Abstract
The Cupressaceae clade has the broadest diversity in habitat and morphology of any conifer family. This clade is characterized by highly divergent physiological strategies, with deciduous swamp-adapted genera-like Taxodium at one extreme, and evergreen desert genera-like Cupressus at the other. The size disparity within the Cupressaceae is equally impressive, with members ranging from 5-m-tall juniper shrubs to 100-m-tall redwood trees. Phylogenetic studies demonstrate that despite this variation, these taxa all share a single common ancestor; by extension, they also share a common ancestral habitat. Here, we use a common-garden approach to compare xylem and leaf-level physiology in this family. We then apply comparative phylogenetic methods to infer how Cenozoic climatic change shaped the morphological and physiological differences between modern-day members of the Cupressaceae. Our data show that drought-resistant crown clades (the Cupressoid and Callitroid clades) most likely evolved from drought-intolerant Mesozoic ancestors, and that this pattern is consistent with proposed shifts in post-Eocene paleoclimates. We also provide evidence that within the Cupressaceae, the evolution of drought-resistant xylem is coupled to increased carbon investment in xylem tissue, reduced xylem transport efficiency, and at the leaf level, reduced photosynthetic capacity. Phylogenetically based analyses suggest that the ancestors of the Cupressaceae were dependent upon moist habitats, and that drought-resistant physiology developed along with increasing habitat aridity from the Oligocene onward. We conclude that the modern biogeography of the Cupressaceae conifers was shaped in large part by their capacity to adapt to drought.
Keywords: cavitation resistance, photosynthesis, plant water transport, xylem structure
Global climate underwent dramatic changes ∼33.7 Mya when a warm and almost universally equable world rapidly became much colder and drier (1–3). This shift is attributed in part to a dramatic decrease in atmospheric CO2 that caused global mean temperatures to drop 3–4 °C in only ∼300,000 years (4, 5). As might be expected, the subsequent distribution of plants and animals changed dramatically (5–7), leading to the development of entirely new biomes, such as grasslands (8) and cactus-dominated deserts (9). Other groups of plants became restricted to habitats that reflected the previous tropical conditions of their ranges (10, 11). However, it is rare to find groups that reflect adaptation to both the Cretaceous-Early Paleogene greenhouse and the cooler, drier post-Eocene world. The Cupressaceae is an exception, known for both moisture-loving paleoendemics, such as the Dawn Redwood (Metasequoia glyptostroboides), and exceptionally drought-tolerant desert trees, such as Juniperus californica and the South African Widdringtonia cedarbergensis. Here, we offer an analysis that was aimed at unraveling the physiological underpinnings of these two dramatically different lineages, and show that they were the likely result of Cenozoic climate change.
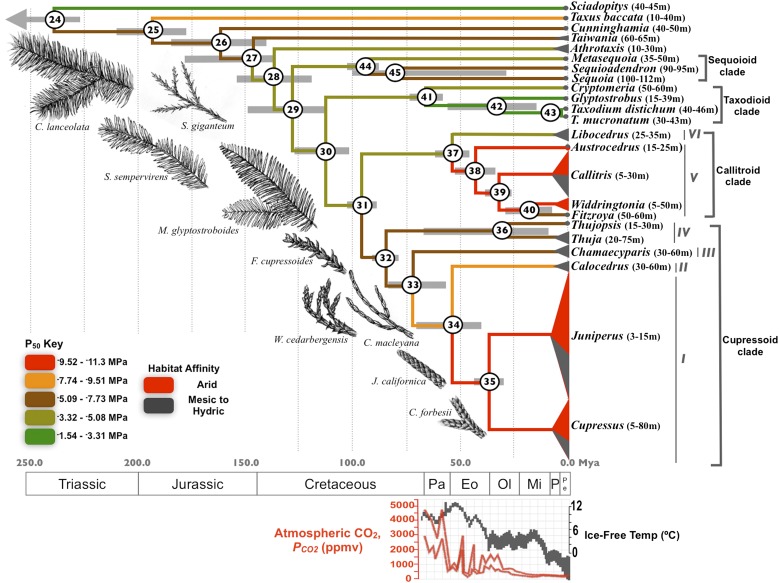
The Cupressaceae family is remarkable for the diversity of morphology, phenology, and habitat preference exhibited by its members, and it is the only conifer family with a circumglobal distribution. The phylogenetic structure of the Cupressaceae suggests that mesic-hydric habitats are likely ancestral within this group. Indeed, early-diverging Cupressaceae—such as Cunninghamia, Taiwania, Metasequoia, and Sequoia—occupy mesic habitats, and close relatives—such as Glyptostrobus and Taxodium—can tolerate water-logged soils. In contrast, many members of the two crown clades, the Cupressoids and Callitroids (Fig. 1), are adapted to arid climates across the northern and southern hemispheres, respectively, and also comprise most of the species within the Cupressaceae. Shorter and often shrub-like, these conifers exhibit small, imbricate leaves that minimize water loss (Fig. 1) (12, 13).
Fig. 1.
