Abstract
Introduction
Pegylated-interferon (PEG-IFN) and ribavirin (RBV), current standard treatment for hepatitis C virus (HCV) infection, is frequently associated with neutropenia and anemia, leading to high treatment discontinuation rates in HIV/HCV coinfected patients. Our objective was to compare the effectiveness of intervening with hematologic growth factors versus dose reductions of standard HCV therapy for the management of treatment-induced hematologic disorders.
Methods
Ninety-two HIV/HCV coinfected, therapy-naive subjects received PEG-IFN alfa-2b 1.5 μg/kg/wk and RBV 13 ± 2 mg/kg/day for up to 48 weeks. Before treatment initiation, subjects were randomized to subsequently receive growth factors, recombinant human erythropoietin (rHuEPO) and/or granulocyte-colony stimulating factor (G-CSF), or dose reduction (RBV and/or PEG-IFN) for anemia and neutropenia management, respectively. We analyzed the ability of each management strategy to control anemia and neutropenia and the percentage of subjects who achieved a successful treatment outcome among subjects according to the different management strategies.
Results
During treatment, 43 subjects developed anemia (HuEPO, n=24; dose reduction, n=19) while 25 subjects developed neutropenia (G-CSF, n=10; dose reduction, n=15). Following the intervention, the increase in both hemoglobin and absolute neutrophil counts also did not differ between the two side effect management strategies. Sustained response percentages were similar comparing anemic and neutropenic subjects regardless of management strategy (anemia: rHuEPO, 29% versus dose reduction, 21%, p=0.92; neutropenia: G-CSF, 40% versus dose reduction, 20%, p=0.46).
Conclusions
Growth factor supplementation and dose reduction do not appear to differ as management strategies for anemia and neutropenia in HIV/HCV co-infected individuals treated with PEG-IFN/RBV.
Keywords: Hepatitis C virus/HIV coinfection, anemia, neutropenia, recombinant human erythropoietin, granulocyte colony stimulating factor
Introduction
Because of shared routes of viral transmission, nearly one third of HIV-infected patients in the United States are co-infected with the hepatitis C virus (HCV)1, 2. Standard combination therapy for chronic HCV with pegylated-interferon (PEG-IFN) alfa plus ribavirin (RBV) results in sustained virological responses (SVR) in 27% to 40% of HIV/HCV coinfected patients3–5. However, SVR percentages are markedly lower in the more difficult-to-treat patients infected with HCV genotype 1 (14% to 29%)3–5. This contrasts markedly with HCV monoinfected patients where SVR percentages range from 54% to 56% for all genotypes and from 42% to 46% in genotype 1 patients6, 7.
Hematologic abnormalities, specifically anemia and neutropenia, are the most common reasons for PEG-IFN/RBV dose reductions and premature treatment discontinuation. In APRICOT, the largest HIV/HCV coinfection treatment trial performed to date, neutropenia and anemia were responsible for dose reduction or treatment discontinuation in 28% and 17% of PEG-IFN/RBV-treated patients, respectively4. In the same trial, PEG-IFN alfa-2a /RBV treatment resulted in a mean 3.1 g/dl decline in hemoglobin levels in patients who were not receiving zidovudine (AZT)1. In general, anemia (hemoglobin < 10 g/dl) is reported in up to 31% of coinfected patients receiving PEG-IFN/RBV4, 8, 9 although much higher rates have been reported in patients concomitantly receiving AZT10. Neutropenia, defined as a neutrophil count <1,500 × 103 cells/μl, is encountered in up to 60% of PEG-IFN/RBV-treated coinfected patients10. Overall, 5% to 28% of HIV-infected patients with HCV enrolled in HCV clinical trials require dose modification due to neutropenia4, 8, 11.
Hematological adverse events in PEG-IFN/RBV-treated patients have traditionally been managed by dose reduction12. However, factors that affect medication adherence can drastically diminish the likelihood of achieving an SVR. For example, HCV genotype-1-infected patients who receive at least 80% of their PEG-IFN alfa and RBV doses for at least 80% of the prescribed treatment duration are significantly more likely to attain an SVR than less adherent patients13. Similar observations regarding the importance of adherence have also been confirmed in HIV/HCV coinfected patients 3. Consequently, alternative strategies to treat hematologic abnormalities during PEG-IFN/RBV therapy, as well as for patients treated with direct acting antivirals (DAAs) against HCV, could have important clinical utility. Growth factor supplementation is an alternative management strategy for the treatment of anemia and neutropenia in patients with chronic HCV undergoing therapy with PEG-IFN/RBV. For example, recombinant human erythropoietin (rHuEPO) has been shown to effectively increase peripheral hemoglobin concentrations in patients with RBV-induced anemia14–18. Similarly, granulocyte-colony stimulating factor (G-CSF) is effective in increasing absolute neutrophil counts (ANC) in patients with neutropenia18, 19.
The optimal therapeutic strategy for anemia and neutropenia that occur during PEG-IFN/RBV therapy, however, has not been defined. Whether growth factors or dose reduction is more effective at maintaining hematologic parameters in treated patients is an important clinical question, which is particularly relevant to HIV/HCV coinfected patients in whom low SVR percentages and treatment-related anemia are common. To address this question, we conducted a superiority prospective, two-arm, open-label, multicenter randomized trial. Our goal was to compare the ability of dose reduction (RBV or PEG-IFN) to that of growth factor supplementation (rHuEPO or GCSF) to maintain hemoglobin levels >10.5 gm/dl or ANC levels >750 cells/mm3, respectively, thresholds at which interventions are deemed necessary.
