estimated the proportion of TB transmission originating from a high-incidence hotspot (6.0% of the city’s population, 16.5% of TB incidence) and the relative impact of TB control measures targeting the hotspot vs. the general community. If each case of active TB in the hotspot caused 0.5 secondary transmissions in the general community for each within-hotspot transmission, the 6.0% of people living in the hotspot accounted for 35.3% of city-wide TB transmission. Reducing the TB transmission rate (i.e., number of secondary infections per infectious case) in the hotspot to that in the general community reduced city-wide TB incidence by 9.8% in year 5, and 29.7% in year 50—an effect similar to halving time to diagnosis for the remaining 94% of the community. The importance of the hotspot to city-wide TB control depended strongly on the extent of TB transmission from the hotspot to the general community. High-incidence hotspots may play an important role in propagating TB epidemics. Achieving TB control targets in a hotspot containing 6% of a city’s population can have similar impact on city-wide TB incidence as achieving the same targets throughout the remaining community.
Keywords: infectious disease transmission, theoretical models, urban population, epidemiology, communicable disease control
Tuberculosis (TB) remains a leading infectious cause of morbidity and mortality, with over 8.8 million cases and 1.4 million deaths annually worldwide (1). TB is known to cluster in hyperendemic “hotspots” often characterized by crowding (2), poverty (3), HIV infection (4), and other social determinants (5). However, compared with other infectious diseases [e.g., sexually transmitted diseases (6) and vector-borne diseases (7)], where 20% of the population may generate 80% of transmission (7), TB transmission appears relatively more homogeneous. As a result, though spatial targeting is often advocated as an efficient method for achieving control of diseases such as malaria (8), the degree to which hotspots contribute to community-wide transmission of TB remains uncertain. The concept of geographically defined hotspots driving TB transmission has biological plausibility. Preventing TB cases in high-transmission areas (e.g., crowded urban slums, poorly ventilated hospitals) may avert many more secondary transmissions than similar efforts in low-transmission areas. Similarly, cases in high-transmission areas are more likely to represent recent infection (which is more amenable to intervention) than reactivation of latent disease (9). Prior explorations of heterogeneity in TB transmission (10–12) have studied the relative importance of TB reinfection vs. reactivation, but have not explicitly evaluated the contribution of hotspots to broader TB epidemics and their control. Rio de Janeiro boasts substantial geographic heterogeneity in TB incidence, high-quality surveillance data, and, due to the upcoming Olympics and World Cup, active efforts to disrupt the social underpinnings (e.g., drug trade) contributing to TB transmission (13). Thus, we constructed a mathematical model of TB transmission in Rio de Janeiro to explore the importance of geographic hotspots to community-wide TB control.
Results
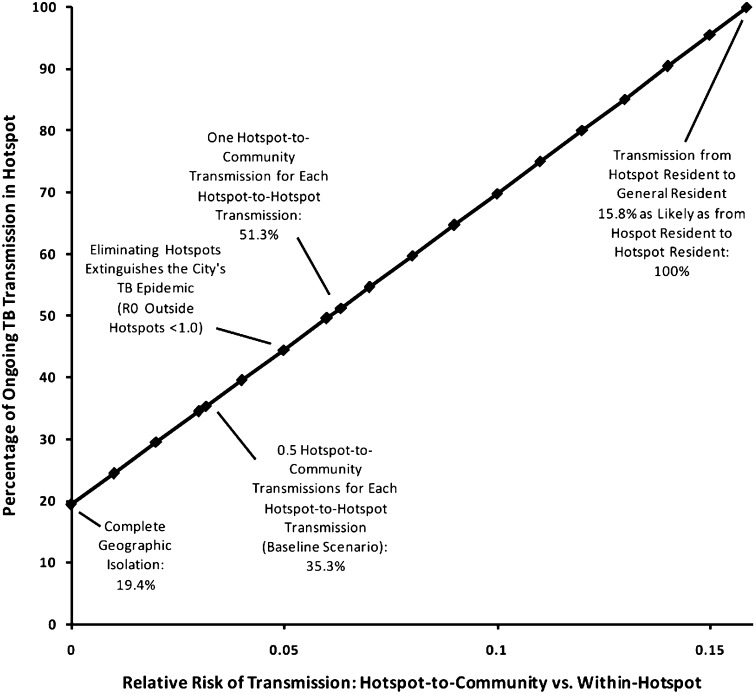
In this simplified model of TB transmission in Rio de Janeiro (Fig. 1), a subpopulation (hotspot) comprising 6.0% of the city’s population accounted for a disproportionate amount of ongoing TB transmission. If each case of active TB in the hotspot caused 0.5 secondary transmissions in the general community for each secondary transmission within the hotspot (i.e., a randomly selected resident of the hotspot was 32× more likely to be infected by a case of active TB in the hotspot than a randomly selected resident of the general community), the hotspot accounted for 35.3% of all TB transmission in the city (Fig. 2). When the relative risk of transmission within the hotspot relative to the general community was lowered from 32 to 20, ongoing transmission in the community became dependent on the hotspot (i.e., R0 <1 in the community). Under these conditions, reducing rates of TB transmission in the hotspot to those in the general community was sufficient to eliminate TB from the population in the long term. By contrast, when no transmission events were assumed to occur between the hotspot and the general community, the hotspot accounted for 19.4% of ongoing TB transmission (Fig. 2). The common modeling assumption of geographic homogeneity in TB transmission (i.e., relative risk of 1.0 on the x axis of Fig. 2) was grossly untenable; if hotspot-to-community transmission is even one-sixth as likely as hotspot-to-hotspot transmission, the hotspot accounted for all TB transmission in the community.
