ABSTRACT
To identify roles in spermatogenesis for major subclasses of N- and O-glycans and Notch signaling, male mice carrying floxed C1galt1, Pofut1, Notch1 or Mgat1 alleles and a testis-specific Cre recombinase transgene were generated. T-synthase (C1GALT1) transfers Gal to generate core 1 and core 2 mucin O-glycans; POFUT1 transfers O-fucose to particular epidermal growth factor-like repeats and is essential for canonical Notch signaling; and MGAT1 (GlcNAcT-I) transfers GlcNAc to initiate hybrid and complex N-glycan synthesis. Cre recombinase transgenes driven by various promoters were investigated, including Stra8-iCre expressed in spermatogonia, Sycp1-Cre expressed in spermatocytes, Prm1-Cre expressed in spermatids, and AMH-Cre expressed in Sertoli cells. All Cre transgenes deleted floxed alleles, but efficiencies varied widely. Stra8-iCre was the most effective, deleting floxed Notch1 and Mgat1 alleles with 100% efficiency and floxed C1galt1 and Pofut1 alleles with ∼80% efficiency, based on transmission of deleted alleles. Removal of C1galt1, Pofut1, or Notch1 in spermatogonia had no effect on testicular weight, histology, or fertility. However, males in which the synthesis of complex N-glycans was blocked by deletion of Mgat1 in spermatogonia did not produce sperm. Spermatogonia, spermatocytes, and spermatids were generated, but most spermatids formed giant multinucleated cells or symplasts, and apoptosis was increased. Therefore, although core 1 and 2 mucin O-glycans, NOTCH1, POFUT1, O-fucose glycans, and Notch signaling are dispensable, MGAT1 and complex N-glycans are essential for spermatogenesis.
Keywords: glycosyltransferases, Notch signaling, sperm maturation, spermatid, testis
Spermatogenesis is blocked when the Mgat1 gene is conditionally deleted in spermatogonia, but proceeds normally when Notch signaling is inhibited by deletion of Notch1 or Pofut1, or mucin O-glycan synthesis is prevented by deletion of C1galt1, in these cells.
INTRODUCTION
During spermatogenesis, spermatogonia proliferate and differentiate in clonal syncytia in close contact with Sertoli cells to generate spermatozoa in the lumen of testicular tubules [1]. Mechanisms regulating spermatogenesis in mammals are important to define as they generally apply to fertility in man, and infertility in young men is a major public health concern [2, 3]. Several classes of N-glycans and mucin O-glycans are differentially expressed during spermatogenesis in the mouse [4–8], and the Notch signaling pathway is active [9–11]. Functional importance for glycosylation during spermatogenesis has been observed in mice with targeted gene mutations. Thus, males lacking α-mannosidase IIX, one of two α-mannosidases involved in the synthesis of hybrid and complex N-glycans [12]; polypeptide GALNT3, one of numerous ppGalNAcTs that initiate mucin O-GalNAc glycan synthesis [13]; or certain classes of glycosphingolipids [14], are infertile. Identifying how and why these glycans affect spermatogenesis will reveal basic mechanisms of glycan functions and provide insights into male reproduction. However, this is difficult in gene knockout mice with a universal deficiency in a glycosyltransferase. Therefore, to define functions for specific classes of N- and O-glycans and Notch signaling in spermatogenesis, we used several Cre recombinase transgenes to cause cell-specific deletion of key glycosyltransferase genes and Notch1 in testicular germ cells.
The synthesis of both core 1 and core 2 mucin O-glycans is initiated by T-synthase or C1GALT1 [15] (Fig. 1). Mucin O-glycans are crucial for angiogenesis and development beyond ∼E13.5 in the mouse [16]. They have a regulatory role in oocyte development [17, 18], but little is known about their functional roles during spermatogenesis. Core 1 O-glycans, as detected by specific binding of the lectin peanut agglutinin (PNA), are expressed by round and elongated spermatids, but not by spermatogonia or spermatocytes, in rat [4] and in lesser deer mouse testes [19], indicating regulated expression of core 1 mucin O-glycans during spermatogenesis. More recently, PNA binding to spermatogonia and spermatocytes was observed in mouse testis [8].
FIG. 1. .
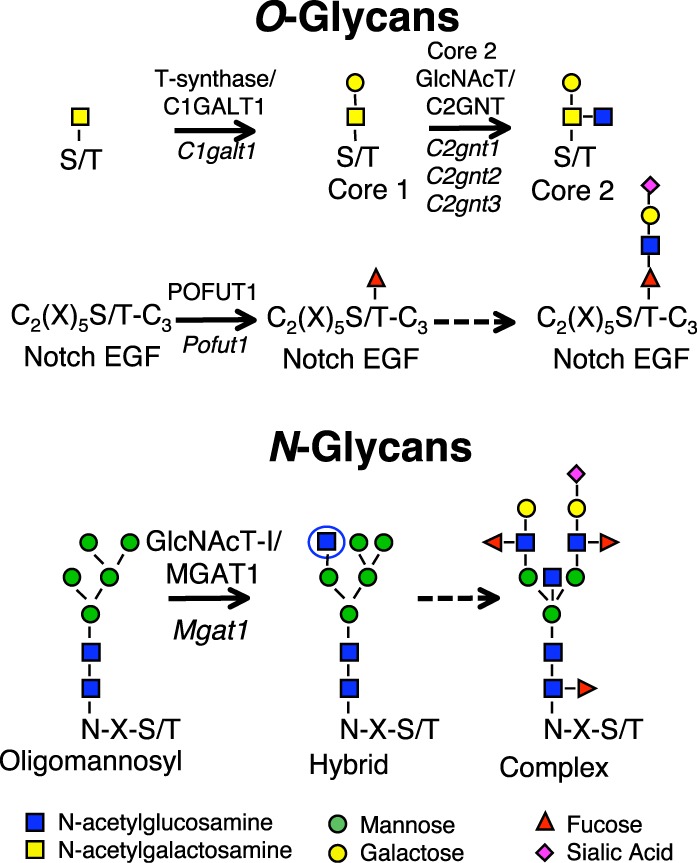
Glycosyltransferase genes conditionally deleted in testis. Each reaction shown is catalyzed by the respective glycosyltransferase. Core 1 β(1,3)galactosyltransferase 1 or T-synthase/C1GALT1, encoded by the C1galt1 gene, transfers Gal to O-GalNAc on Ser/Thr residues in mucin O-glycan synthesis. Core 2 O-glycans are generated by the action of any of three core 2 β(1,6)N-acetylgucosaminyltransferases (Core 2 GlcNAcT/C2GNT) encoded by the C2gnt1, C2gnt2, or C2gnt3 genes as shown. Protein O-fucosyltransferase 1 (POFUT1), encoded by the Pofut1 gene, transfers O-fucose to EGF repeats that contain the consensus motif shown [60]; the O-fucose can be extended by the addition of GlcNAc, Gal, and sialic acid in mammalian cells [61, 62]. N-acetylglucosaminyltransferase I (GlcNAcT-I/MGAT1), encoded by the Mgat1 gene, transfers GlcNAc (circled) to a high-mannose N-glycan at N-X-S/T sequons where X is not P. This intermediate is subsequently processed and extended to form a complex N-glycan. Sugar symbols are as shown.
The addition of O-fucose to specific epidermal growth factor-like (EGF) repeats is initiated by the fucosyltransferase POFUT1 [20] (Fig. 1), and O-fucose glycans are essential for Notch signaling and embryonic development in mouse [21, 22]. Notch signaling is suggested to be of functional importance for spermatogenesis because of the expression of Notch pathway members in testis. Western blot analysis identifies NOTCH1, NOTCH2, and NOTCH3; the Notch ligands Delta1 (DLL1), Jagged1 (JAG1) and Jagged2 (JAG2); and presenilin 1 (PSEN1) in neonatal mouse testis [9]. Notch1, Notch2, Jag1 and the three Fringe genes (Lfng, Mfng, and Rfng) are expressed in germ cells based on RT-PCR [23]. Immunohistochemistry in adult testis sections provided evidence that NOTCH1 is expressed in spermatogonia [24], activated NOTCH1 and NOTCH4 in spermatocytes and spermatids, and activated NOTCH3 in spermatogonia [10]. All four Notch receptors and several Notch pathway members are expressed in the embryonic gonad, and Notch signaling is important for the maintenance of Leydig progenitor cells, but not Sertoli cells [25]. However, a recent in situ hybridization analysis of testis sections finds no Notch receptors to be expressed in germ cells, and only Notch1 transcripts in Sertoli cells [11]. Jag1 and Jag2 of the five canonical Notch ligands are expressed in germ cells. The authors provide evidence that Notch1 signaling occurs in Sertoli cells induced by Jag2 in spermatogonia. Nevertheless, it was observed that deletion of global Notch signaling in Sertoli or germ cells did not inhibit spermatogenesis. In this paper, we use alternative Cre recombinase transgenic mice, a different Pofut1 conditional allele [21], and a Notch1 conditional ligand binding domain mutation [26, 27], to show that O-fucose glycans and Notch signaling in germ cells are dispensable for spermatogenesis. We find that core 1 and core 2 mucin O-glycans are also dispensable. By contrast, complex and hybrid N-glycans are shown to be absolutely required for spermatogenesis. The synthesis of these N-glycans is initiated by MGAT1 (also known as GlcNAcT-I or N-acetylglucosaminyltransferase I), encoded by the Mgat1 gene (Fig. 1).