Methods
Patients
Adults aged 26-66 years with a diagnosis of compensated chronic hepatitis C and HIV-1 infection were enrolled in an investigator-initiated, multi-center, randomized, open-label trial in HIV/HCV co-infected patients at 11 sites throughout the United States between February 2002 and May 2004. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as revised in 2000. The study was approved by the institutional review board at each investigative site. All participants provided written informed consent.
Patients were included in the study if they were HCV and HIV seropositive, had detectable serum HCV RNA, were naïve to IFN and RBV, and for a minimum of 4 weeks preceding PEG-IFN/RBV were either on a highly active antiretroviral regimen or had untreated HIV. Baseline CD4+ cell counts were required to be ≥ 100 cells/mm3, and patients were not permitted to use rHuEPO and G-CSF growth factors in the 30 days immediately preceding study entry.
Patients with significant hematological abnormalities at baseline, such as neutropenia (ANC <1,200 × 103 cells/μl), thrombocytopenia (<70 × 103 cells/μl), and anemia (<11.0 g/dl), were excluded. Patients were also excluded from study participation if they had serum creatinine ≥1.70 mg/dl (150 μmol), hepatitis B surface antigen positivity, decompensated cirrhosis, or other forms of liver disease not attributable to HCV. Patients with severe depression or psychosis, uncontrolled seizures, poorly controlled cardiovascular disease, diabetes mellitus, or autoimmune disorders were also ineligible. Patients who had actively used illicit drugs three months prior to enrollment or who reported a daily alcohol intake >40 g (women) or >50 g (men) were also ineligible. Women were ineligible for study participation if they were pregnant or unwilling to use at least two forms of effective contraception during the entire study period.
Patients had necroinflammatory grade and fibrosis stage assessed within 36 months prior to study entry using the Scheuer system20. Liver biopsy was not required for patients with documented cirrhosis by imaging.
Study Design
Patients received PEG-IFN alfa-2b (1.5 μg/kg/week) and weight-based RBV (800-1400 mg/d; 13 ± 2 mg/kg/day) for 24 weeks if they were infected with HCV genotype 2 or 3 and for 48 weeks if they were infected with HCV genotype 1. RBV was administered according to the patient’s weight (<65kg, 800mg/day; 65-85kg, 1000mg/day; 86-105kg, 1200mg/day; >105kg, 1400mg/day). Patients who did not achieve an undetectable HCV RNA level by week 24 were discontinued from the treatment regimen.
Patients were randomly assigned to one of two strategies, either growth factor supplementation or dose reduction, for the management of hematologic adverse events, i.e., anemia or neutropenia. We used a centralized randomization schedule with a four-block design and a 1:1 randomization ratio. Target enrollment was initially set at 200 patients, but the study was discontinued prematurely by the study sponsor after 103 patients had enrolled. Anemia was defined as hemoglobin <10.5 g/dl. Patients who developed anemia during HCV therapy received either rHuEPO 40,000 U per week or RBV dose reduction to 10 ± 2 mg/kg/day. Patients receiving rHuEPO whose hemoglobin levels did not increase by ≥ 1g/dl during the first four weeks of treatment were eligible to have their rHuEPO dose increased by 20,000 units every four weeks to a maximum of 80,000 U. Likewise, patients whose hemoglobin did not achieve target increases were eligible for additional RBV dose reductions or temporary or permanent RBV discontinuation. Neutropenia was defined as ANC ≤ 750× 103 cells/μl l at any point during treatment. Patients who developed neutropenia during HCV therapy received either G-CSF 5 μg/kg/d twice weekly or had PEG-IFN alfa-2b dose reduced to 1.0μg/kg/wk. Patients who did not achieve target increases in ANC were eligible for subsequent increases in G-CSF or temporary or permanent PEG-IFN discontinuation.
Determination of HCV and HIV RNA levels
Serum HCV RNA levels were measured by PCR (Roche Amplicor, Branchberg, NJ-lower limit of detection of 600 IU) at study weeks 0, 24, and 48 (genotype 1 only) and at week 24 post-treatment. HIV RNA levels were also measured by PCR (lower limit of detection 400 copies/ml) at study weeks 0, 4, 24 and 48 (genotype 1 only) and at weeks 4 and 24 post-treatment.
Safety
Safety and tolerability were evaluated at weeks 1, 2, 4, 8, 12, and every six weeks thereafter until PEG-IFN/RBV was completed. Post-treatment, patients were evaluated at weeks, 4, 12, and 24.
Statistical Analysis
Statistical analysis was performed using SAS (version 9.2, SAS Institute Inc., Cary, NC, USA), R (version 2.10.1, The R Foundation for Statistical Computing) and S-Plus (version 7.0, Insightful Corp, Palo Alto, CA, USA). Efficacy analyses were based on the intention-to-treat population defined as all randomized patients who received at least one dose of PEG-IFN and RBV. Wilcoxon rank-sum test was used for non-parametric comparisons among continuous variables. Fisher’s exact test was used for categorical variables. Multivariate modeling was performed using logistic regression. All statistical tests are two-sided and the level of significance is 0.05.