The evolutionary trajectory of cavitation resistance (P50) in the Cupressaceae over deep time. Branch colors indicate ancestral reconstruction of P50 values. Clades are identified according to Gadek et al. (13) and the Cenozoic climate data are replotted from Zachos et al. (4). Node bars represent 95% credible intervals at each node. Tip expansions approximate the number of species found in each of the major clades per Farjon (12) (Cupressus n = 16, Juniperus n = 52, Callitris n = 15) and the proportion of species found in arid habitats (see text). Branch tips without expansions indicate monotypic genera. The node numbers correspond to the contrasts data in Fig. 3. Sketches provide examples of polymorphic foliage in the Cupressaceae with appressed, imbricate leaves found in more arid-adapted taxa. The height ranges of adult trees are presented alongside genus names (12).
Temperature and water availability constrain the distribution of most woody plants, primarily because the xylem must balance efficient water delivery against safety from water stress. For example, prolonged dehydration can cause water potential to become negative enough to cause air to leak into xylem conduits (cavitation). In conifers, cavitation occurs when air is sucked into a water-filled conduit through a broken seal in a pit membrane (14, 15). This air expands (embolism) and replaces the water in the conduit. Air-filled conduits can significantly impair xylem conductivity, so selection favors vascular traits and leaf phenologies that optimize water transport with respect to expected water deficits in any given environment (16–18).
Given the well-resolved phylogeny (12, 13, 19, 20), deep fossil record (21–24), global distribution, and morphological diversity of the Cupressaceae, this family presents a rare opportunity to investigate how comparative physiology evolved with respect to the Cenozoic paleoclimate in a woody plant lineage. Here, we use a “common garden” approach, standardizing the plants’ growing environment by sampling the hydraulic and gas-exchange response of individuals growing in San Francisco Bay Area arboreta (Table 1 and Table S1). By analyzing standardized physiological data within a phylogenetic framework, this study fills an important gap in our mechanistic understanding of how selection shaped the biogeography of the Cupressaceae since the Mid-Jurassic, and demonstrates that retention of ancestral character traits may explain the present limited distribution of the early-diverging Cupressaceae.
Table 1.
Study species, figure abbreviations, and species’ natural history
| Species | Abbr. | Phenology, native habitat |
| Athrotaxis laxifolia | AL | E, Montane forests, Tasmania |
| Austrocedrus chilensis | AC | E, The Andes, Chile and Argentina |
| Calocedrus decurrens | CD | E, Montane forests, United States–Mexican Pacific Coast |
| Chamaecyparis lawsoniana | CL | E, Mixed forests, Oregon to northern California |
| Callitris macleayana | CM | E, Mesic-dry forests, eastern Australia |
| Calocedrus decurrens | CD | E, Mixed forests, Oregon to northern Mexico |
| Cryptomeria japonica | CJ | E, Mixed evergreen forests, Japan |
| Cunninghamia lanceolata | CL | E, Mixed broad-leaved forests of southeast Asia |
| Cupressus forbesii | CF | E, Chaparral, southern California, northern Mexico |
| Fitzroya cupressoides | FC | E, Evergreen rainforest, Chile |
| Glyptostrobus pensilis | GP | D, Riparian, southern China |
| Juniperus californica | JC | E, Desert, southern California to northern Mexico |
| Libocedrus plumosa | LP | E, Mixed conifer rainforests, New Zealand |
| Metasequoia glyptostroboides | MG | D, Mesic mixed forests, central China |
| Sequoiadendron giganteum | SG | E, Sierra Nevada, California |
| Sequoia sempervirens | SS | E, northern coastal California |
| Taxodium distichum | TD | D, Riparian regions in southeastern United States |
| Taxodium mucronatum | TM | D, Southern Texas, Mexico, Central America |
| Taiwania cryptomeroides | TC | E, Cool temperate forests, Asia |
| Thuja plicata | TP | E, Mixed coniferous forests, United States Pacific northwest |
| Thujopsis dolabrata | TO | E, Coastal and montane Japan |
| Widdringtonia cedarbergensis | WC | E, Fynbos, South Africa |
| Sciadopitys verticillata (Sciadopityaceae) | SC | E, Temperate moist forests, Japan |
| Taxus baccata (Taxaceae) | TB | E, Broadly distributed across Europe |
D, deciduous; E, evergreen.
Results
We first characterized the climate affinities of all extant Cupressaceae according to detailed descriptions of species habitat (12). Eighty-one percent of Cupressus and 69% of Juniperus, the two most speciose genera, are found in climates with arid conditions during the growing season (Fig. 1, subclade I). Similarly, 57% of species within the crown Callitroids (Fig. 1, subclade V) have xeric associations. Of the remaining Callitroid and Cupressoid species, the majority inhabit mesic habitats. With the exception of Sequoiadendron giganteum, the early-diverging Cupressaceae occupy chiefly mesic-hydric habitats.
We used the centrifuge method of inducing cavitation to determine the water potential at which xylem exhibits a 50% loss of hydraulic conductivity due to embolism (17) (P50). Even when grown under common conditions, species’ P50 values reflected the water availability of their native habitats. For example, the riparian Glyptostrobus pensilis had a P50 of −2.8 ± 0.62 MPa (mean ± SD), but W. cedarbergensis, a xeric species, showed extreme cavitation resistance of −11.27 ± 3.52 MPa (Fig. 2). A strong genetic influence was also observed in the relationship between P50 and the minimum growing-season precipitation of these species’ native ranges (r2 = 0.34, P = 0.0025) (Fig. S1). We would expect a stronger correlation between P50 and climate if the hydraulic measurements were performed on plants collected from their native habitats.