MATERIALS AND METHODS
Mice
Mice carrying a Prm1-Cre transgene [28] strain 129S/Sv-Tg (Prm-Cre)58Og/J were kindly provided by Stephen O'Gorman (Case Western Reserve University); mice with the Sycp1-Cre transgene [29, 30] strain B6;D2-Tg (Sycp1-Cre)4Min/J were obtained from Jackson Labs; mice carrying the Stra8-iCre transgene strain FVB;Tg (Stra8-iCre)1Reb/J [31] were kindly provided by Robert Braun (Jackson Labs); and mice with the AMH-Cre transgene [32] were kindly provided by Florian Guillou (INRA/CNRS). C1galt1F/F or TsynF/F [16, 18], Mgat1F/F or Mgat1tm2Jxm/tm2.Jxm [33, 34], Pofut1F/F or Pofut1tm2.Pst/tm2.Pst [21, 35] and Notch112F/12F or Notch1tm2.Pst/tm2.Pst mice [27] strains were described previously. Mice were genotyped by PCR of tail genomic DNA by the methods described in the papers quoted above and using new primers as follows: C1galt1 primers FB33, GTCCACAGAGGGCCATTAGA and FB34, TGCTGATCCACAGAGGAAGA gave products of 398 bp (wild type) and 445 bp (floxed); Pofut1 primers FB21, CCAGGCTGATCACTTCTTGG and FB22, CCCTGTCTCGAAAAAGCAAA, gave products of 450 bp (wild type) and 530 bp (floxed); a deleted Pofut1 allele product (450 bp) was obtained with primers FB23, CAATGCCGTGCTGAGAGTAA and FB24, CAGAGAAACCCTGTCTCGAA; Notch1 primers FB15, CGGAGTGGACGGGTATGTAT and FB16, GTGTGTGTTGTGGCAGGTTC gave products of 458 bp (wild type) and 561 bp (floxed); a Notch1 deleted allele product (615 bp) was obtained with primers FB17, CGGAGTGGACGGGTATGTAT and FB19, AGTGGCCATTGTGCAGACAG. All mice were bred within the Institute for Animal Studies at the Albert Einstein College of Medicine, and experimental protocols were approved by the Albert Einstein Animal Institute Committee. Mice were killed by an intraperitoneal injection of 2.5% Avertin solution followed by cervical dislocation. Testes and epididymides were dissected free of surrounding tissue and weighed prior to fixation. Sperm were squeezed from epididymides and capacitated in 3% bovine serum albumin (BSA) in PBS pH 7.4 with 1 mM Ca2+, 1 mM Mn2+, and 1 mM Mg2+ (PBS), examined by light microscopy, and counted using a hemocytometer.
Testis Fixation, Paraffin Embedding, and Sectioning
Testes and epididymides were incubated in fresh 4% paraformaldehyde (PFA) in PBS overnight at 4°C. PFA was removed by incubating the tissue in 70% ethanol for at least 4 h at room temperature (RT) prior to paraffin embedding. The left testis of each mouse was paraffin embedded, and serial sections of 5 μm were cut and collected on positively charged slides.
Hematoxylin and Eosin Staining
Prior to analysis, sections were incubated successively for 4, 3, and 3 min in Histo-Clear (National Diagnostics). Sections were then incubated for 1 min successively in 100%, 90%, 70%, and 50% ethanol and rehydrated by incubation for 3 min in double-distilled water. Testis sections were stained with hematoxylin solution (Sigma) at RT for 1 min. After rinsing in tap water, sections were dipped twice in acid ethanol (0.3% HCl in 100% ethanol), rinsed in tap water, dipped in ammonium hydroxide (0.42 g/L), rinsed in tap water, and incubated in eosin solution (EM Diagnostics) for 2 min. After staining, sections were incubated for 1 min successively in 50%, 70%, 90% and 100% ethanol, incubated successively for 4, 3, and 3 min in Histo-Clear (National Diagnostics), and mounted using Permount solution (Fisher Scientific).
Lectin Histochemistry
After dewaxing and rehydration of tissue sections as described above, endogenous peroxidase was blocked in a solution containing 0.5% H2O2 and 0.4% HCl in absolute methanol for 30 min at RT. After two washes in double-distilled water and two washes in Tris-buffered saline pH 7.5 (TBS), sections were incubated in 20 mg/ml proteinase K in TBS at RT for 5 min in a humidified chamber. After two washes in TBS pH 7.5, sections were incubated in 10 μg/ml biotinylated plant lectin leukoagglutinin from Phaseolus vulgaris (L-PHA), agglutinin from Griffonia simplicifolia (GSA), concanavalin A (Con A), PNA, or agglutinin from Vicia villosa (VVA) (Vector Laboratories) for 30 min at 18°C. After two washes in TBS pH 7.5, sections were incubated in 5 μg/ml streptavidin-peroxidase for 1 h at 18°C. After three washes in TBS pH 7.5, slides were developed using DAB reagent (Vector Laboratories), ensuring equal exposure for each section. The reaction was terminated in water. Lectin staining was counterstained using hematoxylin as described above. Sections were dehydrated, cleared, and mounted as described above.
TUNEL Assay
The TUNEL assay was performed using the DeadEnd Fluorimetric TUNEL System (Promega). Briefly, after dewaxing and rehydration, testis sections were fixed in 4% formalin for 10 min at RT. After two washes in PBS, sections were incubated in 20 mg/ml proteinase K in TBS for 5 min in a humidified chamber at RT. After two washes in PBS, sections were incubated in the reaction mix for 1 h at 37°C in the dark. Sections were then incubated in 2× saline-sodium citrate buffer for 15 min at RT in the dark and then washed three times in PBS before mounting in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) mounting medium (Vector Laboratories).
Sertoli Cell Preparation
The method for isolating and growing Sertoli cells was adapted from Chang et al. [36]. Briefly, testes from five 3-wk-old mice were dissected and placed in a Petri dish with sterile PBS containing 10% penicillin and streptomycin. Following decapsulation of the testes, the seminiferous tubules from each mouse were pooled in a 50-ml conical tube and washed with PBS three times. Tubules were treated with 2 mg/ml collagenase I (Sigma) and 0.5 mg/ml DNase I (Sigma) in Dulbecco modified Eagle medium (DMEM) at 37°C for 30 min on a shaker with occasional agitation by gentle pipetting. Tubules were allowed to settle and the supernatant containing Leydig cells was removed. Tubules were washed twice with DMEM and further digested with 2 mg/ml collagenase I (Sigma), 0.5 mg/mL DNase I (Sigma) and 1 mg/ml hyaluronidase type III (Sigma) at 37°C for 30 min on a shaker. Cell suspensions were collected, washed twice with DMEM, and filtered through an 80-μm nylon mesh (Falcon). Cells were plated in DMEM containing 10% fetal bovine serum (FBS) and penicillin and streptomycin at 37°C. The next day, unattached cells were removed and the medium replaced.
Immunocytochemistry
Sertoli cells grown on 12-mm glass coverslips treated with poly-l-lysine (Fisher Scientific) were fixed with 3% PFA in PBS. After incubation for 20 min at RT with blocking solution containing 0.5% BSA, 0.2% saponin, and 1% FBS in PBS, cells were incubated with rabbit anti-Sox9 polyclonal antibody (Chemicon) at a dilution of 1:200 in blocking solution for 1 h at RT in a humidifying chamber. After washing in PBS six times, cells were incubated with Alexa Fluor 488 conjugated goat anti-rabbit antibody (1:400 dilution in TBS) for 1 h at RT in the dark. After washing with PBS, 200 ng/ml of DAPI, Sigma) was used to stain cell nuclei. Samples were mounted on slides using Permount solution (Fisher Scientific) and imaged using a Carl Zeiss Axiovert 200 inverted microscope.
Testis Homogenization and Cell Lysate Preparation
Frozen testes were thawed at RT and mechanically decapsulated. The tubular fraction was incubated with homogenization buffer (1% NP40; 0.5% deoxycholate; 50 mM Tris HCl, pH 7.5; 150 mM NaCl) in an Eppendorf tube and homogenized using a Pellet Pestle (Kontes Glass Co.). The homogenate was centrifuged at 3000 rpm for 10 min at 4°C to remove nuclei. The supernatant (lysate) was assayed for protein concentration using the Dc protein assay (Bio-Rad).
Endoglycosidase Digestions
Endoglycosidase H (Endo H) from S. plicatus (Roche; 5 mU/μl) and Peptide N-glycosidase F (PNGase F; 500 U/μl) from F. meningosepticum (New England Biolabs, Inc.) were used to treat lysates as recommended by the manufacturers. Lysate containing 40 μg protein was treated with 500 U of PNGase F or 5 mU Endo H in a reaction volume of 20 μl containing buffer provided by the manufacturer, at 37°C for ∼2 h. Glycosidase was replaced by water for control reactions. Reactions were stopped by the addition of gel loading buffer, lysates were heated at 95°C for 10 min, and proteins were separated by SDS-PAGE 10% Tris-HCl polyacrylamide gels at 30 mA for 2 h.