Results
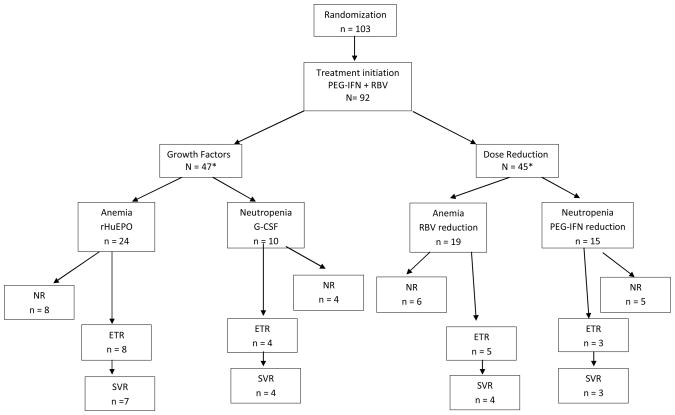
In total, 103 HIV/HCV coinfected patients were randomized to a hematologic management strategy (Figure 1). Of these, 10 patients declined to participate prior to receiving study medication. One patient was lost to follow up at the initiation of the study. Therefore, the study population comprises 92 patients who received once weekly PEG-IFN alfa-2b plus daily RBV. Baseline characteristics did not differ significantly between randomization groups (Table 1a). Study participants had well-controlled HIV disease; 86% were receiving antiretroviral therapy and 71% had HIV RNA <400 copies/ml (Table 1). Median (inter-quartile range [IQR]) CD4+ cell count was 466 (275, 595) cells/mm3. At baseline, significantly more individuals who later developed anemia had undetectable HIV RNA levels in comparison to those who did not (p = 0.001). This association only persisted for those taking anti-HIV therapy. The other baseline characteristics did not differ significantly among any of the groups when comparing anemic to non-anemic and neutropenic to non-neutropenic patients, although CD4+ cell counts tended to be lower in neutropenic patients (p = 0.059).
Figure. 1.
Table 1a.
Demographic characteristics of patients*
| Characteristic | All patients n=92 | Growth Factors n=47 | Dose Reduction n=45 | Anemic n=43 | Non-Anemic n=49 | Neutropenic n=25 | Non-neutropenic n=67 |
|---|---|---|---|---|---|---|---|
| Gender, n (%) | |||||||
| Male | 71 (77) | 37 (79) | 34 (76) | 35 (81) | 36 (73) | 17 (68) | 54 (81) |
| Female | 21 (23) | 10 (21) | 11 (24) | 8 (19) | 13 (27) | 8 (32) | 13 (19) |
| P = .81 | P = .46 | P = .26 | |||||
|
| |||||||
| Race, n (%) | |||||||
| Caucasian | 28 (30) | 13 (28) | 15 (33) | 14 (28) | 16 (33) | 8 (32) | 20 (30) |
| African-American | 39 (42) | 21 (45) | 18 (40) | 20 (47) | 19 (39) | 10 (40) | 29 (43) |
| Hispanic | 20 (22) | 9 (19) | 11 (24) | 9 (21) | 11 (22) | 6 (24) | 14 (21) |
| Other | 5 (5) | 4 (9) | 1 (2) | 2 (5) | 3 (6) | 1 (4) | 4 (6) |
| P = .85 | P = .92 | P =.96 | |||||
|
| |||||||
| Median age, years (IQR) | 47.0 (43.5, 52) | 47 (44, 52) | 47 (43, 51) | 48 (45, 52) | 45 (42, 51) | 48 (45, 53) | 46 (42, 52) |
| P = .79 | P = .13 | P = .37 | |||||
|
| |||||||
| Median body mass index cm/kg2 (IQR)† | 25.2 (23.2,28.1) | 25.7 (23.2,28.9) | 25.1 (23.2,27.8) | 24.9 (22.8, 27.6) | 26 (23.4, 29.9) | 26.5 (24.8,29.4) | 24.9 (22.8, 27.6) |
| P = .88 | P = .16 | P = .11 | |||||
|
| |||||||
| HCV genotype 1, n (%) | 77 (84) | 38 (81) | 39 (87) | 34 (79) | 43 (88) | 23 (92) | 54 (81) |
| P = .58 | P = .28 | P = .34 | |||||
|
| |||||||
| HCV-RNA level (IU/ml), n (%) | |||||||
| >1 million | 46 (50) | 22 (47) | 24 (53) | 18 (42) | 28 (57) | 14 (56) | 32 (48) |
| 100,000–1 million | 35 (38) | 18 (38) | 17 (38) | 20 (47) | 15 (31) | 10 (40) | 25 (37) |
| 10,000–100,000 | 5 (5) | 4 (9) | 1 (2) | 1 (2) | 4 (8) | 1 (4) | 4 (6) |
| <10,000 | 2 (2) | 1 (2) | 1 (2) | 2 (5) | 0 (0) | 0 (0) | 2 (3) |
| >7500 (BDNA) | 4 (4) | 2 (4) | 2 (4) | 2 (5) | 2 (4) | 0 (0) | 4 (6) |
| P = .80 | P = .18 | P = .84 | |||||
|
| |||||||
| Median necroinflammatory grade‡ | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
|
| |||||||
| Median fibrosis score§ | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
|
| |||||||
| Cirrhosis (stage 4), n (%)§ | 15/86 (17) | 6/45 (13) | 9/41 (22) | 7/39 (18) | 8/47 (17) | 6/23 (26) | 9/63 (14) |
| P = .40 | P = 1.0 | P = .21 | |||||
|
| |||||||
| Median CD4 cell count (No./mm3) | 466 (275, 595) | 479.5 (271,595) | 452 (289.5,592) | 490 (328, 605) | 449 (273, 595) | 367 (252, 509.5) | 487 (308, 641) |
| P = .88 | P = .63 | P = .059 | |||||
|
| |||||||
| HIV-1 RNA level (<400 copies/ml), n (%) | 65 (71) | 31 (67) | 34 (76) | 38 (88) | 27 (56) | 21 (84) | 44 (67) |
| P = .49 | P < .001 | P = .12 | |||||
|
| |||||||
| Patients receiving antiretroviral therapy, n (%) | 79 (86) | 39 (83) | 40 (91) | 39 (93) | 40 (82) | 22 (92) | 57 (85) |
| P = .36 | P = .13 | P = .51 | |||||
Some patients were counted twice; patients with anemia and neutropenia are included in the total for anemic and neutropenic patients. Data available on all patients unless indicated below.