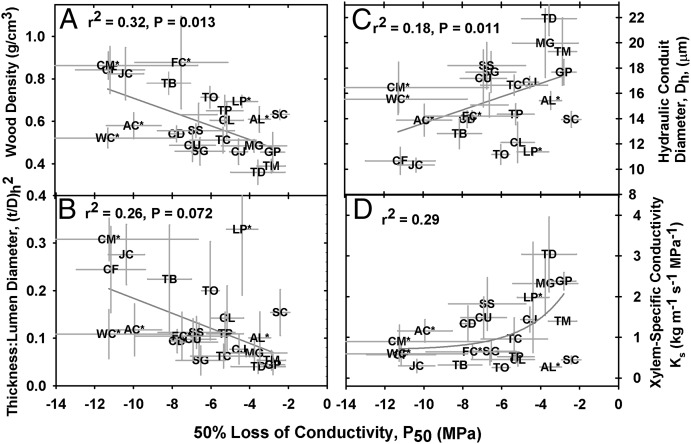
Fig. 2.
The relationship between cavitation resistance and wood density (A), tracheid double-wall thickness:lumen diameter (B), hydraulic conduit diameter (C), and xylem-specific conductivity (D) in the Cupressaceae (means ± SD). T. baccata and S. verticillata were not included in the analysis. Asterisks indicate Cupressaceae taxa endemic to habitats in the southern hemisphere.
In north-temperate conifers, a key cost of xylem adaptation to drought stress is the need for increased wood density, which represents a significant carbon investment per unit height gained (Fig. 2A) (25, 26). Dense wood arises from the need for increased conduit implosion resistance [(t/D)h2] to prevent conduit collapse under very negative xylem tensions. Increased (t/D)h2 is achieved through both thicker conduit walls (t) and reduction in hydraulic conduit diameter (Dh), although Dh typically plays a larger role (Fig. 2 B and C) (26). As a result, cavitation-resistant species typically show a greater number of smaller, thick-walled conduits per unit xylem area, resulting in denser, more costly wood (Fig. S2).
Given that drought-resistance necessitates the development of smaller conduits, it follows that xylem-specific conductivity (Ks) declines with P50 (Fig. 2 C and D), representing a significant transport cost associated with adaptation to arid habitats. Fig. 2 shows the correlations between P50, Dh, and Ks for the combined northern and southern hemisphere Cupressaceae, but the strength of these relationships increases considerably if only the north temperate taxa are considered (for example, P50 vs. Dh: r2 = 0.51, P = 0.017; P50 vs. Ks: r2 = 0.43, P = 0.019). This distinction was observed for many of the anatomical and physiological data presented here, as well as in other studies concerned with conifer xylem structure and function, but the reasons for this remain unclear (26, 27).
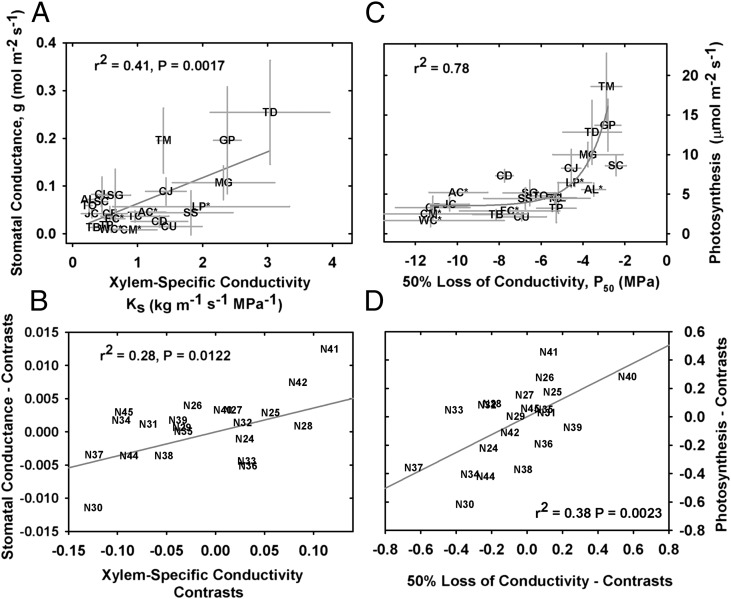
Leaf stomatal conductance (g) is closely coupled with Ks in the Cupressaceae (Fig. 3A) because xylem efficiency places a limit on water availability to the leaf, thus constraining maximum leaf water loss (Fig. S3) (28). Leaf and stem capacitance can modulate the stomatal response in situ (29, 30), but our measurements support the hydraulic coordination of xylem and leaf-level water transport, with cavitation-resistant species exhibiting lower rates of g because of narrow, implosion-resistant conduits. Analysis of phylogenetically independent contrasts (PICs) shows evolutionary coordination of g and Ks (r2 = 0.28, P = 0.0122) (Fig. 3B). We observed a dramatic reduction in CO2 assimilation rates in more cavitation-resistant Cupressaceae (Fig. 3C) (r2 = 0.78) because of the coupling between g and photosynthesis (r2 = 0.79, P < 0.0001) (Fig. S4). PIC analyses suggest that ∼40% of variation can be explained by coevolution between P50 and photosynthesis (r2 = 0.38, P = 0.0023) (Fig. 3D).