Western Blot Analysis
Proteins were transferred from polyacrylamide gel to polyvinylidene difluoride (Perkin Elmer, Inc.) membrane overnight at 50 mA in a transfer buffer containing 10% methanol. For immunoblotting, primary and secondary antibodies were diluted in Tris-buffered saline (10 mM Tris HCl, pH 7.4, 150 mM NaCl) containing 0.05% Tween 20 (Sigma-Aldrich) and 3% nonfat dry milk (wt/vol). Anti-basigin monoclonal antibody OX114 (LifeSpan Biosciences) was diluted 1:1000 and horseradish peroxidase-conjugated rat anti-mouse secondary antibody was used at 1:10 000 dilution. The membrane was washed with the same Tris-buffered saline containing 0.05% Tween 20, incubated with Super Signal West Pico chemiluminescence reagent (Pierce), and exposed to film (Denville Scientific, Inc.).
RESULTS
Core 1 and 2 Mucin O-Glycans Are Dispensable for Spermatogenesis
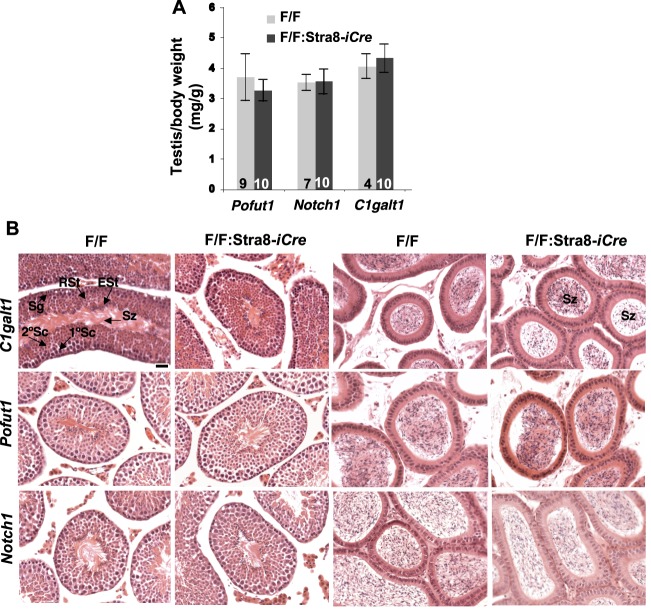
In order to identify potential roles for core 1- and 2-derived mucin O-glycans in spermatogenesis, C1galt1F/F:Stra8-iCre males were generated. The Stra8-iCre transgene is expressed specifically in spermatogonia at 3 dpp [31]. The ratio of testis:body weight (Fig. 2A), the external morphology of the testes, and histology of the testes and epididymides in sections from adult testes showed no apparent abnormalities (Fig. 2B). To detect the mucin O-glycans expressed by C1galt1F/F:Stra8-iCre testicular germ cells, lectin histochemistry was performed using PNA to identify the T antigen (Galβ3GalNAcβ-O-Ser/Thr) and VVA to detect the Tn antigen (GalNAcβ-O-Ser/Thr) expected when C1GALT1 activity is absent. C1galt1F/F testes exhibited PNA staining in all round and elongated spermatids, whereas spermatogonia and spermatocytes either did not stain, or had poor staining (Fig. 3, A and C). In contrast, ∼65% of C1galt1F/F:Stra8-iCre tubules had no staining in spermatids (Fig. 3, B and D, and Table 1), and ∼35% had partial staining with a distribution that varied from less than one quarter to three quarters of the tubule (Fig. 3B and Table 1). The partial staining was distributed in a clonal manner (arrows in Fig. 3B), suggesting that those spermatogonia in which iCre recombinase activity was not functional generated spermatids expressing the T antigen, typical of wild-type spermatids. To detect mutant germ cells that lacked C1GALT1 activity and expressed the Tn antigen, histochemical staining using VVA was performed. As expected, complementary results were obtained: ∼74% C1galt1F/F:Stra8-iCre tubules were completely positive for VVA staining (Fig. 3, H and J, and Table 1), consistent with complete loss of C1GALT1, and ∼25% tubules contained some germ cells with no VVA staining (Fig. 3, H and J, dotted lines, and Table 1). By contrast, control tubules showed no staining with VVA (Fig. 3, G and I).
FIG. 2. .
Deletion of C1galt1, Pofut1, or Notch1 in spermatogonia. A) Testis:body weight (mg/g) in 7-wk-old control littermates (F/F) and C1galt1F/F:Stra8-iCre, Pofut1F/F:Stra8-iCre or Notch1F/F:Stra8-iCre males. Error bars are SD. B) H&E of testis (left) and epididymal (right) sections from control (F/F) and conditional mutant males at 7 wk as marked. All panels at the same magnification; bar in A = 10 μm. Top left panel also identifies tubule regions containing spermatogonia (Sg), 1° spermatocytes (1°Sc), 2°Sc, round spermatids (RSt), elongated spermatids (ESt), and spermatozoa (Sz). Top right panel identifies Sz in epididymal sections.
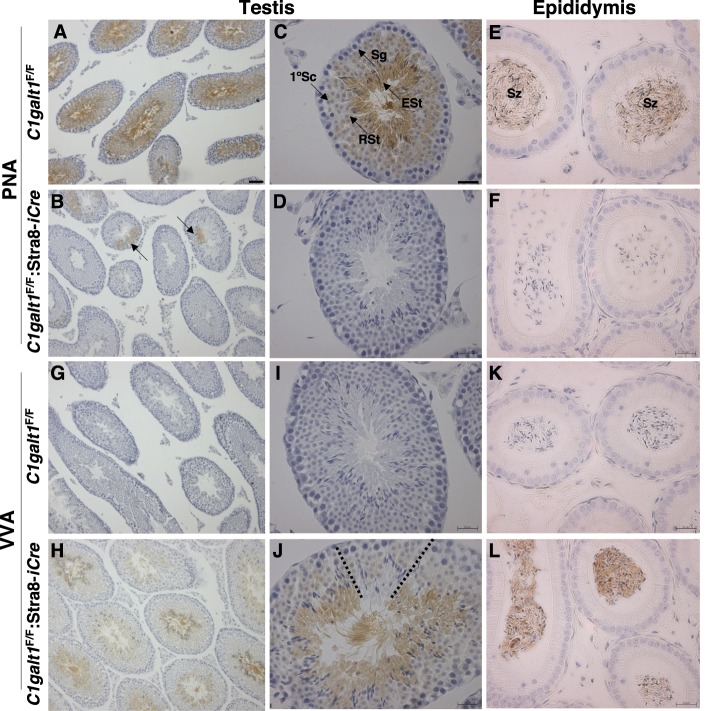
FIG. 3. .
O-glycans expressed in C1galt1F/F:Stra8-iCre testis. A, B) Binding of PNA to testis sections from 7-wk-old control and C1galt1F/F:Stra8-iCre mice. Bar in A = 50 μm. Arrows in B show small clonal regions of PNA binding in mutant testis sections. C, D) Binding of PNA to testis sections as in A, B. Bar in C = 20 μm. E, F) Binding of PNA to epididymal sections from 22-wk-old control and C1galt1F/F:Stra8-iCre mice. Top middle panel identifies tubule regions spermatogonia (Sg), 1° spermatocytes (1°Sc), round spermatids (RSt), and elongated spermatids (ESt). Top right panel identifies spermatozoa (Sz) in epididymal sections. G, H) Binding of VVA to testis sections from 7-wk-old control and C1galt1F/F:Stra8-iCre males. I, J) Binding of VVA to testis sections as in G, H. Dotted lines in J outline small clonal region in mutant testis that does not bind VVA. K, L) Binding of VVA to epididymal sections from 22-wk-old control and C1galt1F/F:Stra8-iCre mice.
TABLE 1. .
T and Tn antigen expression in C1galt1F/F:Stra8-iCre testes.*
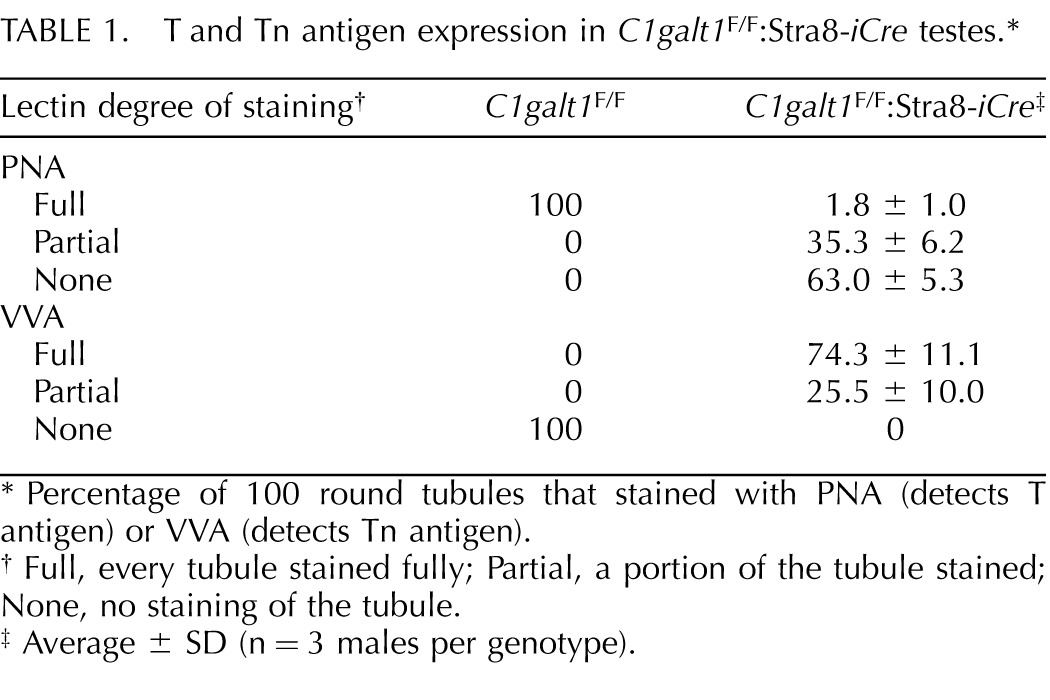
|
Lectin degree of staining† |
C1galt1F/F |
C1galt1F/F:Stra8-iCre‡ |
| PNA | ||
| Full | 100 | 1.8 ± 1.0 |
| Partial | 0 | 35.3 ± 6.2 |
| None | 0 | 63.0 ± 5.3 |
| VVA | ||
| Full | 0 | 74.3 ± 11.1 |
| Partial | 0 | 25.5 ± 10.0 |
| None | 100 | 0 |
Percentage of 100 round tubules that stained with PNA (detects T antigen) or VVA (detects Tn antigen).