Body mass index unavailable for 2 patients.
Scores range from 0 to 4, with higher scores indicating more activity.20 Grade unavailable for 6 patients.
Scores range from 0 to 4, with higher scores indicating more extensive fibrosis.20 Stage unavailable for 6 patients.
CD4 count unavailable for 2 patients.
Fifty-three patients (58%) underwent a full course of HCV therapy according to the study protocol. Out of the remaining 39 patients, HCV treatment was discontinued in 8 (9%) patients due to severe anemia, 3 (3%) due to severe neutropenia, and 12 (13%) due to other severe side effects. Nine patients (10%) were discontinued due to violation of the study protocol while 6 (7%) withdrew consent.
Effect of management strategies on hematological parameters
Because anemia most often occurs secondary to RBV and neutropenia secondary to PEG-IFN alfa, they were considered independent processes and were analyzed separately even if they both occurred in the same patient.
Anemia
At baseline, median hemoglobin levels were comparable among patients who received either dose reduction or growth factor supplementation: growth factor supplementation group: 14.0 g/dl (IQR: 12.8, 15.3), RBV dose reduction group: 14.1 g/dl (IQR: 13.3, 15.2), P >0.05. Among all patients, hemoglobin levels decreased approximately 25% from baseline after initiation of PEG-IFN/RBV in both groups, resulting in median (IQR) nadir hemoglobin levels of 10.4 (9.6, 12.1) g/dl and 10.6 (10.2, 12.2) g/dl, respectively.
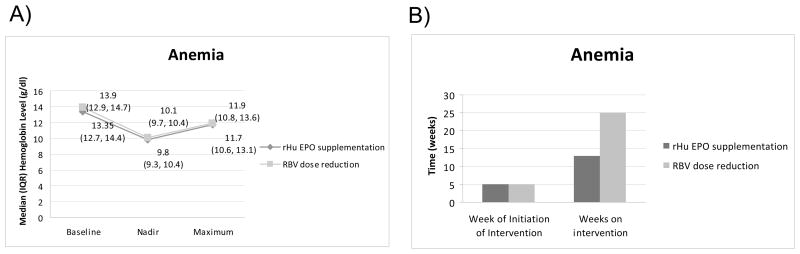
A total of 43 patients developed anemia: 24 (51%) of whom were randomized to receive growth factor supplementation versus 19 (42%) randomized to receive RBV dose reduction (p = 0.41). Among anemic patients, median baseline hemoglobin levels were comparable among those who received either growth factor supplementation or RBV dose reduction: 13.3 g/dl (IQR: 12.7, 14.4) versus 13.9 g/dl (IQR: 12.8, 14.7), respectively (p =0.54). Their hemoglobin levels decreased before intervention to median (IQR) nadir hemoglobin levels of 9.8 (9.3, 10.4) g/dl and 10.1 (9.6, 10.3) g/dl, respectively (p=0.32) (Figure 2A).
Figure. 2.
Treatment week 5 was the median initiation time for treatment with rHuEPO or RBV reduction, IQR (3, 18) and (4, 8), respectively (Figure 2B). Following the intervention, median (IQR) hemoglobin levels peaked at 11.7 (10.5, 13.0) g/dl in anemic patients who received growth factor supplementation and at 11.9 (10.8, 13.6) g/dl in those who had RBV dose reduction (p = 0.46). The median (IQR) time on the interventions was 13 (7.0, 24.5) weeks and 25 (9.0, 41.0) weeks, respectively (p = 0.36). In order to mitigate variations in individual hemoglobin levels over time, we also evaluated mean hemoglobin levels post intervention while patients remained on PEG-IFN/RBV. The median (IQR) level of the mean hemoglobin values after initiation of intervention was 10.8 (9.9, 12.2) for the growth factor group and 11.3 (10.4, 11.9) for the RBV reduction group (p = 0.74).
Fifteen anemic patients (63%) in the growth factor arm completed a full course of HCV treatment versus 9 (47%) in the dose reduction arm (p = 0.37). More patients were discontinued from the study due to severe anemia in the growth factor arm compared to those in the dose reduction arm (6 [25%] versus 1 [5%]), although the difference was not statistically significant (p = 0.11).