Fig. 3.
The relationship between leaf- and xylem-level traits in the Cupressaceae. Leaf stomatal conductance is positively correlated with stem water supply, Ks (A; means ± SD). Reduced photosynthesis rates arise from the inverse relationship between P50 and Ks, and by extension, g (C; see text for details). Asterisks indicate Cupressaceae taxa endemic to habitats in the southern hemisphere. PIC analyses (B and D) demonstrate the correlated evolution of leaf and xylem traits. Each independent contrast corresponds to trait divergence at a specific node; node numbers shown here (labeled “N”) correspond to nodes, or phylogenetic divergence events, in Fig. 1.
The rich Cupressaceae macrofossil record offers the opportunity to examine these results in the context of deep-time approximations of clade divergence (Table S2), allowing us to present a hypothesis for the evolution of physiological traits against a backdrop of Cenozoic climate. Ancestral reconstruction of P50 on the phylogenetic topology recovered here indicates that adaptation to mesic and riparian environments with high water availability is ancestral within the Cupressaceae. This reconstruction also suggests at least two separate evolutions of adaptations to arid environments, one within the Cupressoids (Fig. 1, subclades I–IV) and one within the Callitroids (Fig. 1, subclades V and VI). The earliest-diverging members within each of these clades have more mesic habitat preferences than the crown groups, supporting this conclusion. The 95% highest posterior density interval for the divergence between Juniperus and Cupressus (node 35) is 38.68–31.98 Mya, indicating that diversification within these two groups began after that time. Diversification of the arid-adapted Callitroids begins at node 38, which has a 95% highest posterior-density interval of 52.58–33.92 Mya. Both time intervals include the Eocene-Oligocene boundary (∼33.7 Mya), suggesting that the relatively high species diversity observed in each clade developed after this time.
Discussion
The macrofossil record indicates that the morphology of the Cupressaceae has remained mostly static for the past 70 million years (21–24). Both the coupling of form and function, and the ancestral reconstructions presented here, support the view that the physiology of early-diverging clades is to a large degree conserved. In contrast, the Cupressoid and Callitroid clades show adaptive shifts in xylem and leaf-level physiology that are consistent with expansion into increasingly arid, late Cenozoic habitats.
Reconstructions of Cretaceous climates concur that warm temperatures, elevated levels of atmospheric CO2, and minimal glaciation characterized this period in the Earth’s history, as well as much of the Paleocene and Eocene climates (1–4, 31, 32). The Cupressaceae have their origins in the mid-Mesozoic, with taxa such as Elatides williamsonii appearing in the Jurassic and Sphenolepis kurriana appearing in the Cretaceous. Consistent with this, ancestral reconstructions suggest that early members of the clade likely exhibited increased vulnerability to drought-induced cavitation, and affinities for habitats with higher precipitation (Figs. 1–3, Fig. S5, and SI Text).
Similarly, close relatives of the Taxodioids and Sequoioids were most abundant during the Cretaceous and Paleocene, with populations occupying the once humid, warm climates of the high northern latitudes (21–24, 33). Most early-diverging Cupressaceae, such as M. glyptostroboides and Sequoia sempervirens, have experienced dramatic range contractions since the Paleocene, and their present habitats are thought to be relictual (12, 21–23). These range reductions have been attributed to the cooling and drying of the global climate that followed the early Eocene climatic optimum (4, 5, 23). Consistent with this finding, paleosol evidence documents a transition from forest to woodland to grassland during the Eocene and Oligocene in the Eastern Rockies (7, 34), as well as Old and New World vegetation shifts during the Miocene (6, 8, 9).
With its rich fossil record, the once-abundant Metasequoia is a useful model for understanding the climate-related biogeographical patterns of early-diverging Cupressaceae. In its narrow present-day range in central China, Metasequoia occupies a moderate climate with an average 950–2,300-mm annual precipitation (21, 23). This finding is consistent with this species’ vulnerability to cavitation (P50 = −3.76 ± 1.67 MPa). Planted Metasequoia trees grow well in Alaska and parts of Canada, which suggests that this species is tolerant of freezing temperatures, although cool temperatures may affect seed germination (21, 23). We suggest, therefore, that the drought sensitivity of Metasequoia, like that of other basal Cupressaceae, may explain in part the dramatic diminution of its population range since the Late Cretaceous (21–23).
In contrast, the geographic expansion and diversification of the arid-adapted Cupressoids and Callitroids occurred over the same time period (12, 13, 22, 35). We hypothesize that the physiological shift toward greater drought tolerance, specifically cavitation resistance, observed in these lineages (15, 36, 37) allowed them to radiate into increasingly dry environments during the Mid- to Late-Cenozoic. Although this trend was observed in both Northern and Southern Hemisphere Cupressaceae, the expansion and speciation of Juniperus and Cupressus across North America, Europe, and North Africa is particularly dramatic, with over 70% of subclade I species exhibiting xeric affinities (Fig. 1) (12). Juniperus is the most widely distributed member of the Cupressaceae in North America, and its rapid diversification during the Miocene can be attributed in some part to its inherent drought resistance (19, 36). In contrast, early-diverging mesic or hydric taxa within each of the Cupressoid and Callitroid clades have also been restricted to climatically favorable refugia: examples include Chamaecyparis, Fitzroya, and Thuja, all of which once occupied much broader ranges (12, 22).