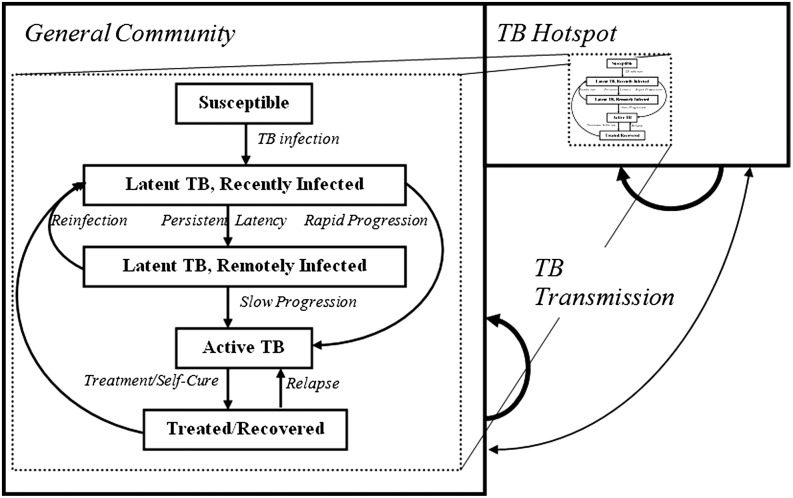
Fig. 1.
Compartmental model of TB epidemic in Rio de Janeiro. The population is divided into two geographic compartments: the general population (94% of the total population) and a TB hotspot (6% of the total population, accounting for 16.5% of TB incidence). TB transmission occurs within and, to a lesser extent, across geographic compartments. In each geographic compartment, individuals fall into one of five TB states. Not shown here, but also included in the model, are two HIV states (HIV infected and uninfected), with HIV prevalence also higher in the hotspot. Each geographic compartment has its own births and deaths; for simplicity, migration between compartments is not explicitly modeled, but can be conceptualized as one mechanism of cross-compartment infection.
Fig. 2.
Proportion of TB transmission events in Rio de Janeiro arising from cases in the hotspot. The x axis describes the relative risk of transmission from an active TB case in the hotspot to a randomly selected resident of the general population, compared with a random hotspot resident. Because the general population is 0.94/0.06 = 15.7× larger than the hotspot, a relative transmission risk of 1/15.7 = 0.064 assumes that a TB case in the hotspot generates as many secondary transmissions in the general population as in the hotspot. The baseline scenario in the text assumes one-half this rate of cross-transmission (i.e., two hotspot-to-hotspot transmissions for every hotspot-to-community transmission). Under this assumption, the hotspot generated 35.3% of all transmission events, compared with 19.4% if cross-transmission were disallowed.
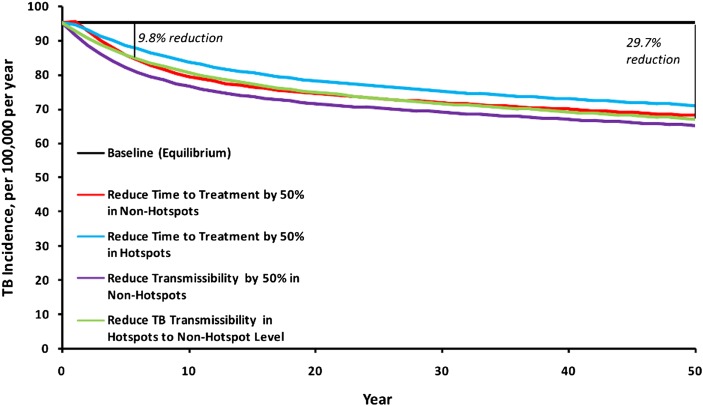
Under the baseline scenario (0.5 hotspot-to-community transmissions for each hotspot-to-hotspot transmission), TB control interventions carried out only in the hotspot (6% of residents) had a similar impact on city-wide TB rates as the same interventions deployed to the remaining 94% of the population in the general community (Fig. 3). For example, we considered an intervention resulting in a 50% reduction in time to treatment (e.g., household case-finding that detects all TB cases twice as quickly as passive case-finding). Carried out in the hotspot alone, this intervention reduced city-wide TB incidence by a mean 1.6% per year over the first 5 y, and by 25.4% by the end of year 50 (Fig. 3, light blue line). By contrast, when applied to the remaining 94% of the population, the intervention reduced city-wide TB incidence by 2.5% per year over the first 5 y, and 28.5% by year 50 (Fig. 3, red line).