Neutropenia
Similar to the changes observed in hemoglobin levels, median baseline ANC levels were comparable among patients who received either growth factor supplementation or dose reduction: 2,220 (IQR: 1,634, 2,622) × 103 cells/μl versus 2,317 (IQR: 1,786, 3,192) × 103 cells/μl, respectively (p = 0.20). Taking all patients together, median ANC levels decreased ~57% in both intervention groups, resulting in nadir ANC levels of 960 (IQR: 756, 1,288) × 103 cells/μl and 1,000 (IQR: 700, 1,312) × 103 cells/μl, respectively (p = 0.93).
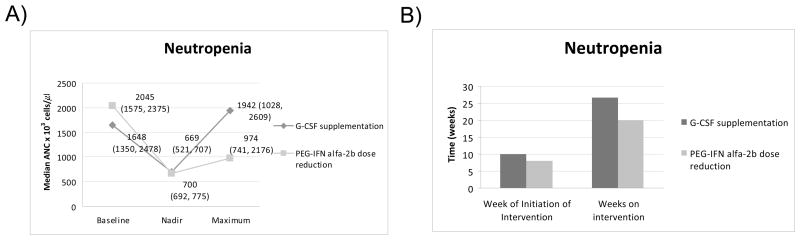
Following PEG-IFN/RBV initiation, 10 (21%) patients randomized to growth factor supplementation became neutropenic versus 15 (33%) patients randomized to PEG-IFN alfa-2b dose reduction (p = 0.24). Among neutropenic patients, baseline median ANC levels were comparable among those who received either growth factor supplementation or PEG-IFN dose reduction: 1,648 (IQR: 1,350, 2,478)× 103 cells/μl and 2,045 (IQR: 1,575, 2,375) × 103 cells/μl, respectively (p =0.62) (Figure 3A). Their ANC levels decreased before intervention to median (IQR) nadir ANC levels of 700 (691.5, 775) × 103 cells/μl and 669 (520.5, 707) × 103 cells/μl, respectively (p = 0.25).
Figure. 3.
G-CSF or PEG-IFN reduction were initiated at a median (IQR) of week 10 (1, 19) or week 8 (4, 11), respectively (p = 0.50) (Figure 3B). Following the intervention, median (IQR) ANC levels peaked at 1,942 (1,028, 2,609) × 103 cells/μl in neutropenic patients who received growth factor supplementation and at 974 (741, 2,176) × 103 cells/μl in those who had PEG-IFN dose reduction (p = 0.20). The median (IQR) time on the intervention was 26.5 (8, 34.5) weeks and 20 (6, 26) weeks, respectively (p = 0.73). We also evaluated mean ANC levels post G-CSF intervention while patients remained on a stable dose of PEG-IFN/RBV. The median (IQR) level of the mean ANC values after initiation of intervention was 1,071 (926.9, 1,886) × 103 cells/μl for the growth factor group and 862 (690.8, 1,270) × 103 cells/μl for the PEG-IFN reduction group (p = 0.14).
Eight neutropenic patients (80%) in the growth factor arm completed a full course of HCV treatment versus 8 (53%) in the dose reduction arm (p = 0.21). Three patients who were discontinued from the study due to severe neutropenia were in the PEG-IFN reduction arm.
Virologic Responses
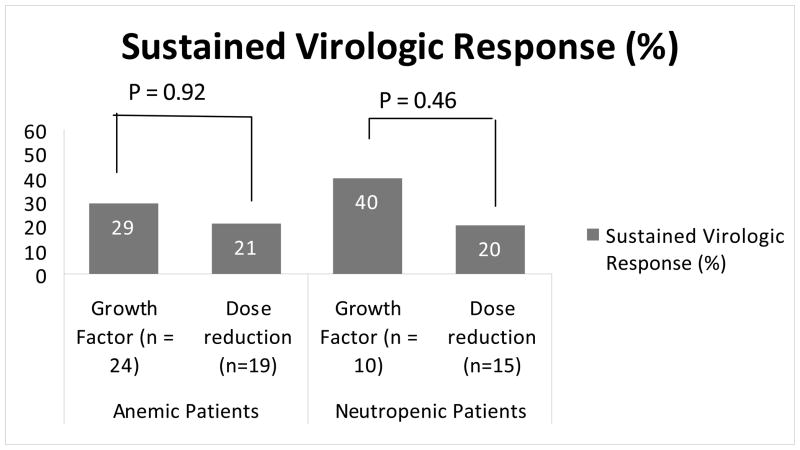
A full course of HCV therapy, as defined in the study protocol, was completed by 53 (58%) patients. Virologic outcome data were also available for an additional four patients, all of whom were discontinued from the treatment protocol due to side effects. SVR was defined as undetectable HCV RNA levels 6 months post-treatment cessation. Of 57 patients with known HCV treatment response, 19 (33%) achieved an SVR (21% by intention to treat). Of these 57 patients, 27 developed anemia and 16 developed neutropenia. Of 27 anemic patients, 16 received growth factor supplementation, and 11 had RBV dose reduction; of 16 neutropenic patients, 8 received growth factor supplementation and 8 had PEG-IFN reduction. Eleven of 43 (26%) patients who became anemic during PEG-IFN/RBV attained an SVR. SVR percentages were similar among anemic patients in the growth factor supplementation group and the RBV dose reduction group (29% versus 21%, respectively, P = 0.92) (Figure 4). Seven of 25 (28%) patients who became neutropenic during PEG-IFN/RBV attained an SVR. SVR percentages were similar among neutropenic patients in the growth factor group and the PEG-IFN reduction group (40% versus 20%, respectively, P = 0.46) (Figure 4).