The drought tolerance of the Cupressoid and Callitroid lineages came at the expense of reduced hydraulic efficiency and lower rates of photosynthesis (Figs. 1–3), and these costs arise from anatomical, tissue, and whole-plant adjustments. At the level of the interconduit pit membrane, increasing cavitation resistance is coupled with smaller pit apertures, the cost of which is up to a sixfold reduction in pit conductivity (15). Within the xylem tissue, a more negative P50 exerts significant carbon and hydraulic penalties, both of which contribute to reduced photosynthetic capacity. Finally, imbricate leaves are favored in drier habitats over bilaterally flattened foliage, a trend that is consistent with adjustments in sapwood to leaf-area ratios that prevent xylem tensions from becoming low enough to cause cavitation (38). Taken together, these data indicate that the cost of survival in arid environments is slow growth, a trend reflected by a reduction in stature from the tall early-diverging Cupressaceae to the often shrubby, arid-adapted Callitroids and Cupressoids (Fig. 1).
It is less clear how other conifer lineages responded to Cenozoic climate change, but some were apparently excluded from the newly developed arid habitats. In the southern hemisphere for example, members of the Podocarpaceae were subject to selective pressures arising from increasing aridity as well as greater ecological competition from angiosperms (10, 11, 39). Several of the drought-intolerant Podocarp lineages disappeared during the mid-to-late Cenozoic, and others found refugia in mesic tropical forests by evolving flattened leaves that allowed them to compete with angiosperms for light (10, 11, 39). Although the Podocarpaceae retreated, we speculate that Callitroid lineages may have succeeded in colonizing available arid habitats. In the northern hemisphere, the Oligocene saw a dramatic reduction in the relative abundance of Metasequoia (and perhaps other mesic Cupressaceae) as the Pinaceae became increasingly dominant (21, 23). Indeed, the Pinaceae is a family whose members exhibit fast growth and high reproductive output combined with a tolerance to freezing temperatures, drought, and fire (40, 41). There is evidence that post-Eocene climate deterioration shaped the biogeography of all conifer families, not just the Cupressaceae, and that conifer persistence depended on proximity to refugia, shade tolerance, and in the case of the Pinaceae, aggressive life-history strategies. The extent to which competition, nutrient acquisition, dispersal, and geologic events have shaped the relative abundances of the Earth’s conifers will require further study.
The physiological data presented here accord well with other data suggesting that the Cenozoic was an era of large-scale habitat sorting that saw angiosperm success in climatically favorable regions, while conifers competed more effectively in marginal habitats (42–44). The tremendous success of the angiosperms can be partially attributed to their developmentally flexible and hydraulically efficient xylem, which supports a high degree of morphological variation and rapid growth, something the conifers never have been able to match (42–44). Because of their low tolerance to drought and limited ability to compete with angiosperms, we suspect that the continued presence of the early-diverging Cupressaceae may be attributed to their longevity and to once-abundant distributions that increased the probability of their occurrence in refugial habitats.
In conclusion, our data indicate that drought-adapted lineages within the Cupressaceae have undergone profound vascular structure-function adjustments consistent with their presumed radiation into the increasingly dry habitats of post-Eocene climates. This radiation was coupled with greater cavitation resistance but at the cost of increased investment in xylem and reduced rates of photosynthesis. In contrast, ancestral reconstruction suggests that the narrowly distributed Taxodioids and Sequoioids have retained their ancestral leaf and xylem features, and that the physiology of these plants has changed little. Consequently, we propose that the dramatic range reduction of the mesic Cupressaceae in response Cenozoic climate change can be explained in part by physiological limitations, such as low resistance to drought-induced cavitation.
It is rare that a single group of related organisms offers an opportunity to examine physiology on both sides of a dramatic shift in global climate. The Cupressaceae, with its combination of morphologically static mesic paleoendemics and its radiation into newer arid environments, provides a chance to look at evolution in progress. Future generations may witness the limits of adaptive flexibility in this ancient plant lineage as greenhouse gas-induced climate change brings warmer temperatures and altered hydrologic regimes to the Anthropocene world (45, 46).
Materials and Methods
Plant Material.
Twenty-one Cupressaceae species, plus Sciadopitys verticillata and Taxus baccata, were collected from arboreta on California’s central coast (Table S1). Specimens grew in close proximity to scheduled irrigation systems but were not watered preferentially. The climate is characterized by mild temperatures, wet winters, abundant summer fog, and very rare freezing events [San Francisco, mean annual temperature (MAT), 13 ± 0.23 °C, mean annual precipitation (MAP), 502 ± 150 mm; Berkeley MAT: 13.9 ± 0.23 °C, MAP: 599 ± 196 mm; Santa Cruz MAT: 13.8 ± 0.25 °C, MAP: 763 ± 243 mm). Juvenile stems ranging from 5 to 8 mm in diameter were collected from sun-exposed regions of mature tree canopies from heights of 1.5–4 m. Five to eight branches were clipped from two to six individuals (depending on permission). Species were randomly sampled from April to August from 2006 to 2009.
Hydraulic Measurements.