Full, every tubule stained fully; Partial, a portion of the tubule stained; None, no staining of the tubule.
Average ± SD (n = 3 males per genotype).
T and Tn antigen expression were also examined in the epididymides. Sperm in the epididymal ducts of C1galt1F/F controls exhibited abundant PNA signal (Fig. 3E), whereas no signal was detected in C1galt1F/F:Stra8-iCre epididymal ducts (Fig. 3F), showing that the vast majority of sperm produced by C1galt1F/F:Stra8-iCre mice did not express the T antigen. By contrast, VVA histochemistry showed robust staining in the epididymal ducts of C1galt1F/F:Stra8-iCre males (Fig. 3L), whereas control epididymides exhibited no detectable signal (Fig. 3K).
C1galt1F/F:Stra8-iCre males mated with wild-type females were fertile, and the C1galt1 floxed allele was deleted efficiently (Table 2 and Supplemental Table S1, available online at www.biolreprod.org). Although most litters had at least one pup with a C1galt1 floxed allele, one litter had only C1galt1+/Del progeny. Taken together, the combined data show that the majority of sperm did not express the T antigen, and that transmission of a mutant allele occurred ∼80% of the time. Therefore, we conclude that deletion of C1galt1 in a large proportion of spermatogonia had no significant effect on spermatogenesis, fertility, or litter size.
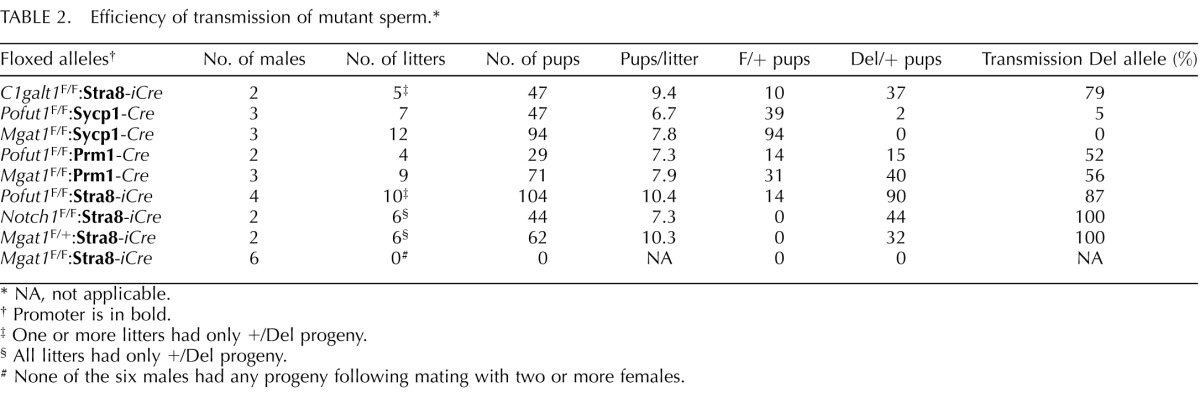
TABLE 2. .
Efficiency of transmission of mutant sperm.*
|
Floxed alleles† |
No. of males |
No. of litters |
No. of pups |
Pups/litter |
F/+ pups |
Del/+ pups |
Transmission Del allele (%) |
| C1galt1F/F:Stra8-iCre | 2 | 5‡ | 47 | 9.4 | 10 | 37 | 79 |
| Pofut1F/F:Sycp1-Cre | 3 | 7 | 47 | 6.7 | 39 | 2 | 5 |
| Mgat1F/F:Sycp1-Cre | 3 | 12 | 94 | 7.8 | 94 | 0 | 0 |
| Pofut1F/F:Prm1-Cre | 2 | 4 | 29 | 7.3 | 14 | 15 | 52 |
| Mgat1F/F:Prm1-Cre | 3 | 9 | 71 | 7.9 | 31 | 40 | 56 |
| Pofut1F/F:Stra8-iCre | 4 | 10‡ | 104 | 10.4 | 14 | 90 | 87 |
| Notch1F/F:Stra8-iCre | 2 | 6§ | 44 | 7.3 | 0 | 44 | 100 |
| Mgat1F/+:Stra8-iCre | 2 | 6§ | 62 | 10.3 | 0 | 32 | 100 |
| Mgat1F/F:Stra8-iCre | 6 | 0# | 0 | NA | 0 | 0 | NA |
NA, not applicable.
Promoter is in bold.
One or more litters had only +/Del progeny.
All litters had only +/Del progeny.
None of the six males had any progeny following mating with two or more females.
Notch Signaling Is Dispensable for Spermatogenesis
Deletion of the Pofut1 and Notch1 genes was investigated using various germ cell-specific Cre recombinase transgenes. Male mice carrying two Pofut1 floxed alleles [21, 35] and the Sycp1-Cre transgene expressed in spermatocytes [29] or Prm1-Cre expressed in spermatids [28] were mated with C57Bl/6 females. The deletion of Pofut1F alleles occurred only in the first litter from Sycp1-Cre males, whereas deletion by the Prm1-Cre transgene was ∼50% efficient (Table 2 and Supplemental Table S1). Nevertheless, no differences from controls were observed in litter size, in body or testis weights, or in the morphology or histology of testes or epididymides of Pofut1F/F:Prm1-Cre males (data not shown).
Floxed Pofut1 alleles were deleted in spermatogonia using the Stra8-iCre transgene [31]. Deletion efficiency was much improved with this transgene (Table 2 and Supplemental Table S1). Three litters from two Pofut1F/F:Stra8-iCre males mated to wild-type females gave rise to solely Pofut1+/Del progeny. Inactivation of Pofut1 had no apparent effect on testis morphology or weight (Fig. 2A), number of litters or pups per litter (Table 2 and Supplemental Table S1). Testis sections also showed no histological defects in seminiferous tubules from Pofut1F/F:Stra8-iCre males (Fig. 2B). Epididymal ducts from Pofut1F/F:Stra8-iCre males were full of sperm and indistinguishable from controls (Fig. 2B). Similar results were obtained following inactivation of Notch1 in spermatogonia using Stra8-iCre, which occurred with 100% efficiency in six litters from two Notch1F/F:Stra8-iCre males (Table 2 and Supplemental Table S1). There was no effect of the loss of NOTCH1 on fertility, and testis:body weight ratios at 7 wk revealed no significant differences (Fig 2A). Hematoxylin and eosin (H&E) staining of testis sections from 7-wk-old mice showed no abnormalities and epididymides contained abundant sperm (Fig. 2B). Therefore, signaling via NOTCH1 in germ cells is not required for spermatogenesis. In addition, signaling via the other Notch receptors is not required because loss of POFUT1, a necessary component of Notch signaling through all four receptors [21, 22], had no apparent effect on spermatogenesis, as recently also observed with a different Pofut1 mutation and Cre transgene [11].
A role for Notch signaling in Sertoli cells was examined by deletion of Pofut1 using an Amh-Cre transgene [32]. Conditional mutant males at 6–8 mo showed deletion of Pofut1 in testis but not in tail DNA (Fig. 4A). Sertoli cell preparations from 3-wk-old Pofut1F/F:Amh-Cre males expressed Sox9, a transcription factor specific to Sertoli cells in testis [37], in > 50% of nuclei of Sertoli cell preparations from both control and Pofut1F/F:AMH-Cre males (data not shown). However, the deletion efficiency of Pofut1 in Sertoli cells cultured from 3-wk-old Pofut1F/F:AMH-Cre mice was markedly lower than in 6-mo-old testis (Fig. 4B). Testis sections from 6-mo-old males indicated that the stages of spermatogenesis in Pofut1F/F:Amh-Cre and control males were indistinguishable (not shown). Sperm counts from caudal epididymides in 6- to 8-mo-old mice were similar in Pofut1F/F:Amh-Cre and controls (Fig. 4C). Fertility was assessed by mating 1.5- to 6-mo-old Pofut1F/F:Amh-Cre (n = 9) or control (n = 8) males with C57BL/6 females. Litter sizes from both groups were similar (Fig. 4D). However, body weight in some Pofut1F/F:Amh-Cre males was reduced compared to controls, increasing the average testis:body weight (Fig. 4E). The number of apoptotic cells per 100 round tubules was comparable between 6- to 8-mo-old Pofut1F/F:Amh-Cre+ males and controls (Fig. 4F). Therefore, deletion of Pofut1 in a substantial proportion of Sertoli cells did not significantly affect spermatogenesis or fertility.