Fig. 3.
Impact on TB incidence of hotspot-focused vs. population-based interventions. The upper solid line reflects the equilibrium assumption used to fit the model, in the absence of any intervention. The light blue and red lines correspond to 50% reductions in the mean infectious period (e.g., through active case-finding or improved diagnosis) of cases residing in the hotspot and general community, respectively. The purple line shows a reduction of 50% in the transmission rate of TB cases (i.e., number of secondary infections per infectious person-year) residing outside the hotspot. By comparison, the green line shows “normalization” of the hotspot (reduction in TB transmission per infectious hotspot person-year to the mean level in the general population). This scenario generates a mean 2.0% annual reduction in TB incidence per year over the first 5 y, or 9.8% reduction in TB incidence by the end of year 5 (29.7% by year 50). In general, interventions targeting the 6% of people living in the hotspot have similar impact to interventions targeting the remaining 94% of the population.
Reducing TB transmission rates in the hotspot to those in the general community (Fig. 3, green line) reduced city-wide TB incidence by a mean 2.0% per year over the first 5 y, or a 9.8% reduction by year 5 (95% uncertainty range: 6.9%, 11.4%). By year 50, TB incidence was reduced by 29.7%, reflecting a 62.8% reduction in incidence in the hotspot and a 23.1% reduction in the remaining community. Corresponding reductions in city-wide TB mortality were slightly lower: 7.8% reduction by year 5, and 29.1% by year 50. This impact was relatively robust to changes in parameters other than TB transmission rates (Fig. 4). Assigning all such parameters to their least-favorable values resulted in a projected 5-y reduction in TB incidence of 4.7%; the corresponding best-case scenario achieved a 22.2% reduction. Removing HIV from the model increased the 5-y incidence reduction to 13.2%, and splitting the single hotspot into three noncommunicating hotspots did not change the projected reduction in incidence, to within 0.1%.
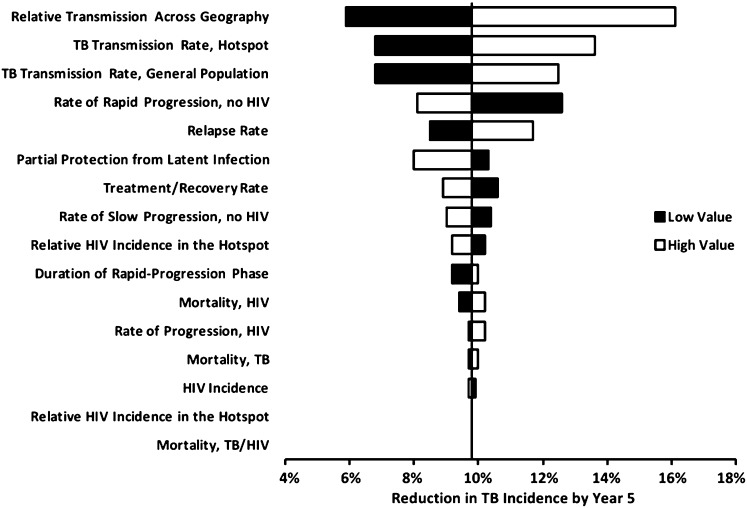
Fig. 4.
One-way sensitivity analysis showing the reduction in transmission at year 5 from eliminating hotspots. Values on the x axis represent the relative reduction in city-wide TB incidence achieved at the end of year 5 after normalizing the hotspot (see text and Fig. 3, green line). Relative transmission across geography is varied from 0.01 to 0.063 (one hotspot-to-community transmission for each hotspot-to-hotspot transmission; Fig. 2); other parameters are varied to produce a 25% change in the corresponding epidemiological parameter (Table 1). The top three parameters were subsequently varied in multiway sensitivity analysis that also included hotspot size (Fig. 5), whereas the remaining parameters were simultaneously varied in best-case and worst-case uncertainty analysis (see text for more information).
