Abstract
There has been a dramatic shift of attention from the ciliary axoneme to the ciliary membrane, much of this driven by the appreciation that cilia play a widespread role in sensory reception and cellular signaling. This Perspective focuses attention on some of the poorly understood aspects of ciliary membranes, including the establishment of ciliary and periciliary membrane domains, the trafficking of membrane components into and out of these membrane domains, the nonuniform distribution of ciliary membrane components, the regulation of membrane morphogenesis, functional collaboration between the axoneme and the membrane, and the evolving field of therapeutics targeted at the ciliary membrane.
INTRODUCTION
Cilia and flagella 1 are membrane-bounded organelles with unique membrane, soluble, and cytoskeletal compartments. Although the study of cilia has a long and interesting history (Bloodgood, 2009), for much of this history they were believed to function solely as motile organelles translocating entire cells (gametes, protistans) through a medium or materials (water, mucus, gametes, food) along the surface of ciliated epithelia. This motility-centric view of the organelle was solidified by the discovery that one could isolate cilia, remove and discard the membrane and soluble components, and reactivate motility in the axoneme by addition of ATP (Hoffman-Berling, 1955). This focus on the axoneme (Gibbons, 2011) contributed to the long neglect of the membrane; when early workers did focus on the ciliary membrane, it tended to be in the context of the ionic regulation of axonemal beating (Kung and Salmi, 1982). Although there were clearly exceptional cases that drew attention to the membrane (e.g., odorant receptors on olfactory cilia; flagella-based mating interactions in ciliated protozoa and flagellated green algae; Bloodgood, 1990), the axonemal-centric view of cilia was to be dramatically altered only upon the clear demonstration that nonmotile primary cilia function as antennae for sensory reception (Pazour and Witman, 2003), as well as integrators of important cellular signaling pathways, such as hedgehog signaling (Huangfu et al., 2003). This millennial renaissance of primary cilia as sensory organelles and signaling centers led to an enormous increase in research on cilia and flagella and the blossoming of the ciliopathy field, drawing in new investigators (including clinically oriented ones) not previously interested in cilia and flagella. The final step was the recent realization that motile cilia also exhibit sensory functions (Shah et al., 2009; Bloodgood, 2010). Despite enormous recent progress, our understanding of the multiple functions of ciliary and flagellar membranes (in regulation of ciliary beat, adhesion, mechanoreception, chemoreception, thermoreception, photoreception, and cell signaling) is still incomplete. It is my goal to highlight a few of the unsolved or only partially solved issues related to ciliary and flagellar membranes.
FIELDS AND FENCES. MEMBRANE DOMAINS AND MEMBRANE DOMAIN BARRIERS
Tight junctions, coupled with directed targeting of vesicular traffic, create plasma membrane (PM) domains with specialized compositions and specialized functions within polarized epithelial cells. The ciliary membrane is another such PM domain, within which many membrane proteins involved in sensory reception and signaling are freely diffusible (Hu et al., 2010). In at least some cells, there exists another PM domain surrounding the ciliary membrane domain, morphologically described as the ciliary pocket (Field and Carrington, 2009; Molla-Herman et al., 2010; Ghossoub et al., 2011) and functionally defined as a periciliary sorting domain (Vieira et al., 2006; Pazour and Bloodgood, 2008; Nachury et al., 2010). In trypanosomes, it functions as the only location in the PM for endocytosis and exocytosis, as well as the sorting domain for proteins destined for the flagellar membrane or the general cell body PM (Field and Carrington, 2009). Membrane proteins in trypanosomes can localize to all three domains, to only the pocket and the flagellar membrane, or to only the pocket and general PM, suggesting the presence of two functional barriers that can be independently regulated. Although ciliary pockets occur in mammalian primary cilia (Ghossoub et al., 2011), evidence for a functional periciliary sorting domain, which would be the target for fusion of membrane transport vesicles, is scarce. Although a periciliary domain has been defined in the apical surface of mammalian epithelial cells (Vieira et al., 2006), it has been suggested that this may be an artifact of fixation (Francis et al., 2011). In any event, much work remains to demonstrate the ubiquity of such a PM domain. The one protein that has been localized thus far to the periciliary sorting domain boundary is an actin cytoskeleton–associated protein (Bonhivers et al., 2008).