FIG. 4. .
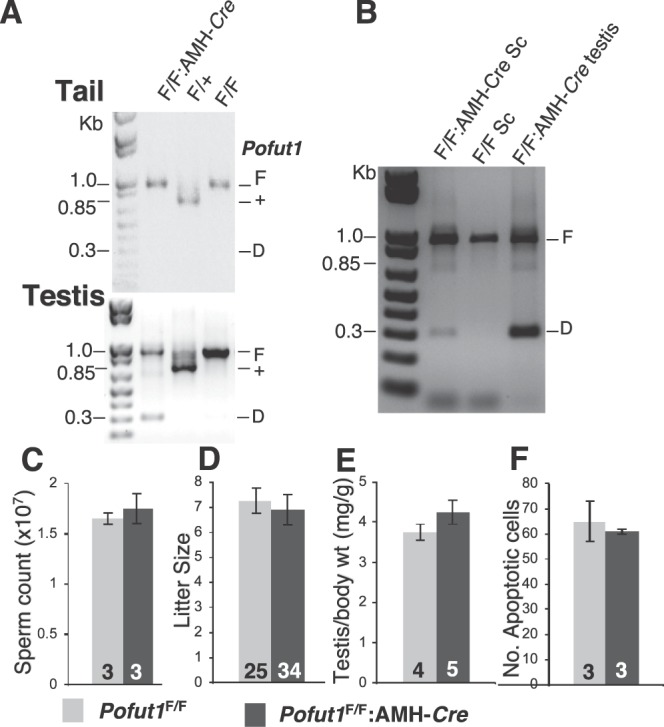
Deletion of Pofut1 in Sertoli cells. A) Deletion of floxed Pofut1 alleles in testes of Pofut1F/F:AMH-Cre males. Genomic DNA from mouse tail or testes of 6- to 8-mo-old control and Pofut1F/F:AMH-Cre males was genotyped by PCR using primers PS644 and PS645 [21, 35] that amplify a 960-bp product from the floxed Pofut1 allele (F), a 700-bp product from wild-type allele (+), and a 300-bp product from the deleted allele (D). B) PCR genotyping of DNA from Sertoli cells (Sc) isolated from 3-wk-old mice and from 6-mo-old testis using primers PS644 and PS645. C) Sperm count from one caudal epididymis per mouse was examined in 6- to 8-mo-old control and Pofut1F/F:AMH-Cre mice (n = 3 each genotype). D) Litter sizes from matings of Pofut1F/F:AMH-Cre or control Pofut1F/F males with C57Bl/6 females. Eight controls had 25 litters and nine Pofut1F/F:AMH-Cre males had 34 litters. E) Testis:body weight in 6- to 8-mo-old control and Pofut1F/F:AMH-Cre males. F) Apoptosis was quantified as the average number of apoptotic cells per 100 round testis tubules. Error bars are SEM.
MGAT1 and Complex N-Glycans Are Essential for Spermatogenesis
Deletion of Mgat1 in spermatogonia gave a dramatic phenotype. Mgat1F/F:Stra8-iCre males were completely infertile (Table 2 and Supplemental Table S1). Importantly, the infertility of Mgat1F/F:Stra8-iCre males was not observed in heterozygous Mgat1F/+:Stra8-iCre males (Table 2 and Supplemental Table S1). Deletion efficiency was 100% in all litters from two heterozygous males, giving 62 pups with no transmission of a floxed allele. Histology of testes and epididymides from Mgat1F/+:Stra8-iCre males revealed no differences from controls; intratubular structure and germ cell distribution were normal, and epididymal ducts were full of sperm (data not shown). Therefore a single Mgat1 wild-type allele is sufficient for normal spermatogenesis, and the Stra8-iCre transgene has no effect on fertility, as expected [31].
At 7 wk, Mgat1F/F:Stra8-iCre males exhibited a significant decrease in testis:body weight due to a decrease in testis weight of ∼33% (Fig. 5A). At 22 wk, Mgat1F/F:Stra8-iCre males exhibited a reduction in both testis and body weights (Fig. 5B). As a potential basis of testicular weight loss, apoptosis was investigated using a TUNEL assay. Compared to control testis sections from 7-wk-old males, the number of apoptotic germ cells was increased within the tubules of sections from Mgat1F/F:Stra8-iCre males, whereas parenchymal cells outside the tubules were not affected (Fig. 6, A–F). Two parameters were significantly increased in males with germ cells lacking MGAT1: 1) the number of tubules containing apoptotic cells rose from ∼25% in controls to ∼90% in mutant tubules (Fig. 6G); and 2) the number of apoptotic cells per positive tubule was increased by ∼3.5-fold in mutant compared to control tubules (Fig. 6H).
FIG. 5. .
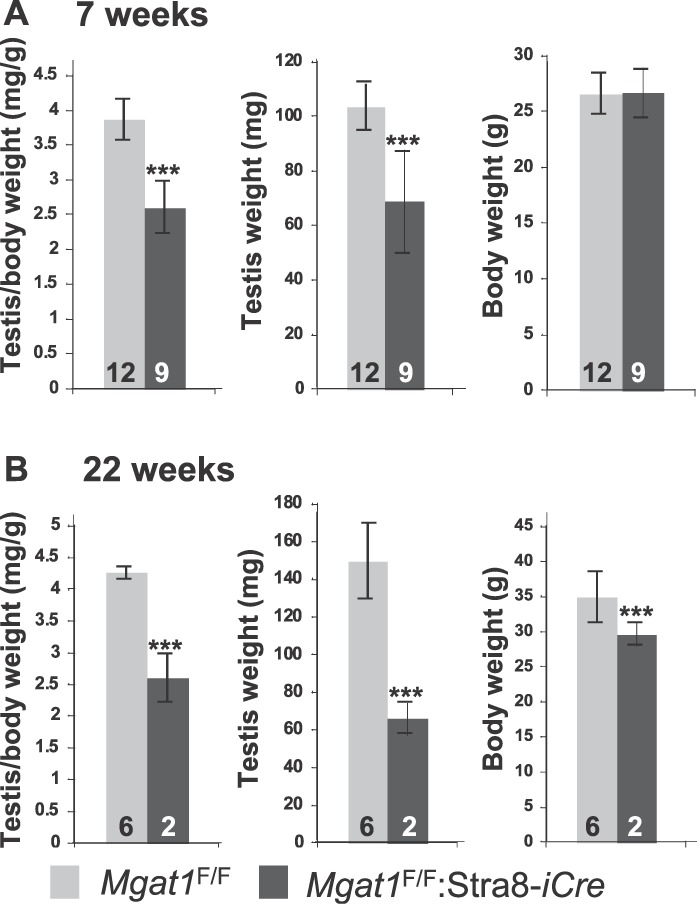
Deletion of Mgat1 in spermatogonia reduces testis weight. A) Testis and body weights of control and Mgat1F/F:Stra8-iCre males at 7 wk of age. B) Testis and body weights of control and Mgat1F/F:Stra8-iCre males at 22 wk of age. Error bars are SD; ***P value below 0.001 based on the Student t-test.
FIG. 6. .
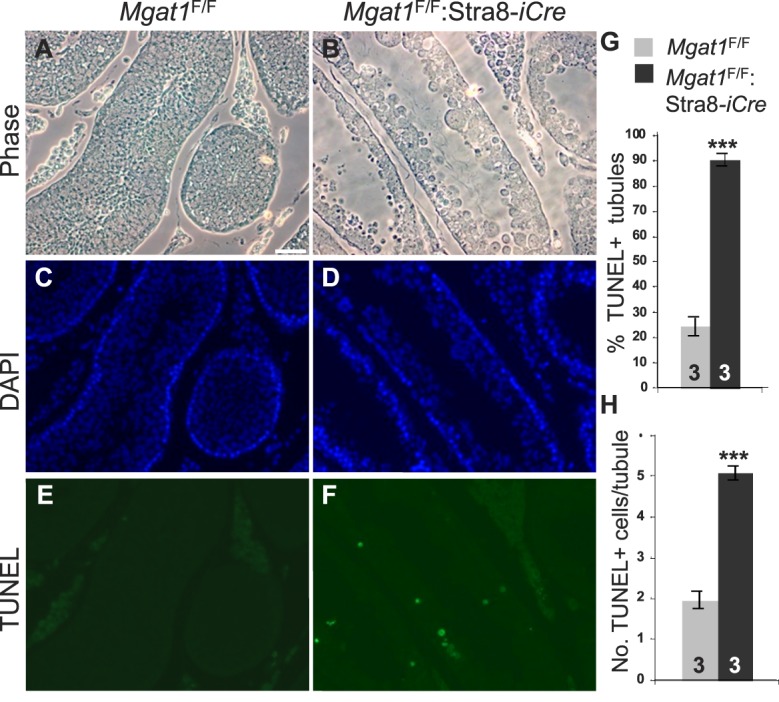
Apoptosis is increased in Mgat1F/F:Stra8-iCre testes. A–F) Testis sections from 7-wk-old males subjected to the TUNEL assay and imaged by phase or fluorescence microscopy for apoptotic cells (green) and nuclei stained by DAPI (blue). Bar in A = 50 μm. G) The percentage of tubules containing apoptotic cells. H) The total number of apoptotic cells per apoptotic tubule. Three control and mutant testes and two independent sections for each were analyzed. Error bars are SD; ***P value below 0.001 based on the Student t-test.