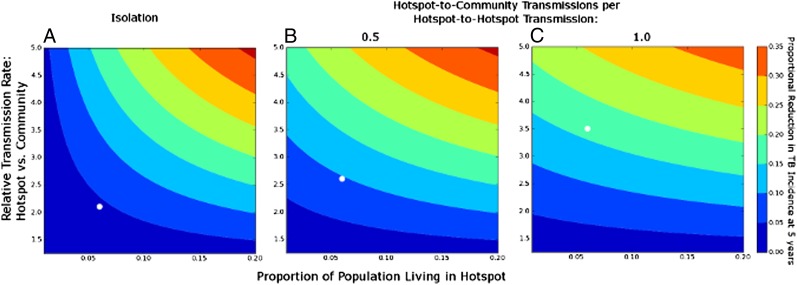
The contribution of the hotspot to city-wide TB transmission was most sensitive to hotspot size, hotspot intensity (relative rate of hotspot-to-hotspot vs. community-to-community transmission), and level of cross-transmission (relative rate of hotspot-to-hotspot vs. hotspot-to-community transmission). Fig. 5 shows the 5-y reduction in TB incidence projected from normalizing a hotspot (i.e., reducing its per-case TB transmission rates to those of the general community), according to these parameter values. Normalizing a geographically isolated, low-intensity hotspot (6% of the city’s population, per-case TB transmission rate 2.1× that in the community, and no cross-transmission) reduced city-wide TB incidence by only 4.3%. By contrast, normalizing a nonisolated, high-intensity hotspot of the same size (per-case transmission rate 3.5× the community rate, generating one hotspot-to-community transmission for every hotspot-to-hotspot transmission) reduced city-wide incidence in year 5 by 16.3% (Fig. 5, white circles). Both of these scenarios were consistent with the observed hotspot and community-wide TB incidence rates in Rio de Janeiro.
Fig. 5.
Projected reduction in city-wide TB incidence after normalizing hotspots, according to size and intensity of hotspot. Values of contour lines show the proportional reduction in city-wide TB incidence at the end of year 5 achieved by lowering TB transmission in hotspots to the mean value in the rest of the city (Fig. 3, green line). Box A assumes complete geographic isolation of the hotspot (i.e., no cross-transmission from hotspot to community), box B assumes 0.5 transmission events from hotspot to community for every hotspot-to-hotspot transmission, and box C assumes one hotspot-to-community transmission for every hotspot-to-hotspot transmission. The baseline scenario in the text corresponds to box B, with a relative transmission rate of 2.6 and hotspot size of 0.06 (9.8% reduction). Other scenarios that replicate the TB incidence seen in Rio de Janeiro hotspots are a relative transmission rate of 2.1 in box A (4.3% reduction) and 3.5 in box C (16.3% reduction). These scenarios are shown with open circles.
Discussion
This simple model of TB transmission in Rio de Janeiro uses plausible conditions to demonstrate that a disproportionate share of community-wide TB transmission likely occurs in small, geographically defined hotspots. In our baseline scenario, a hotspot containing 6% of the city’s population and 16.5% of its TB incidence generated 35.3% of all ongoing TB transmission events. Because the burden of TB transmission in such hotspots is greater even than the burden of TB incidence, TB control efforts focusing on these areas are likely to have greater-than-anticipated impact on community-wide TB control. Equalizing TB transmission rates between our modeled hotspot and the general community reduced city-wide TB incidence by 2.0% per year over the first 5 y, and nearly 30% over a 50-y time horizon—an impact similar to halving time to treatment among the remaining 94% of the city’s population.
This modeling exercise shows that, though TB may not follow the “80/20 rule” typical of some other infectious diseases, epidemics of TB may nonetheless depend on a core group, defined by conditions (e.g., crowding, poor access to health care) that engender high rates of ongoing transmission (7). Prior geospatial mapping in Brazil has demonstrated the existence of geographically defined incidence clusters that correlate with low socioeconomic status (14). Control strategies (e.g., contact investigation, reducing socioeconomic disparities) that target such clusters may have disproportionate impact, whereas those that ignore these hotspots will have a ceiling to their effectiveness. The plausibility of this argument is easily demonstrated; our model fitting suggests that a case of active TB in the hotspot generates 2.6× as many secondary infections as a similar case in the general community (e.g., because the hotspot case is exposed to crowded conditions and encounters individuals with greater susceptibility to TB). Thus, over two rounds of secondary transmission, preventing one hotspot case could have 2.6 × 2.6 = 6.8× greater impact on future TB incidence than preventing one community case. This equation is an oversimplification of actual dynamics, in which populations reach new equilibria (and is not assumed in our model). For example, hotspot cases may “saturate” their contacts after a given time (10), or transmission rates may be so high that a prevented transmission event is quickly followed by another transmission event (15). However, in the absence of complete geographic isolation, hotspots should contribute a greater proportion of ongoing community-wide TB transmission than would be estimated by comparing relative incidence rates alone.