Figure. 4.
SVR percentages were generally lower among the subgroups of patients traditionally regarded as more difficult to treat. As per intention to treat, SVR percentages were 16% (12 of 77) among genotype 1 patients, 8% (3 of 39) among African-Americans, and 20% (3 of 15) among cirrhotics (Table 1b). A total of 34 genotype 1-infected patients developed anemia: 19 were treated with dose reduction and 15 were treated with growth factor supplementation. SVR outcome in anemic genotype 1-infected patients was achieved in 4 (21%) of those who received growth factor supplementation and in 2 (13%) of those managed through dose reduction (p = 0.9). A total of 23 genotype 1-infected patients developed neutropenia: 14 were treated with dose reduction and 9 were treated with growth factor supplementation. SVR percentages were similar among neutropenic genotype 1-infected patients treated with either dose reduction (n=3 [21%]) or G-CSF supplementation (n=3 [33%], p = 0.65).
Discussion
The current standard treatment for chronic HCV infection, PEG-IFN in combination with RBV, is frequently associated with hematologic abnormalities, especially in HIV/HCV coinfected patients. Treatment-induced anemia and neutropenia are traditionally managed by dose reduction and more recently with growth factor supplementation. To compare these two management strategies, we performed a prospective, randomized preliminary study in HIV/HCV coinfected patients treated with weight-based PEG-IFN alfa and weight-based RBV. Based upon our results, these two strategies do not appear to differ in their ability for management of hematologic abnormalities in HIV/HCV co-infected patients, although these conclusions may be affected by low power. Overall, SVR percentages were similar in patients who received dose reduction compared with those treated with growth factor supplementation. In addition, the proportion of patients who completed a full course of HCV therapy was comparable between the two strategies for management of anemia and neutropenia.
The main limitation of this study is the cessation of recruitment prior to full enrollment. Additional limitations include withdrawal from the study due to violation of the study protocol in 10% of the patients and consent withdrawal in 7%, which contributed to lower power to prove the apriori hypothesis. Therefore, our results should not be considered as a definitive statement of the management practice for hematologic abnormalities in HIV/HCV coinfected patients treated with PEG-IFN/RBV.
Treatment of HIV/HCV coinfected patients with PEG-IFN and RBV results in suboptimal therapeutic outcomes compared to HCV monoinfected patients3–7. In addition, coinfected patients are twice as likely to discontinue treatment prematurely in comparison with monoinfected patients, with hematological toxicities being cited as one of the most common causes for treatment discontinuation 3, 21–23. The prevalence of anemia varies from 18% to 50% in patients with HIV without AIDS to 68% to 92% in advanced AIDS patients24. Antiviral medications can also contribute to anemia. RBV is directly toxic to red blood cells resulting in hemolysis,25, 26 and zidovudine is a well recognized cause of anemia in HIV infection. In our study, only two patients received zidovudine, both of whom developed anemia. We also observed a significant association between undetectable HIV RNA at baseline and development of anemia. One possible explanation for this finding is that those patients with undetectable HIV RNA may have been more adherent to their medications leading to increased RBV adherence and more anemia. Although higher RBV doses have increased antiviral activity27, 28, higher doses have also been associated with more profound hemoglobin declines during treatment29. Similarly, neutropenia occurs in, approximately 50% of patients with AIDS30.
Historically, dose reduction was the standard management strategy for hematological side effects of PEG-IFN and RBV. However, recent studies have shown that the response to therapy is strongly influenced by adherence to optimal medication doses31. Treatment of anemia, besides improving adherence to combination antiviral therapy, may also improve patients’ health-related quality of life16. Therefore, the use of growth factors, such as G-CSF and rHuEPO to stimulate bone marrow production of leukocytes and erythrocytes, respectively, has been advocated to enhance patients’ ability to tolerate optimal doses of PEG-IFN alfa and RBV.
Growth factors are not without limitations. rHuEPO can cause hypertension, pure red cell aplasia, injection site erythema, erythropoietin resistance, and venous thromboembolism32, 33. Additionally, several large randomized clinical trials have recently shown a potential detrimental effect of rHuEPO administration on tumor progression and survival in patients treated for oncologic conditions34. Both G-CSF and rHuEPO can substantially increase total medication costs. Despite these limitations, a recent analysis suggested that the use of hematopoietic growth factors, especially darbepoetin, was a cost effective intervention during PEG-IFN/RBV treatment of HCV monoinfected patients32, 35. However, this analysis assumed that growth factor supplementation resulted in improved SVR rates, which has not yet been conclusively determined36.