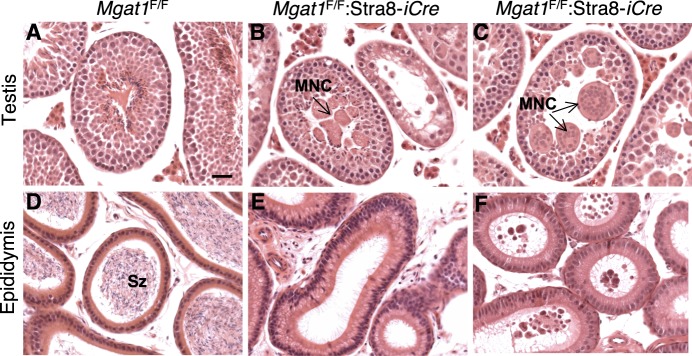
The architecture of seminiferous tubules in sections from Mgat1F/F:Stra8-iCre mice was greatly disrupted compared to controls (Fig. 7, A–C). All tubules from 7-wk-old Mgat1F/F:Stra8-iCre mice contained multinucleated giant cells (MNCs) or symplasts located in the region normally occupied by spermatids (Fig. 7, B and C). The MNCs were composed of either round or elongating spermatids, suggesting that differentiation proceeded to some extent. Histological analysis of sectioned epididymides from 7-wk-old mice showed that Mgat1F/F:Stra8-iCre mice are unable to produce sperm (Fig. 7, E compared to D). At 22 wk, Mgat1F/F:Stra8-iCre epididymides were completely devoid of spermatozoa but occasionally contained small MNCs or aberrant spermatids (Fig. 7F). We also analyzed the simultaneous deletion of Mgat1 and C1galt1 in spermatogonia. Mgat1F/F:C1galt1F/F:Stra8-iCre males (n = 8) showed the same testis and epididymal defects observed in sections from Mgat1F/F:Stra8-iCre males (data not shown). Therefore, loss of C1GALT1 along with MGAT1 did not worsen or ameliorate the phenotype observed with loss of MGAT1 alone.
FIG. 7. .
Complex N-glycans are essential for spermatogenesis. A–C) H&E-stained testis sections of 7-wk-old Mgat1F/F and Mgat1F/F:Stra8-iCre males. MNC labels multinucleated cells. D–F) H&E-stained epididymal sections of 7-wk-old Mgat1F/F and Mgat1F/F:Stra8-iCre males (D, E) or 22 wk of age (F). Degenerate germ cells accumulated in epididymides of adult Mgat1F/F:Stra8-iCre males (F). Sz, spermatozoa. Bar in A = 10 μm.
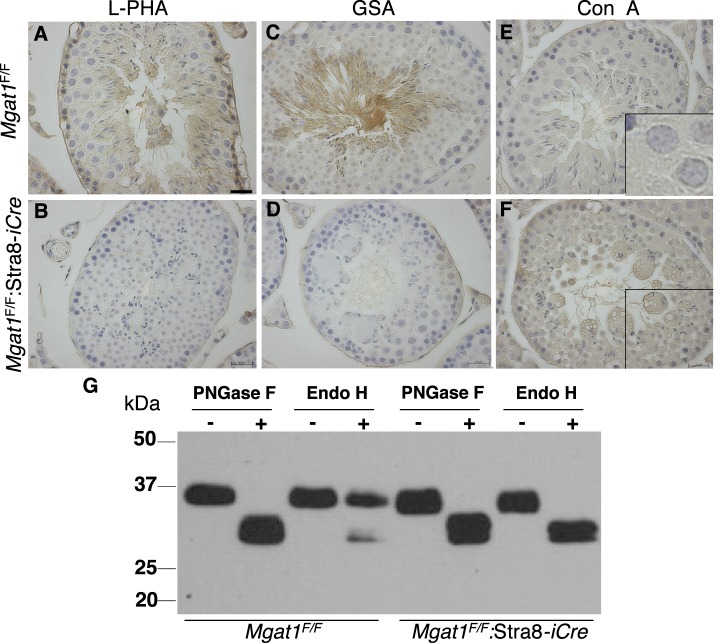
To determine that deletion of Mgat1 in spermatogonia led to loss of complex N-glycans on germ cell glycoproteins, testis sections from 7-wk-old control and Mgat1F/F:Stra8-iCre males were investigated for staining by the lectins L-PHA, GSA, and Con A. L-PHA and GSA bind to complex N-glycans, but not to high-mannose N-glycans that predominate in cells lacking MGAT1 (Fig. 1). By contrast, Con A is a lectin that binds preferentially to high-mannose N-glycans. As previously reported in rats [4, 5], L-PHA bound with greatest intensity to the cell surface of spermatogonia and elongated spermatids, whereas spermatocytes bound less L-PHA (Fig. 8A). However, sections from Mgat1F/F:Stra8-iCre males showed only background intratubular L-PHA binding, consistent with loss of complex N-glycans (Fig. 8B). For GSA, binding to control sections was most prominent for spermatids and spermatozoa, as observed previously [12, 38] (Fig. 8C). Consistent with the absence of complex N-glycans, GSA binding was not observed in sections from Mgat1F/F:Stra8-iCre males (Fig. 8D). By contrast, Con A binding was slightly less in control compared to Mgat1F/F:Stra8-iCre males (Fig. 8, E and F). Moreover, at higher power, the intensity of the Con A signal was more easy to observe in germ cells that had lost MGAT1 (Fig. 8, E and F, insets). There was no evidence of partial deletion of Mgat1. Thus germ cells in all tubules from Mgat1F/F:Stra8-iCre males lacked binding of L-PHA and GSA, and bound Con A.
FIG. 8. .
Mgat1F/F:Stra8-iCre males do not produce complex N-glycans in germ cells. A, B) Binding of L-PHA to testis sections from 7-wk-old control and Mgat1F/F:Stra8-iCre males. C, D) Binding of GSA to testis sections as in A, B. E, F) Binding of Con A to testis sections as in A, B. Bar in A = 20 μm. G. Lysates from control and Mgat1F/F:Stra8-iCre decapsulated testes were digested with (+) PNGase F or Endo H, or incubated without (−) enzyme, and subjected to immunoblotting using anti-basigin mAb.
To further characterize deletion efficiency of the Mgat1 floxed allele in testis, the glycans present on basigin, an N-glycosylated protein belonging to the immunoglobulin superfamily expressed in germ cells and important for spermatogenesis [39], were examined. Decapsulated testis extracts were treated with PNGase F to remove all N-glycans, or Endo H to remove only oligomannosyl N-glycans, and subjected to Western blot analysis using an anti-basigin monoclonal antibody. In control Mgat1F/F lysate digestion with PNGase F reduced the MW of both basigin bands by ∼4 kDa (Fig. 8G). Endo H treatment showed that the majority of testis basigin carries complex N-glycans that are resistant to Endo H. However, a small fraction was digested to a band of the size lacking all N-glycans and obtained with PNGase F digestion. Therefore, a portion of basigin carries only oligomannosyl N-glycans (Fig. 8G). These are likely to be present on basigin in spermatocytes where MGAT1 transcripts are low and GnT1IP, a physiological inhibitor of MGAT1, is maximally expressed [40, 41]. Importantly, Mgat1F/F:Stra8-iCre testis extracts were completely sensitive to Endo H, showing that Mgat1F/F:Stra8-iCre germ cells produce no detectable complex N-glycans (Fig. 8G).
DISCUSSION
Several classes of glycans are differentially expressed during spermatogenesis in rodents as shown by the binding of various lectins to germ and supporting cells of the testis [4–8]. Here we investigated requirements for major subclasses of N- and O-glycans, and for Notch signaling, by conditional deletion of glycosyltransferase genes or Notch1 using a variety of promoters to express Cre recombinase in specific germ cells or Sertoli cells. Deletion of floxed alleles with 100% efficiency was obtained only with the Stra8-iCre transgene expressed in Type A spermatogonia beginning at 3 dpp [31], and only at two loci: Notch1 and Mgat1. At the Pofut1 and C1galt1 loci 100% transmission of a deleted allele was observed only in some litters.
O-glycans generated by C1GALT1 are strongly expressed by round and elongated spermatids in mammals [5, 19]. The T antigen detected by PNA is a long-used acrosomal marker of spermatids [42]. The mucin O-glycans produced by C1galt1F/F:Stra8-iCre germ cells show the expected loss of expression of T antigen in all tubules. However, ∼35% of tubules had partial, apparently clonal, expression of T antigen, indicating incomplete deletion of both C1galt1 alleles in some spermatogonia. However, few unaffected sperm were observed in epididymides. Thus, we conclude that core 1- and 2-derived mucin O-glycans are not required for spermatogenesis. This is consistent with the finding that mice lacking all three core 2 branching N-acetylglucosaminyltransferases that act subsequently to C1GALT1 are fertile [43]. However, males lacking polypeptide N-acetylgalactosaminyltransferase 3 that transfers GalNAc to protein, and acts prior to C1GALT1, are infertile, with delayed spermatogenesis, azoospermia, and defective spermatozoa formation [13]. This suggests either that ppGalNAcT-3 does not provide a substrate for core 1- and 2-derived O-glycans in germ cells, or that ppGalNAcT-3 has important functions in Sertoli cells. Conditional deletion of ppGalNAcT-3 would be necessary to determine in which cell type(s) it is required during spermatogenesis. Finally, it is interesting that germ cells lacking C1GALT1, and having terminal GalNAc instead of Gal at O-glycan sites, are not noticeably compromised in functional terms. It will be important in future studies to identify O-glycan sites in germ cell glycoproteins, and to determine if the GalNAc recognized by VVA in C1galt1F/F:Stra8-iCre glycoproteins is modified in any way.