It has been well-demonstrated that reducing TB transmission in hyperendemic hotspots is far more difficult than in the general community (16). Preventing a case of TB in a resource-poor hotspot is almost certainly more difficult and costly than preventing a case in the general community. However, our model predicts that in the long term [and similar to other diseases, including malaria (8) and sexually transmitted infections (6)], the community-wide impact of preventing hotspot cases is likely to be disproportionately large. In our model, achieving TB control targets (e.g., 50% reduction in time to detection) in a hotspot comprising 6% of the population had similar community-wide impact as achieving the same target in the remaining 94%. Thus, even if the hotspot-based target was 10× more expensive to achieve on a per-capita basis, its impact on TB incidence per dollar spent would be greater than the hotspot-exclusionary target. This finding has particular relevance as national TB control programs consider how best to scale-up novel diagnostic interventions [e.g., Xpert MTB/RIF, a cartridge-based, automated diagnostic test that can identify Mycobacterium tuberculosis (MTB) and resistance to rifampicin (RIF) (17, 18)]; our model suggests that targeting such interventions—and the necessary infrastructure for reporting and treatment (19, 20)—to hotspots of high TB transmission may be a more cost-effective use of resources, even if fewer units can be deployed than when targeted to the general population.
The projected impact of hotspot-targeted interventions depends strongly on the rate of TB transmission from cases in the hotspot to members of the general community. Such cross-transmission events may be common in the setting of nosocomial transmission, incarceration, migration/transportation, and occupational exposures. Prior studies have shown that the amount of transmission that occurs in the household may be small (21, 22), but other transmission events (e.g., at social events) (23) may be far more likely to occur within geographically defined boundaries than across them. This may also be true of hotspots (e.g., townships in Cape Town, South Africa) (24) that are geographically isolated from low-incidence areas. By contrast, the areas of highest TB incidence in Rio de Janeiro are centrally located and in close geographic proximity to commercial districts and other residential areas, making them potentially more important to ongoing city-wide TB transmission. Similar findings, in which the majority of cases attributed to recent transmission are not geographically aggregated, have been reported in Baltimore (25) and Harare, Zimbabwe (26). Although molecular epidemiology studies are unlikely to be undertaken on a sufficient scale to ascertain the relative rates of transmission within vs. across hotspots on a population level (10), further modeling and mapping studies may shed light on this question, which is of critical importance in determining the appropriate prioritization of hotspot-centered interventions in TB control.
As with any mathematical model, our analysis has important limitations. First, for purposes of conducting a parsimonious experiment, we adopted a deterministic framework with two geographically defined compartments. Though this method allows us to investigate geographic heterogeneity in transmission with minimal reliance on extraneous parameter estimates, more complex models (e.g., agent-based models) would be necessary to elucidate important relationships on a finer scale, including social networks and household structure. Furthermore, our summation of three population clusters into a single transmission hotspot may be overly simplistic, although a model with three separate hotspots gave identical results. Second, as a proof-of-concept study, we did not evaluate the absolute impact of any specific TB control intervention. Thus, our numerical estimates of potential impact should be interpreted with caution. Third, we chose to model the population of Rio de Janeiro as a site with known geographic heterogeneity in TB incidence and existing high-quality surveillance systems. Though we believe the concepts elucidated by this model may be broadly applicable, caution should be exercised in generalizing specific estimates (e.g., the size or intensity of the transmission hotspot) to other epidemiological situations. Finally, the quality of our surveillance data may be lower in hotspots than in other districts of the city (e.g., underdetection of cases in hotspots); to the extent this is true, our data may underestimate the contribution of hotspots to ongoing city-wide TB transmission.
In conclusion, geographic heterogeneity in TB transmission results in hotspots that may play a disproportionate role in propagating TB epidemics. Under plausible conditions, a hotspot containing 6% of a city’s population can be responsible for 35% or more of its ongoing TB transmission. Achieving given TB control targets in a hotspot of this size may have similar impact on long-term, community-wide TB incidence as achieving the same targets in the remaining 94% of the population. To achieve maximum community-wide impact, TB control programs should focus their efforts disproportionately on the areas of highest transmission, even if it is more resource-intensive on a per-capita basis to do so.