To date, very few studies have compared growth factor supplementation and dose reduction for management of hematologic abnormalities in HCV-infected or in HIV/HCV coinfected patients treated with PEG-IFN and RBV. A study performed in HCV monoinfected patients found that the use of rHuEPO from the beginning of HCV treatment did not increase SVR rates17. Although EPO use significantly reduced the incidence of anemia and RBV dose reductions, it did not affect treatment responses. Another study demonstrated that erythropoiesis-stimulating agents significantly improved treatment outcome in those with early onset anemia (≤ 8 weeks of therapy)37. In the AACTG-A5071 study, rHuEPO and G-CSF support was also associated with an improved clinical response to therapy in HIV/HCV co-infected patients38. Although we observed higher SVR percentages in those who received G-CSF (40% versus 20%), our sample size precluded the observation of a significant relationship. Twenty-one percent of our patients achieved an SVR, which is slightly lower than SVR percentages reported in other clinical trials in HIV/HCV co-infected patients receiving PEG-IFN/RBV therapy3–5, 10, 39. The inclusion of a relatively high proportion of patients who are traditionally regarded as difficult-to-treat (such as African-American [43%], genotype 1 [84%], and cirrhotic individuals [17%]) likely contributed to the low overall SVR percentage in the current study. In addition, poor patient adherence coupled with high early discontinuation rates likely also contributed to a poor antiviral response.
In summary, in HIV/HCV coinfected patients, the use of growth factors did not significantly enhance hemoglobin and ANC values compared with dose reduction although this conclusion may be influenced by our low numbers of subjects. Our results on the timing and extent of hemoglobin and ANC decline and recovery before and after intervention with growth factors or dose reduction may be useful in the clinical management of HIV/HCV coinfected patients and can also be used for planning larger trials.
Table 1b.
Sustained virological response (SVR) stratified by fibrosis stage for the total study population.
| Growth Factors | Dose Reduction | |||
|---|---|---|---|---|
| Stage | Total (N=45) | SVR, N (%) | Total (N=41) | SVR, N (%) |
| 0 | 0 | 0 | 2 | 1 (50) |
| 1 | 14 | 3 (21.4) | 10 | 1 (10) |
| 2 | 14 | 2 (14.3) | 18 | 5 (28) |
| 3 | 11 | 4 (36.4) | 2 | 0 (0) |
| 4 | 6 | 2 (33.3) | 9 | 1 (11) |
| Total | 45 | 11 (24.4) | 41 | 8 (19.5) |
Acknowledgments
We acknowledge Jay Kadam MD, Kristina. Jones MD, Preeti Golia MD, Sandra Flynn RN, Michelle Pappas, and Ray Peterson for assistance with the conduct of the study. We also acknowledge Larry Heller for assisting in the study design and for helpful conversations.
Footnotes
Presented at: 107th Annual Meeting of American Gastroenterological Association (DDW); Los Angeles, 2006.
Financial disclosure: This study was conducted as an investigator-initiated study. The protocol was authored solely by the principal investigator although the study sponsor approved the final protocol. The sponsor had no input whatsoever into the data collection, analysis of the data, or manuscript preparation. The authors had full access to the data and take full responsibility for its accuracy.
ClinicalTrials.gov: Study is registered as NCT00194857.
References
- 1.Brau N. Treatment of chronic hepatitis C in human immunodeficiency virus/hepatitis C virus-coinfected patients in the era of pegylated interferon and ribavirin. Semin Liver Dis. 2005;25:33–51. doi: 10.1055/s-2005-864780. [DOI] [PubMed] [Google Scholar]
- 2.Puoti M, Manno D, Nasta P, et al. The burden of HIV and hepatitis C virus coinfection. Curr Opin HIV AIDS. 2007;2:460–465. doi: 10.1097/COH.0b013e3282f11906. [DOI] [PubMed] [Google Scholar]
- 3.Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. Jama. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 4.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 5.Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 7.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 8.Laguno M, Murillas J, Blanco JL, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. Aids. 2004;18:F27–36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 9.Mauss S. Treatment of viral hepatitis in HIV-coinfected patients-adverse events and their management. J Hepatol. 2006;44:S114–118. doi: 10.1016/j.jhep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Moreno L, Quereda C, Moreno A, et al. Pegylated interferon alpha2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. Aids. 2004;18:67–73. doi: 10.1097/00002030-200401020-00008. [DOI] [PubMed] [Google Scholar]
- 11.Crespo M, Sauleda S, Esteban JI, et al. Peginterferon alpha-2b plus ribavirin vs interferon alpha-2b plus ribavirin for chronic hepatitis C in HIV-coinfected patients. J Viral Hepat. 2007;14:228–238. doi: 10.1111/j.1365-2893.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 12.Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264. doi: 10.1053/j.gastro.2005.11.010. quiz 214–237. [DOI] [PubMed] [Google Scholar]
- 13.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 14.Talal AH, Weisz K, Hau T, et al. A preliminary study of erythropoietin for anemia associated with ribavirin and interferon-alpha. Am J Gastroenterol. 2001;96:2802–2804. doi: 10.1111/j.1572-0241.2001.04149.x. [DOI] [PubMed] [Google Scholar]