Despite predictions that Notch signaling would be essential for spermatogenesis [9–-11,] 23], there were no apparent consequences for testicular morphology, germ cell production, or fertility from deletion of Pofut1 and O-fucose glycans in germ cells. Deletion of Pofut1 in a significant proportion of Sertoli cells also did not perturb spermatogenesis at the histological level, nor in terms of fertility. Similar findings were recently obtained using a different conditional Pofut1 allele, deleted using different Cre transgenes targeted to either Sertoli or germ cells, respectively [11]. Notch signaling was similarly predicted to be necessary for oogenesis because Notch pathway genes are expressed in oocytes and follicle cells [23, 44, 45]. However, the generation of females lacking POFUT1 or CSL (RBP-Jκ) in primary oocytes showed that loss of canonical Notch signaling does not disrupt oogenesis [35, 46].
Our findings that deletion of Notch1 in germ cells has no effect on spermatogenesis are consistent with the Pofut1 deletion data, and with the fact that Notch1 transcripts are not detected in germ cells by in situ hybridization [11]. Rescue due to redundant properties of other Notch receptors is very unlikely, especially because expression of Notch2, Notch3, and Notch4 was not detected by in situ hybridization [11]. However, Jag1 and Jag2 are expressed in germ cells and are substrates of POFUT1 [47]. Because NOTCH1 in Sertoli cells is active in Notch signaling that is proposed to be induced by JAG2 in germ cells [11], the loss of POFUT1 and O-fucose glycans on JAG2 EGF repeats has no obvious functional affects. Testicular germ cells therefore provide an excellent opportunity to investigate functions of JAG1 and JAG2 in the absence of cis-interactions with Notch receptors.
MGAT1 and complex N-glycans are essential for embryonic development beyond midgestation [48, 49]. We show here that complex N-glycans on glycoproteins in male germ cells are also essential for spermatogenesis. Testis weight in Mgat1F/F:Stra8-iCre adult males was ∼33% less than controls, and apoptosis was increased ∼3.5-fold. Interestingly, apoptosis was observed not only in spermatogonia but also in spermatocytes and spermatids, presumably reflecting their inability to develop in association with Sertoli cells. There was essentially no binding of L-PHA to germ cells in Mgat1F/F:Stra8-iCre males, showing highly efficient inhibition of complex N-glycan synthesis. All tubules in Mgat1F/F:Stra8-iCre males were aberrant and contained MNCs, some with spermatids having elongated nuclei. This suggests that the program of round-to-elongated spermatid differentiation begins in the absence of functional MGAT1, but is impaired before completion. The MNC phenotype has been observed in males lacking complex glycolipids in germ and supporting cells [14, 50], and is usually attributed to the premature opening of intercellular bridges following loss of spermatid contact with Sertoli cells [51]. A related phenotype was observed with the inactivation of the Man2a2 gene that acts after MGAT1 in the generation of complex N-glycans [12]. However, this mouse lacks α-mannosidase IIX in all cells, and is also complicated by the presence of Man2a1 in testis [52], an enzyme that may partially rescue the loss of Man2a2 [53]. To investigate a direct role for the loss of complex N-glycans generated by deletion of α-mannosidase II activities, a conditional knockout of Man2a1 and Man2a2 would be necessary. Another related MNC phenotype in seminiferous tubules was observed with the inactivation of the Cadm1 gene [54]. CADM1 is a glycoprotein that carries N-glycans contributing ∼25% to molecular weight [55]. It is expressed at the surface of spermatogonia and mediates binding to Sertoli cells [56]. CADM1 is a potential target of MGAT1 as it may need complex N-glycans to function during spermatogenesis.
A recently identified physiological inhibitor of MGAT1 termed GlcNAcT-I inhibitory protein (GnT1IP) is also predicted to be functionally important in spermatogenesis [41]. The GnT1IP gene is expressed almost solely in testis [57, 58]. It is transcriptionally up-regulated in spermatocytes at 22 dpp [41], when Mgat1 transcripts are down-regulated [40], and GnT1IP is translationally activated at the same stage [59]. A membrane-anchored form, GnT1IP-L, specifically inhibits MGAT1 and the synthesis of complex and hybrid N-glycans. The inhibition of MGAT1 causes the expression of high-mannose N-glycans on spermatocytes, and this may be reflected in the fraction of basigin with N-glycans that are fully sensitive to Endo H (Fig. 8G). Cells expressing GnT1IP-L adhere more avidly to Sertoli cell lines in cell adhesion assays, suggesting a role for GnT1IP-L in germ-Sertoli cell interactions [41]. Elucidating the mechanisms by which complex and oligomannosyl N-glycans are required for spermatogenesis will reveal critical MGAT1 and GnT1IP-L glycoprotein targets and novel roles for complex N-glycosylation during germ cell maturation.
ACKNOWLEDGMENT
The authors are very grateful to, and thank, all those who provided mouse strains (see Materials and Methods), Rani Sellers and Paula Cohen for expert advice, and Huimin Shang, Wen Dong, and Subha Sundaram for excellent technical assistance.
Footnotes
Supported by NCI grant RO1 CA030645 from the National Cancer Institute to P.S. and in part by the Albert Einstein Cancer Center grant from the NCI PO1 CA013330.
Current address: Department of Pediatrics-Hematology/Oncology, Baylor College of Medicine, Houston, TX 77030.
Current address: Nuffield Department of Obstetrics & Gynaecology, University of Oxford, Women's Centre, Level 3, John Radcliffe Hospital, Oxford, UK, OX3 9DU.
REFERENCES
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech 2010; 73: 241 278 [DOI] [PubMed] [Google Scholar]
- Nalam RL, Matzuk MM. Local signalling environments and human male infertility: what we can learn from mouse models. Expert Rev Mol Med 2010; 12: e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsenko AN, Iwamori N, Iwamori T, Matzuk MM. The power of mouse genetics to study spermatogenesis. J Androl 2010; 31: 34 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ, Morrison CA, Stoddart RW. Histochemical analysis of rat testicular glycoconjugates. 2. Beta-galactosyl residues in O- and N-linked glycans in seminiferous tubules. Histochem J 1992; 24: 327 336 [DOI] [PubMed] [Google Scholar]
- Jones CJ, Morrison CA, Stoddart RW. Histochemical analysis of rat testicular glycoconjugates. 1. Subsets of N-linked saccharides in seminiferous tubules. Histochem J 1992; 24: 319 326 [DOI] [PubMed] [Google Scholar]
- Lee MC, Damjanov I. Anatomic distribution of lectin-binding sites in mouse testis and epididymis. Differentiation 1984; 27: 74 81 [DOI] [PubMed] [Google Scholar]
- Lemaire L, Heinlein UA. Stage-specific mouse testis cell surface alterations detected by fluorescence-labeled lectins. Cell Biol Int Reports 1992; 16: 675 677 [DOI] [PubMed] [Google Scholar]
- Lohr M, Kaltner H, Schwartz-Albiez R, Sinowatz F, Gabius HJ. Towards functional glycomics by lectin histochemistry: strategic probe selection to monitor core and branch-end substitutions and detection of cell-type and regional selectivity in adult mouse testis and epididymis. Anat Histol Embryol 2010; 39: 481 493 [DOI] [PubMed] [Google Scholar]
- Dirami G, Ravindranath N, Achi MV, Dym M. Expression of Notch pathway components in spermatogonia and Sertoli cells of neonatal mice. J Androl 2001; 22: 944 952 [DOI] [PubMed] [Google Scholar]
- Mori S, Kadokawa Y, Hoshinaga K, Marunouchi T. Sequential activation of Notch family receptors during mouse spermatogenesis. Dev Growth Differ 2003; 45: 7 13 [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Okamura Y, Saga Y. Notch signaling in Sertoli cells regulates cyclical gene expression of Hes1 but is dispensable for mouse spermatogenesis. Mol Cell Biol 2011; 32: 206 215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama TO, Nakagawa H, Sugihara K, Narisawa S, Ohyama C, Nishimura S, O'Brien DA, Moremen KW, Millan JL, Fukuda MN. Germ cell survival through carbohydrate-mediated interaction with Sertoli cells. Science 2002; 295: 124 127 [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Sorenson AH, Austin AM, Mackenzie DS, Fritz TA, Moh A, Hui SL, Econs MJ. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology 2009; 150: 2543 2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff R, Geyer R, Jennemann R, Paret C, Kiss E, Yamashita T, Gorgas K, Sijmonsma TP, Iwamori M, Finaz C, Proia RL, Wiegandt H. et al. Novel class of glycosphingolipids involved in male fertility. J Biol Chem 2005; 280: 27310 27318 [DOI] [PubMed] [Google Scholar]
- Brockhausen I, Schachter H, Stanley P. O-GalNAc glycans. : Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, GW, Hart, Etzler ME. (eds.), Essentials of Glycobiology, 2nd Ed Cold Spring Harbor (NY); 2009: 115 127 [PubMed] [Google Scholar]
- Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol 2004; 164: 451 459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Stanley P. Mouse fertility is enhanced by oocyte-specific loss of core 1-derived O-glycans. FASEB J 2008; 22: 2273 2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Xia L, Cummings RD, McEver RP, Stanley P. Fertilization in mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans. J Cell Sci 2007; 120: 1341 1349 [DOI] [PubMed] [Google Scholar]