Hydraulic measurements (Ks) were made according to standard plant hydraulic protocols (15, 18, 26). Species’ cavitation resistance (P50) was determined using the centrifuge method and the curves fit with a Weibull Function (26, 27). Conduit diameter distributions and (t/D)h2 measurements were performed according to standard anatomical methods (18, 25, 26).
Gas-Exchange Measurements.
Gas-exchange measurements were performed in June 2010 on the same individuals sampled for hydraulic and anatomical data. We found no significant differences in conductivity or P50 in a subsample of the plants sampled in 2010 vs. 2006–2009.
Sun-exposed twigs (5–15 mm diameter) were clipped from the tree, reclipped under water, and the cut ends smoothed with a razor blade. To ensure maximum hydration, the twigs were placed in water overnight, uncovered. Water potentials ranged from −0.03 to −0.75 MPa when gas-exchange measurements were obtained (11:00 AM–3:00 PM).
A Li-Cor LI-6400XT instrument (Li-Cor Biosciences) with an opaque 2 × 3 cm LED-lighted chamber was used to obtain leaf gas-exchange measurements. Each measurement took between 4 and 6 min with leaf temperature at 22.2 ± 0.8 °C, the light level at 2,000 μmol·m−2·s−1, and a flow rate of 300 mL·min−1 with the CO2 mixer set to 400 μmol·m−2·s−1. Projected leaf area measurements were used across all species.
Species’ Habitat Characterization.
The precipitation regimes for each of the sampled Cupressaceae represent averaged data collected from climate stations located within each species’ known range, detailed with maps and observations by Farjon (12) (Fig. S1). The coordinates of species’ distributions were manually identified using Google Maps and reconciled with the global-regional climate database of Peel et al. (47) (http://www.hydrol-earth-syst-sci.net/11/1633/2007/hess-11-1633-2007-supplement.zip; last accessed in July 2011), who also categorize climate according to standard Koppen–Geiger criteria. This database contains long-term monthly precipitation and temperature values reported alongside station coordinates and elevation. Elevation data, slope, and aspect for the Cupressaceae observations are not provided, and Peel et al.’s (47) climate data may not be from stations in close proximity to these locations. Species may naturally occur outside of our conservative estimates of their climate envelope.
We also relied on Peel et al.’s climate criteria (47) and detailed species’ habitat descriptions (12) for species’ assignments of arid- or mesic/hydric-adapted Cupressaceae in Fig. 1.
Phylogenetic Reconstruction.
Sequences for two molecular markers (matK and rbcL) were downloaded from GenBank (see Table S3 for accession numbers). Sequences were aligned with ClustalW using default settings and concatenated in Mesquite (48, 49). Topology and branch lengths were reconstructed simultaneously using BEAST and interpreted using associated programs (50–52). Each gene was included in a separate partition. Substitution models were allowed to vary separately for each partition. A general time-reversible model of nucleotide substitution with a four-category γ + invariant model of site heterogeneity was used, with empirical base frequencies. A single linked uncorrelated lognormal clock model was estimated for both partitions jointly. Speciation was estimated from a combined birth-death model. Two separate runs of 10,000 samples each from 2,500,000 generations were combined to generate the final phylogeny.
Fossil calibrations drew on a variety of sources (Table S2). Fossils with high resolution dating were rarely available and the average length of the date range was ∼19 Mya. At the same time, the ecological habit of several extant and many fossil Cupressaceae is near freshwater swamps, a taphonomic environment with a relatively high likelihood of preservation. For several specimens, there is therefore a high probability of rapid incorporation into the fossil record, and a relatively large uncertainty about the exact time of incorporation; in light of this, we chose to use normally distributed calibration priors with a mean centered on the middle of the geological period from which the fossil was recovered, and a SD of one-half of the length of the period.
Comparative Phylogenetic Analyses.
The phylogeny was imported into the R statistical environment (R Development Core Team, 2008) using the APE package (54) and the physiological data presented in this article were analyzed using PICs (53). No contrast was calculated for differences between Taxodium mucronatum and Taxodium distichum because of the very short branches diverging from this node. Only data for T. distichum was included in the PIC analysis and figures presented. PICs compare the amount of divergence in trait values that has occurred between branching events. Each value calculated corresponds to the difference in trait values associated with a particular node. In figures, we label each contrast with the number corresponding to its associated node in the phylogeny shown in Fig. 1. Further statistical analysis was conducted on the resulting contrasts using APE and Picante (55). R scripts used are available from S.A.S. upon request. Ancestral trait reconstruction of P50 values was conducted in Mesquite, using a maximum parsimony model. Reconstructed ancestral states are shown as the color of branches leading to each node in Fig. 1.
Supplementary Material
Acknowledgments
We thank the following individuals for their excellent assistance: Holly Forbes and Barbara Keller (University of California Botanical Garden, Berkeley, CA); Tony Morosco, Mona Bourell, and David Kruse-Pickler (Strybing Arboretum, San Francisco Botanical Garden); Deborah Knotts (Japanese Tea Garden, Golden Gate Park, San Francisco); Brett Hall and Steve McCabe (University of California Santa Cruz Arboretum; Emily Limm, Sergei Vickulin, Paul Brooks, Stefania Mambelli, David Barajas, Lucy Lynn, and Chris Rico for assistance with data collection and other phases of the project; and Andrew Knoll and three anonymous reviewers for their insightful comments on the manuscript. Funding was provided by the Miller Institute for Basic Research at the University of California at Berkeley (J.P. and T.E.D.) and University of California Santa Cruz Start-Up funds (J.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114378109/-/DCSupplemental.