Materials and Methods
Using ordinary differential equations, we developed a compartmental model of the TB epidemic in Rio de Janeiro. Because our aim was to explore the implications of heterogeneity in TB transmission (i.e., not to evaluate specific interventions), we strove for simplicity in model structure. We sought to incorporate sufficient complexity to reflect available epidemiological inputs with a minimum of additional assumptions. We therefore adopted a parsimonious model of five TB states (Fig. 1), assigning cases of active TB the weighted average of infectiousness among smear-positive pulmonary, smear-negative pulmonary, and extrapulmonary cases. Latently infected and recovered individuals were assumed to be noninfectious. Population compartments were also defined by HIV status (positive vs. negative), with HIV-infected individuals having higher susceptibility to TB, greater risk of progression to active TB, higher general and TB-specific mortality, and lower TB infectivity. Further details of the model structure are given in SI Materials and Methods.
To fit the model to the population of Rio de Janeiro, we used data from ongoing, high-quality passive surveillance systems for TB, HIV, and mortality. These surveillance systems, which have been described elsewhere (27), collect data at the level of bairros, geographically defined regions with population sizes ranging from 180 to 202,000 individuals. We identified three geographic clusters of bairros with TB incidence that was two- to fourfold higher than that of the general population of Rio de Janeiro. These clusters correspond to (i) the largest slum (favela) in the city (Rocinha), (ii) the city center, and (iii) a second favela (Manguinhos) that borders an aging and industrial area of the city (Bonsucesso). Together, these three clusters accounted for 6.0% of the population of Rio de Janeiro in 2009, but reported 16.5% of all incident TB cases. To facilitate a simple description of transmission dynamics, we then divided our model population into two subpopulations: the general community and a single, artificially defined TB transmission hotspot with a population size and TB incidence equal to that of the combined population of the three high-transmission clusters of bairros. To evaluate the effect of simplifying model structure into a single hotspot, we conducted a structural sensitivity analysis in which the hotspot was broken into three separate hotspots without cross-transmission.
The contribution of hotspots to community-wide TB transmission depends on the relative rate of TB transmission from the hotspot to the general community vs. within the hotspot. We therefore set three independent TB transmission rates: hotspot-to-hotspot, community-to-community, and the relative rate of cross-population (compared with within-population) transmission. A focused review of the literature (SI Materials and Methods) suggested that 30–50% of recent TB transmission at the city level is geographically clustered (25, 26, 28), but a small study in a Rio de Janeiro hotspot (29) found a higher rate of geographic aggregation (70%). Thus, for our baseline scenario, we estimated that a case of active TB in the hotspot would generate 0.5 secondary transmission events outside the hotspot for every transmission event occurring within the hotspot—a scenario that would lead to 67% geographic clustering among secondary cases if linked to the index case. Because the general community was 16× larger than the hotspot, the relative cross-transmission rate (i.e., rate of transmission to a randomly selected person in the other subpopulation vs. a randomly selected member of the same subpopulation) was 0.5/16 = 0.03. We performed sensitivity analyses for the extremes of complete geographic isolation (i.e., relative cross-transmission rate of zero) and one hotspot-to-community transmission event for every hotspot-to-hotspot transmission event (relative rate of 1/16, leading to 50% geographic clustering).
Model parameters were fit using epidemiological data from Rio de Janeiro (Table 1). We mapped model parameters to epidemiological data points in one-to-one fashion, using an iterative routine (30) to fit the model to the epidemiology of TB in Rio de Janeiro in 2007–2009, the last years for which comprehensive data were available. For simplicity in illustration and mathematical rigor, we assumed an equilibrium (steady-state) epidemic at baseline, defining equilibrium as <1% relative (or 1 per 100,000 absolute) change in any epidemiological data point in Table 1 over 5 y, and adequate model fit as matching each data point in Table 1 to within 1% of its recorded value. In Rio de Janeiro, both HIV and TB incidence have remained within a ±10% range over the last 4 y for which data are available, supporting the concept of near equilibrium (31).
Table 1.