- 15.Afdhal NH, Dieterich DT, Pockros PJ, et al. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302–1311. doi: 10.1053/j.gastro.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Pockros PJ, Shiffman ML, Schiff ER, et al. Epoetin alfa improves quality of life in anemic HCV-infected patients receiving combination therapy. Hepatology. 2004;40:1450–1458. doi: 10.1002/hep.20482. [DOI] [PubMed] [Google Scholar]
- 17.Shiffman ML, Salvatore J, Hubbard S, et al. Treatment of chronic hepatitis C virus genotype 1 with peginterferon, ribavirin, and epoetin alpha. Hepatology. 2007;46:371–379. doi: 10.1002/hep.21712. [DOI] [PubMed] [Google Scholar]
- 18.Younossi ZM, Nader FH, Bai C, et al. A phase II dose finding study of darbepoetin alpha and filgrastim for the management of anaemia and neutropenia in chronic hepatitis C treatment. J Viral Hepat. 2008;15:370–378. doi: 10.1111/j.1365-2893.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- 19.Koirala J, Gandotra SD, Rao S, et al. Granulocyte colony-stimulating factor dosing in pegylated interferon alpha-induced neutropenia and its impact on outcome of anti-HCV therapy. J Viral Hepat. 2007;14:782–787. doi: 10.1111/j.1365-2893.2007.00870.x. [DOI] [PubMed] [Google Scholar]
- 20.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 21.Fredrick RT, Hassanein TI. Role of growth factors in the treatment of patients with HIV/HCV coinfection and patients with recurrent hepatitis C following liver transplantation. J Clin Gastroenterol. 2005;39:S14–22. doi: 10.1097/01.mcg.0000145537.66736.38. [DOI] [PubMed] [Google Scholar]
- 22.Myers RP, Benhamou Y, Bochet M, et al. Pegylated interferon alpha 2b and ribavirin in HIV/hepatitis C virus-co-infected non-responders and relapsers to IFN-based therapy. Aids. 2004;18:75–79. doi: 10.1097/00002030-200401020-00009. [DOI] [PubMed] [Google Scholar]
- 23.Cargnel A, Angeli E, Mainini A, et al. Open, randomized, multicentre italian trial on PEG-IFN plus ribavirin versus PEG-IFN monotherapy for chronic hepatitis C in HIV-coinfected patients on HAART. Antivir Ther. 2005;10:309–317. [PubMed] [Google Scholar]
- 24.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):27S–43S. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Russmann S, Grattagliano I, Portincasa P, et al. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem. 2006;13:3351–3357. doi: 10.2174/092986706778773059. [DOI] [PubMed] [Google Scholar]
- 26.Grattagliano I, Russmann S, Palmieri VO, et al. Glutathione peroxidase, thioredoxin, and membrane protein changes in erythrocytes predict ribavirin-induced anemia. Clin Pharmacol Ther. 2005;78:422–432. doi: 10.1016/j.clpt.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Larrat S, Stanke-Labesque F, Plages A, et al. Ribavirin quantification in combination treatment of chronic hepatitis C. Antimicrob Agents Chemother. 2003;47:124–129. doi: 10.1128/AAC.47.1.124-129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson IM, Brown RS, Jr, Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46:971–981. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 29.Jen JF, Glue P, Gupta S, et al. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit. 2000;22:555–565. doi: 10.1097/00007691-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Jaresko GS. Etiology of neutropenia in HIV-infected patients. Am J Health Syst Pharm. 1999;56 (Suppl 5):S5–8. doi: 10.1093/ajhp/56.suppl_5.S5. [DOI] [PubMed] [Google Scholar]
- 31.Everson GT, Hoefs JC, Seeff LB, et al. Impact of disease severity on outcome of antiviral therapy for chronic hepatitis C: Lessons from the HALT-C trial. Hepatology. 2006;44:1675–1684. doi: 10.1002/hep.21440. [DOI] [PubMed] [Google Scholar]
- 32.Dar Santos AE, Partovi N, Ford JA, et al. Use of hematopoietic growth factors as adjuvant therapy for anemia and neutropenia in the treatment of hepatitis C. Ann Pharmacother. 2007;41:268–275. doi: 10.1345/aph.1H169. [DOI] [PubMed] [Google Scholar]
- 33.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. Jama. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 34.Hadland BK, Longmore GD. Erythroid-stimulating agents in cancer therapy: potential dangers and biologic mechanisms. J Clin Oncol. 2009;27:4217–4226. doi: 10.1200/JCO.2008.21.6945. [DOI] [PubMed] [Google Scholar]
- 35.Del Rio RA, Post AB, Singer ME. Cost-effectiveness of hematologic growth factors for anemia occurring during hepatitis C combination therapy. Hepatology. 2006;44:1598–1606. doi: 10.1002/hep.21409. [DOI] [PubMed] [Google Scholar]
- 36.Muir AJ, McHutchison JG. Growth factors during HCV therapy may be “cost-effective”, but are they “effective”? Hepatology. 2006;44:1400–1403. doi: 10.1002/hep.21426. [DOI] [PubMed] [Google Scholar]
- 37.Sulkowski MS, Shiffman ML, Afdhal NH, et al. Hepatitis C virus treatment-related anemia is associated with higher sustained virologic response rate. Gastroenterology. 2010;139:1602–1611. 1611, e1601. doi: 10.1053/j.gastro.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 38.Behler CM, Vittinghoff E, Lin F, et al. Hematologic toxicity associated with interferon-based hepatitis C therapy in HIV type 1-coinfected subjects. Clin Infect Dis. 2007;44:1375–1383. doi: 10.1086/515398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Olmeda M, Nunez M, Romero M, et al. Pegylated IFN-alpha2b plus ribavirin as therapy for chronic hepatitis C in HIV-infected patients. Aids. 2003;17:1023–1028. doi: 10.1097/00002030-200305020-00011. [DOI] [PubMed] [Google Scholar]