References
- 1.Spicer RA, Chapman JL. Climate change and the evolution of high-latitude terrestrial vegetation and floras. Trends Ecol Evol. 1990;5:279–284. doi: 10.1016/0169-5347(90)90081-N. [DOI] [PubMed] [Google Scholar]
- 2.Sluijs A, et al. Warm and wet conditions in the Arctic region during Eocene Thermal Maximum 2. Nat Geosci. 2009;2:777–780. [Google Scholar]
- 3.Breecker DO, Sharp ZD, McFadden LD. Atmospheric CO2 concentrations during ancient greenhouse climates were similar to those predicted for A.D. 2100. Proc Natl Acad Sci USA. 2010;107:576–580. doi: 10.1073/pnas.0902323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zachos JC, Dickens GR, Zeebe RE. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature. 2008;451:279–283. doi: 10.1038/nature06588. [DOI] [PubMed] [Google Scholar]
- 5.Zanazzi A, Kohn MJ, MacFadden BJ, Terry DO. Large temperature drop across the Eocene-Oligocene transition in central North America. Nature. 2007;445:639–642. doi: 10.1038/nature05551. [DOI] [PubMed] [Google Scholar]
- 6.Barreda V, Palazzesi L. Patagonian vegetation turnovers during the Paleogene-early Neogene: Origin of arid-adapted floras. Bot Rev. 2007;73(1):31–50. [Google Scholar]
- 7.Wing SL. Eocene and Oligocene floras and vegetation of the Rocky Mountains. Ann Miss Bot Gar. 1987;74:748–784. [Google Scholar]
- 8.Edwards EJ, et al. C4 Grasses Consortium The origins of C4 grasslands: Integrating evolutionary and ecosystem science. Science. 2010;328:587–591. doi: 10.1126/science.1177216. [DOI] [PubMed] [Google Scholar]
- 9.Arakaki M, et al. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proc Natl Acad Sci USA. 2011;108:8379–8384. doi: 10.1073/pnas.1100628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodribb TJ, Hill RS. In: The Evolution of Plant Physiology. Hemsley AR, Poole I, editors. Elsevier Academic Press, London; 2004. pp. 381–399. [Google Scholar]
- 11.Biffin E, Brodribb TJ, Hill RS, Thomas P, Lowe AJ. Leaf evolution in Southern Hemisphere conifers tracks the angiosperm ecological radiation. Proc Biol Sci. 2011;279:341–348. doi: 10.1098/rspb.2011.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farjon A. A Monograph of Cupressaceae and Sciadopitys. Kew, Richmond, Surrey, UK: Royal Botanic Gardens; 2005. [Google Scholar]
- 13.Gadek PA, Alpers DL, Heslewood MM, Quinn CJ. Relationships within Cupressaceae sensu lato: A combined morphological and molecular approach. Am J Bot. 2000;87:1044–1057. [PubMed] [Google Scholar]
- 14.Sperry JS. Hydraulic constraints on plant gas exchange. Agric For Meteorol. 2000;104:13–23. [Google Scholar]
- 15.Pittermann J, et al. The relationships between xylem safety and hydraulic efficiency in the Cupressaceae: The evolution of pit membrane form and function. Plant Physiol. 2010;153:1919–1931. doi: 10.1104/pp.110.158824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodribb T, Hill RS. The importance of xylem constraints in the distribution of conifer species. New Phytol. 1999;143:364–372. [Google Scholar]
- 17.Pockman WT, Sperry JS, O’Leary JW. Sustained and significant negative pressure in xylem. Nature. 1995;378:715–716. [Google Scholar]
- 18.Jacobsen AL, Pratt RB, Ewers FW, Davis SD. Cavitation resistance among 26 chaparral species of southern California. Ecol Monogr. 2007;77:99–115. [Google Scholar]
- 19.Mao K, Hao G, Liu J, Adams RP, Milne RI. Diversification and biogeography of Juniperus (Cupressaceae): variable diversification rates and multiple intercontinental dispersals. New Phytol. 2010;188:254–272. doi: 10.1111/j.1469-8137.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- 20.Little DP. Evolution and circumscription of the true cypresses (Cupressaceae: Cupressus) Syst Bot. 2006;31:461–480. [Google Scholar]
- 21.LePage BA, Yang H, Matsumoto M. In: The Geobiology and Ecology of Metasequoia. LePage BA, Williams CJ, Yang H, editors. Dordrecht: Springer; 2005. pp. 3–94. [Google Scholar]
- 22.Florin R. The distribution of conifer and taxad genera in time and space. Acta Hort Bergiani. 1963;20:121–312. [Google Scholar]
- 23.Liu Y-J, Arens NC, Li C-S. Range change in Metasequoia: Relationship to palaeoclimate. Bot J Linn Soc. 2007;154:115–127. [Google Scholar]
- 24.Stockey RA, Kvacek J, Hill R, Rothwell GH, Kvacek Z. In: A Monograph of the Cupressaceae and Sciadopitys. Farjon A, editor. Kew, Surrey, UK: Royal Botanic Gardens; 2005. pp. 54–68. [Google Scholar]
- 25.Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia. 2001;126:457–461. doi: 10.1007/s004420100628. [DOI] [PubMed] [Google Scholar]