Model parameters
| Epidemiological data point | Value, Rio de Janeiro | Value, model | Corresponding model parameter | Value in model | Sensitivity analysis range | Source |
| TB incidence, per 100,000 per year | 95.3 | 95.3 | Number of transmissions per case of active TB per year, community | 3.71 | 2.36–4.59 | (1) |
| Proportion of TB incidence in the hotspot | 16.5% | 16.5% | Number of transmissions per case of active TB per year, hotspot | 9.74 | 7.93–11.40 | (1) |
| HIV/TB incidence, per 100,000 per year | 10.7 | 10.7 | Rate of rapid progression after recent infection, HIV-positive, per year | 0.31 | 0.15–0.82 | (2) |
| Rate of slow progression after remote infection, HIV-positive, per year | Held at 25% the rate of rapid progression | (3, 4) | ||||
| TB mortality, per 100,000/y | 5.0 | 5.0 | TB mortality rate, HIV-negative, per year | 0.031 | 0.016–0.047 | (2) |
| HIV/TB mortality, per 100,000/y | 0.7 | 0.7 | TB mortality rate, HIV-positive, per year | 0.074 | 0.041–0.111 | (2) |
| TB prevalence, per 100,000 | 103.1 | 103.1 | TB detection and treatment rate, HIV-negative, per year | 0.87 | 0.79–0.99 | (5) |
| TB detection and treatment rate, HIV-positive, per year | Held at twice the HIV-negative rate | Reflects relative mortality rates | ||||
| HIV prevalence, per 100,000 | 390 | 390 | HIV incidence, per year | 1.5 × 10−4 | 1.0–2.1 × 10−4 | (2) |
| HIV mortality, per 100,000/y | 16.0 | 16.0 | HIV mortality rate (non-TB) per year | 0.026 | 0.001–0.5 | (2) |
| Proportion of retreatment cases | 27.4% | 27.4% | TB relapse rate per year | 0.0083 | 0.0063–0.0187 | (2) |
| Additional parameters not fit to epidemiological data | Relative infectivity of HIV/TB cases | 0.68 | 0–1 | (6) | ||
| Partial immunity to reinfection if latently infected | 0.56 | 0.42–0.70 | (7, 8) | |||
| Duration of recent infection phase | 5 y | 4.2–6.2 y | (9) | |||
| Rate of rapid progression during this phase, HIV-negative, per year | 0.03 | 0.026–0.036 | (9) | |||
| Rate of slow progression of remote TB infection, HIV-negative, per year | 0.0005 | 0.0002–0.0011 | (4) | |||
| Life expectancy | 73 y | 40–100 | (10) | |||
| Relative HIV incidence in hotspot vs. community | 2.13 | 1.0–5.0 | (2) |
After the equilibrium model was constructed, we introduced hypothetical control interventions as described in Results. Our primary outcome was the projected reduction in TB incidence at 5 y after reducing the rate of TB transmission per active case in the hotspot to the mean rate in the general population. Because we did not seek to evaluate specific control interventions, the mechanism of achieving this reduction in transmission (e.g., general improvement in living conditions vs. TB-specific control measures) was not specified.
We performed one-way sensitivity analyses on all model parameters by determining the parameter value that would generate a 25% change (increase or decrease) in its corresponding epidemiological data point after reaching a new equilibrium (defined as the passage of 50 y). Because model sensitivity to parameters describing TB transmissibility was qualitatively greater than to other parameters, we performed an a priori three-way sensitivity analysis on the size of the hotspot, intensity of the hotspot (i.e., relative rate of hotspot-to-hotspot vs. community-to-community transmission), and relative cross-population TB transmission rate (i.e., hotspot-to-community vs. hotspot-to-hotspot). To estimate the remaining uncertainty related to the values of other model parameters, we performed best- and worst-case scenario analysis on the primary outcome, simultaneously setting all nontransmissibility related parameters to their most- and least-favorable values, respectively. We compared these results to those of probabilistic uncertainty analysis, where we simultaneously (using Latin hypercube sampling) varied all parameters other than the relative TB transmission rate over a β distribution (α = 2) defined by the range in Table 1, taking the most likely value as the mode. We report the 95% uncertainty range as the 2.5th and 97.5th percentile of results from 10,000 such simulations. We assessed structural uncertainty by comparing results of our model to those of parallel models incorporating three separate, noncommunicating hotspots as above, and also by excluding compartments related to HIV coinfection.
Supplementary Material
Acknowledgments
This work was supported in part by the Bill and Melinda Gates Foundation Grant 19790.01, Consortium to Respond Effectively to the AIDS/TB Epidemic.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203517109/-/DCSupplemental.