- Agungpriyono S, Kurohmaru M, Kimura J, Wahid AH, Sasaki M, Kitamura N, Yamada J, Fukuta K, Zuki AB. Distribution of lectin-bindings in the testis of the lesser mouse deer, Tragulus javanicus. Anat Histol Embryol 2009; 38: 208 213 [DOI] [PubMed] [Google Scholar]
- Wang Y, Shao L, Shi S, Harris RJ, Spellman MW, Stanley P, Haltiwanger RS. Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J Biol Chem 2001; 276: 40338 40345 [DOI] [PubMed] [Google Scholar]
- Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci U S A 2003; 100: 5234 5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y, Saga Y. Pofut1 is required for the proper localization of the Notch receptor during mouse development. Mech Dev 2008; 125: 663 673 [DOI] [PubMed] [Google Scholar]
- Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development 2005; 132: 817 828 [DOI] [PubMed] [Google Scholar]
- von Schonfeldt V, Wistuba J, Schlatt S. Notch-1, c-kit and GFRalpha-1 are developmentally regulated markers for premeiotic germ cells. Cytogenet Genome Res 2004; 105: 235 239 [DOI] [PubMed] [Google Scholar]
- Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development 2008; 135: 3745 3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Liu T, Hou X, Stanley P. In vivo consequences of deleting EGF repeats 8–12 including the ligand binding domain of mouse Notch1. BMC Dev Biol 2008; 8: 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Stanley P. The O-fucose glycan in the ligand-binding domain of Notch1 regulates embryogenesis and T cell development. Proc Natl Acad Sci U S A 2008; 105: 1539 1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A 1997; 94: 14602 14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal F, Sage J, Cuzin F, Rassoulzadegan M. Cre expression in primary spermatocytes: a tool for genetic engineering of the germ line. Mol Reprod Dev 1998; 51: 274 280 [DOI] [PubMed] [Google Scholar]
- Chung SS, Cuzin F, Rassoulzadegan M, Wolgemuth DJ. Primary spermatocyte-specific Cre recombinase activity in transgenic mice. Transgenic Res 2004; 13: 289 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 2008; 46: 738 742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecureuil C, Fontaine I, Crepieux P, Guillou F. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 2002; 33: 114 118 [DOI] [PubMed] [Google Scholar]
- Ye Z, Marth JD. N-glycan branching requirement in neuronal and postnatal viability. Glycobiology 2004; 14: 547 558 [DOI] [PubMed] [Google Scholar]
- Shi S, Williams SA, Seppo A, Kurniawan H, Chen W, Ye Z, Marth JD, Stanley P. Inactivation of the Mgat1 gene in oocytes impairs oogenesis, but embryos lacking complex and hybrid N-glycans develop and implant. Mol Cell Biol 2004; 24: 9920 9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Stahl M, Lu L, Stanley P. Canonical Notch signaling is dispensable for early cell fate specifications in mammals. Mol Cell Biol 2005; 25: 9503 9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR. Wt1 negatively regulates beta-catenin signaling during testis development. Development 2008; 135: 1875 1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 5: intercellular junctions and contacts between germs cells and Sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins associated with more than one germ cell generation. Microsc Res Tech 2010; 73: 409 494 [DOI] [PubMed] [Google Scholar]
- Fukuda MN, Akama TO. In vivo role of alpha-mannosidase IIx: ineffective spermatogenesis resulting from targeted disruption of the Man2a2 in the mouse. Biochim Biophys Acta 2002; 1573: 382 387 [DOI] [PubMed] [Google Scholar]
- Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O. et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol 1998; 194: 152 165 [DOI] [PubMed] [Google Scholar]
- Chalmel F, Rolland AD, Niederhauser-Wiederkehr C, Chung SS, Demougin P, Gattiker A, Moore J, Patard JJ, Wolgemuth DJ, Jegou B, Primig M. The conserved transcriptome in human and rodent male gametogenesis. Proc Natl Acad Sci U S A 2007; 104: 8346 8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HH, Stanley P. A testis-specific regulator of complex and hybrid N-glycan synthesis. J Cell Biol 2010; 190: 893 910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom KO, Malmi R, Karjalainen K. Binding of fluorescein isothiocyanate conjugated lectins to rat spermatogenic cells in tissue sections. Enhancement of lectin fluorescence obtained by fixation in Bouin's fluid. Histochemistry 1984; 80: 575 579 [DOI] [PubMed] [Google Scholar]
- Stone EL, Ismail MN, Lee SH, Luu Y, Ramirez K, Haslam SM, Ho SB, Dell A, Fukuda M, Marth JD. Glycosyltransferase function in core 2-type protein O glycosylation. Mol Cell Biol 2009; 29: 3770 3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier S, Vandormael-Pournin S, Babinet C, Cohen-Tannoudji M. Developmental expression of the Notch signaling pathway genes during mouse preimplantation development. Gene Exp Patterns 2004; 4: 713 717 [DOI] [PubMed] [Google Scholar]
- Johnson J, Espinoza T, McGaughey RW, Rawls A, Wilson-Rawls J. Notch pathway genes are expressed in mammalian ovarian follicles. Mech Dev 2001; 109: 355 361 [DOI] [PubMed] [Google Scholar]
- Souilhol C, Cormier S, Tanigaki K, Babinet C, Cohen-Tannoudji M. RBP-Jkappa-dependent notch signaling is dispensable for mouse early embryonic development. Mol Cell Biol 2006; 26: 4769 4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panin VM, Shao L, Lei L, Moloney DJ, Irvine KD, Haltiwanger RS. Notch ligands are substrates for protein O-fucosyltransferase-1 and Fringe. J Biol Chem 2002; 277: 29945 29952 [DOI] [PubMed] [Google Scholar]
- Ioffe E, Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci U S A 1994; 91: 728 732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler M, Gertz A, Sarkar M, Schachter H, Schrader JW, Marth JD. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J 1994; 13: 2056 2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamiya K, Yamamoto A, Furukawa K, Zhao J, Fukumoto S, Yamashiro S, Okada M, Haraguchi M, Shin M, Kishikawa M, Shiku H, Aizawa S. Complex gangliosides are essential in spermatogenesis of mice: possible roles in the transport of testosterone. Proc Natl Acad Sci U S A 1998; 95: 12147 12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obholz KL, Akopyan A, Waymire KG, MacGregor GR. FNDC3A is required for adhesion between spermatids and Sertoli cells. Dev Biol 2006; 298: 498 513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr B, Voolstra C, Heinen TJ, Baines JF, Rottscheidt R, Ihle S, Muller W, Bonhomme F, Tautz D. A change of expression in the conserved signaling gene MKK7 is associated with a selective sweep in the western house mouse Mus musculus domesticus. J Evol Biol 2006; 19: 1486 1496 [DOI] [PubMed] [Google Scholar]
- Akama TO, Nakagawa H, Wong NK, Sutton-Smith M, Dell A, Morris HR, Nakayama J, Nishimura S, Pai A, Moremen KW, Marth JD, Fukuda MN. Essential and mutually compensatory roles of {alpha}-mannosidase II and α-mannosidase IIx in N-glycan processing in vivo in mice. Proc Natl Acad Sci U S A 2006; 103: 8983 8988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L, Arends MJ, Chausiaux OE, Ellis PJ, Lange UC, Surani MA, Affara N, Murakami Y, Adams DJ, Bradley A. Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of spermatogenesis. Mol Cell Biol 2006; 26: 3595 3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Yoshida M, Williams YN, Fukami T, Kikuchi S, Masuda M, Maruyama T, Ohta T, Nakae D, Maekawa A, Kitamura T, Murakami Y. Disruption of spermatogenic cell adhesion and male infertility in mice lacking TSLC1/IGSF4, an immunoglobulin superfamily cell adhesion molecule. Mol Cell Biol 2006; 26: 3610 3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Sai Y, Ito A, Kato Y, Kurobo M, Murakami Y, Nakashima E, Tsuji A, Kitamura Y, Iseki S. Heterophilic binding of the adhesion molecules poliovirus receptor and immunoglobulin superfamily 4A in the interaction between mouse spermatogenic and Sertoli cells. Biol Reprod 2007; 76: 1081 1090 [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR. et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 2004; 101: 6062 6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol 2006; 7 (suppl 1): S12 11 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi N, Tobias JW, Hecht NB. Expression profiling reveals meiotic male germ cell mRNAs that are translationally up- and down-regulated. Proc Natl Acad Sci U S A 2006; 103: 7712 7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampal R, Luther KB, Haltiwanger RS. Notch signaling in normal and disease states: possible therapies related to glycosylation. Curr Mol Med 2007; 7: 427 445 [DOI] [PubMed] [Google Scholar]
- Moloney DJ, Shair LH, Lu FM, Xia J, Locke R, Matta KL, Haltiwanger RS. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J Biol Chem 2000; 275: 9604 9611 [DOI] [PubMed] [Google Scholar]
- Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, Wilson R, Wang Y, Stanley P, Irvine KD, Haltiwanger RS, Vogt TF. Fringe is a glycosyltransferase that modifies Notch. Nature 2000; 406: 369 375 [DOI] [PubMed] [Google Scholar]