- 26.Pittermann J, Sperry JS, Wheeler JK, Hacke UG, Sikkema EH. Mechanical reinforcement of tracheids compromises the hydraulic efficiency of conifer xylem. Plant Cell Environ. 2006;29:1618–1628. doi: 10.1111/j.1365-3040.2006.01539.x. [DOI] [PubMed] [Google Scholar]
- 27.Pittermann J, Sperry JS, Hacke UG, Wheeler JK, Sikkema EH. Inter-tracheid pitting and the hydraulic efficiency of conifer wood: The role of tracheid allometry and cavitation protection. Am J Bot. 2006;93:1265–1273. doi: 10.3732/ajb.93.9.1265. [DOI] [PubMed] [Google Scholar]
- 28.Brodribb T. Xylem hydraulic physiology: The functional backbone of terrestrial plant productivity. Plant Sci. 2009;177:245–251. [Google Scholar]
- 29.Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR. Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct Ecol. 2009;23:922–930. [Google Scholar]
- 30.Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003;26:1343–1356. [Google Scholar]
- 31.Crowley TJ, Zachos JC. In: Warm Climates in Earth History. Huber B, MacLeod KG, Wing SL, editors. Cambridge, UK: Cambridge Univ Press; 2001. pp. 50–76. [Google Scholar]
- 32.Barron EJ, Fawcett PJ, Peterson WH, Pollard D, Thompson SL. A “simulation” of Mid-Cretaceous climate. Paleoceanography. 1995;10:953–962. [Google Scholar]
- 33.Beerling DJ, Osborne CP. Physiological ecology of Mesozoic polar forests in a high CO2 environment. Ann Bot (Lond) 2002;89:1–11. doi: 10.1093/aob/mcf045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stromberg CAE. The origin and spread of grass–dominated ecosystems in the Late Tertiary of North America: Preliminary results concerning the evolution of hypsodonty. Palaeogeogr Palaeoclimatol Palaeoecol. 2002;177:59–75. [Google Scholar]
- 35.Crisp MD, Cook LG. Cenozoic extinctions account for the low diversity of extant gymnosperms compared with angiosperms. New Phytol. 2011;192:997–1009. doi: 10.1111/j.1469-8137.2011.03862.x. [DOI] [PubMed] [Google Scholar]
- 36.Willson CJ, Manos PS, Jackson RB. Hydraulic traits are influenced by phylogenetic history in the drought-resistant, invasive genus Juniperus (Cupressaceae) Am J Bot. 2008;95:299–314. doi: 10.3732/ajb.95.3.299. [DOI] [PubMed] [Google Scholar]
- 37.Brodribb TJ, Cochard H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maherali H, DeLucia EH. Influence of climate-driven shifts in biomass allocation on water transport and storage in ponderosa pine. Oecologia. 2001;129:481–491. doi: 10.1007/s004420100758. [DOI] [PubMed] [Google Scholar]
- 39.Brodribb TJ. A functional analysis of podocarp ecology. Smithsonian Contributions to Botany. 2011;95:165–173. [Google Scholar]
- 40.Rejmanek M, Richardson DM. What attributes make some plant species more invasive? Ecology. 1996;77:1655–1661. [Google Scholar]
- 41.Richardson DM. Ecology and Biogeography of Pinus. Cambridge, UK: Cambridge Univ Press; 1998. [Google Scholar]
- 42.Brodribb TJ, Pittermann J, Coomes DA. Impacts of angiosperm evolution on conifers. Int J Plant Sci. 2012 in press. [Google Scholar]
- 43.Bond WJ. The tortoise and the hare: Ecology of angiosperm dominance and gymnosperm persistence. Biol J Linn Soc Lond. 1989;36:227–249. [Google Scholar]
- 44.Feild TS, et al. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc Natl Acad Sci USA. 2011;108:8363–8366. doi: 10.1073/pnas.1014456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnstone JA, Dawson TE. Climatic context and ecological implications of summer fog decline in the coast redwood region. Proc Natl Acad Sci USA. 2010;107:4533–4538. doi: 10.1073/pnas.0915062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon S, Plattner GK, Knutti R, Friedlingstein P. Irreversible climate change due to carbon dioxide emissions. Proc Natl Acad Sci USA. 2009;106:1704–1709. doi: 10.1073/pnas.0812721106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Koppen-Geiger climate classification. Hydrol Earth Syst Sci. 2007;11:1633–1644. [Google Scholar]
- 48.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 49.Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis. Evolution. 2008;62:1103–1118. [Google Scholar]
- 50.Rambaut A, Drummond AJ. 2011. BEAST package v1.6.2, http://beast.bio.ed.ac.uk/Main_Page. Accessed December 1, 2012.
- 51.Rambaut A. 2009. FigTree v1.3.1, http://tree.bio.ed.ac.uk/software/figtree/. Accessed December 1, 2012.
- 52.Rambaut A, Drummond J. 2009. Tracer v1.5, http://tree.bio.ed.ac.uk/software/tracer/. Accessed December 1, 2012.
- 53.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 54.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 55.Kembel SW, et al. 2011. CRAN: Package Picante. Available at http://cran.r-project.org/web/packages/picante/index.html. Accessed December 1, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.