References
- 1.World Health Organization . Global Tuberculosis Control: WHO Report 2011. Geneva: WHO; 2011. [Google Scholar]
- 2.Munch Z, et al. Tuberculosis transmission patterns in a high-incidence area: A spatial analysis. Int J Tuberc Lung Dis. 2003;7:271–277. [PubMed] [Google Scholar]
- 3.Spence DP, Hotchkiss J, Williams CS, Davies PD. Tuberculosis and poverty. BMJ. 1993;307:759–761. doi: 10.1136/bmj.307.6907.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaisson RE, Martinson NA. Tuberculosis in Africa—combating an HIV-driven crisis. N Engl J Med. 2008;358:1089–1092. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]
- 5.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Thomas JC, Tucker MJ. The development and use of the concept of a sexually transmitted disease core. J Infect Dis. 1996;174(Suppl 2):S134–S143. doi: 10.1093/infdis/174.supplement_2.s134. [DOI] [PubMed] [Google Scholar]
- 7.Woolhouse ME, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousema T, et al. Hitting hotspots: Spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JY, et al. Prediction of the tuberculosis reinfection proportion from the local incidence. J Infect Dis. 2007;196:281–288. doi: 10.1086/518898. [DOI] [PubMed] [Google Scholar]
- 10.Cohen T, Colijn C, Finklea B, Murray M. Exogenous re-infection and the dynamics of tuberculosis epidemics: Local effects in a network model of transmission. J R Soc Interface. 2007;4:523–531. doi: 10.1098/rsif.2006.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colijn C, Cohen T, Murray M. Emergent heterogeneity in declining tuberculosis epidemics. J Theor Biol. 2007;247:765–774. doi: 10.1016/j.jtbi.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schinazi RB. On the role of reinfection in the transmission of infectious diseases. J Theor Biol. 2003;225:59–63. doi: 10.1016/s0022-5193(03)00220-0. [DOI] [PubMed] [Google Scholar]
- 13.Devi S. Innovation and education improve health in Rio’s favelas. Lancet. 2010;376:83–84. doi: 10.1016/s0140-6736(10)61064-1. [DOI] [PubMed] [Google Scholar]
- 14.Maciel EL, et al. Spatial patterns of pulmonary tuberculosis incidence and their relationship to socio-economic status in Vitoria, Brazil. Int J Tuberc Lung Dis. 2010;14:1395–1402. [PMC free article] [PubMed] [Google Scholar]
- 15.Uys P, Marais BJ, Johnstone-Robertson S, Hargrove J, Wood R. Transmission elasticity in communities hyperendemic for tuberculosis. Clin Infect Dis. 2011;52:1399–1404. doi: 10.1093/cid/cir229. [DOI] [PubMed] [Google Scholar]
- 16.Kritzinger FE, et al. No decrease in annual risk of tuberculosis infection in endemic area in Cape Town, South Africa. Trop Med Int Health. 2009;14:136–142. doi: 10.1111/j.1365-3156.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 17.Boehme CC, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boehme CC, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans CA. GeneXpert—a game-changer for tuberculosis control? PLoS Med. 2011;8:e1001064. doi: 10.1371/journal.pmed.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowdy DW, Cattamanchi A, Steingart KR, Pai M. Is scale-up worth it? Challenges in economic analysis of diagnostic tests for tuberculosis. PLoS Med. 2011;8:e1001063. doi: 10.1371/journal.pmed.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verver S, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004;363:212–214. doi: 10.1016/S0140-6736(03)15332-9. [DOI] [PubMed] [Google Scholar]
- 22.Guwatudde D, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Classen CN, et al. Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax. 1999;54:136–140. doi: 10.1136/thx.54.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray EJ, et al. A multidisciplinary method to map potential tuberculosis transmission ‘hot spots’ in high-burden communities. Int J Tuberc Lung Dis. 2009;13:767–774. [PubMed] [Google Scholar]
- 25.Bishai WR, et al. Molecular and geographic patterns of tuberculosis transmission after 15 years of directly observed therapy. JAMA. 1998;280:1679–1684. doi: 10.1001/jama.280.19.1679. [DOI] [PubMed] [Google Scholar]
- 26.Easterbrook PJ, et al. High rates of clustering of strains causing tuberculosis in Harare, Zimbabwe: A molecular epidemiological study. J Clin Microbiol. 2004;42:4536–4544. doi: 10.1128/JCM.42.10.4536-4544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda AE, et al. Tuberculosis and AIDS co-morbidity in Brazil: Linkage of the tuberculosis and AIDS databases. Braz J Infect Dis. 2009;13:137–141. doi: 10.1590/s1413-86702009000200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alland D, et al. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 29.Mendes JM, et al. Molecular diversity of Mycobacterium tuberculosis strains in a slum area of Rio de Janeiro, Brazil. J Bras Pneumol. 2008;34:1063–1068. doi: 10.1590/s1806-37132008001200012. [DOI] [PubMed] [Google Scholar]
- 30.Dowdy DW, Chaisson RE, Maartens G, Corbett EL, Dorman SE. Impact of enhanced tuberculosis diagnosis in South Africa: A mathematical model of expanded culture and drug susceptibility testing. Proc Natl Acad Sci USA. 2008;105:11293–11298. doi: 10.1073/pnas.0800965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Municipal Secretary of Health and Civil Defense . Epidemiological Bulletin (Coordination of Infectious Diseases: AIDS, Tuberculosis, Leprosy) Rio de Janeiro: City of Rio de Janeiro; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.