The barrier believed to delimit the ciliary membrane is located at the level of the transitional zone/transitional fibers, also the location of the ciliary necklace, an intramembrane particle array defined by freeze-etch microscopy (Czarnecki and Shah, 2012). Although these are possibly related, we have to distinguish in principle between the barriers that serve as the gateways to the ciliary membrane and the internal compartment of the cilium (axoneme and cilioplasm). It has recently been suggested (without much evidence as yet) that all ciliary proteins enter the cilium in association with membrane (Baldari and Rosenbaum, 2010), something that would require the presence of receptors on the cytosolic side of transport vesicles for binding of axonemal proteins, including tubulin. Progress is being made in identifying candidates for the diffusion barrier establishing the ciliary membrane domain. Septins are oligomeric GTPases known to form a membrane diffusion barrier in yeast and mammalian spermatozoa. Septin-2 localizes to the base of the cilium in mammalian cells, and its knockdown by small interfering RNA (siRNA) results in a functional breakdown of the diffusion barrier, entry of PM proteins, and an inability to concentrate membrane proteins in the cilium (Hu et al., 2010; Chih et al., 2011). Knockdown of CEP290/nephrocystin-6 results in a loss of the transition fibers and compromise of the ciliary “gate” and a change in protein composition of the Chlamydomonas flagellum (Craige et al., 2010); although most of the proteins affected are nonmembrane proteins, there appears to be a reduction in the amount of polycystin-2, a membrane protein critical for sensory transduction. Elimination of a hedgehog signaling regulator interferes with proper localization of many ciliary membrane proteins; not surprisingly, a mutation in this protein results in a ciliopathy (Garcia-Gonzalo et al., 2011). To summarize, the transition zone is a large assembly of many interacting proteins, most of which have been implicated in ciliopathies (Garcia-Gonzalo et al., 2011; Sang et al., 2011; Czarnecki and Shah, 2012), as well as in the control of ciliary membrane composition. Much work remains to determine whether the composition and mechanics of the membrane diffusion barrier are similar across all classes of cilia, to understand why there is so much complexity to this gateway to the cilium, to determine whether it can be regulated in terms of permeability, and to determine the mechanism by which selected membrane proteins are allowed to cross this barrier. Large protein assemblies (intraflagellar transport [IFT] trains, BBSomes, mastigonemes, dynein arm and radial spoke preassemblies) transit through this congested region at the base of the cilium, whereas many PM and cytosolic components (mRNAs, ribosomes, cytosolic proteins) are restricted from entering the cilium.
THE INS AND OUTS OF CILIARY/FLAGELLAR MEMBRANES
Getting In
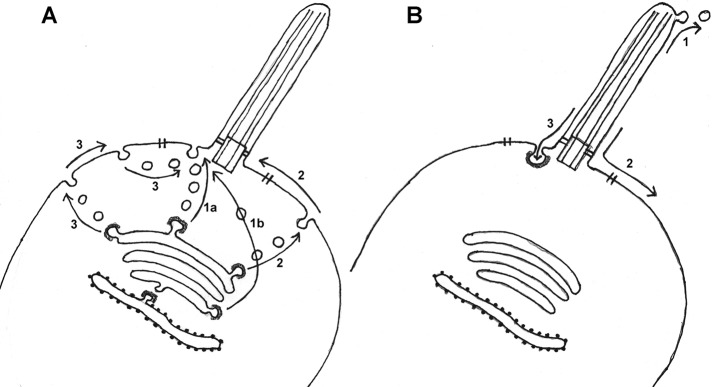
An important goal is to understand the relative importance of the various routes by which membrane components (be it bulk membrane or individual membrane proteins) enter and leave the ciliary membrane (Figure 1), as well as the regulation of this process. There are three possible routes for entry into the ciliary membrane (Milenkovic et al., 2009): 1) Transport vesicles from the Golgi deliver membrane to a specialized region of the PM at the base of the cilium (the periciliary domain), followed by diffusion or motor protein–dependent transport into the ciliary membrane. 2) Transport vesicles from the Golgi deliver membrane to the PM, followed by lateral diffusion or motor protein–dependent transport through the periciliary membrane domain into the ciliary membrane. 3) A combination of the previous two routes in which a transport vesicle is initially targeted from the Golgi to the PM. The endocytic pathway then retrieves material from the PM into a new class of transport vesicle targeted to the periciliary domain, followed by lateral movement into the ciliary membrane. Much remains to be discovered about the relative extent to which each of these pathways contributes to delivery of lipids and proteins to the ciliary membrane, not to mention the associated targeting information. The current assumption is that route 1a (Figure 1A) is the primary route for delivery of bulk membrane and membrane proteins to the cilium. Although this has been supported by transmission electron microscopy (TEM) evidence (Papermaster et al., 1985), by an intriguing IFT-20 “pathway” through the cytoplasm from the Golgi to the base of the cilium (Follit et al., 2006), and by a wealth of recent data on the roles of small G proteins (Lim et al., 2011), evidence is emerging to support alternative routes. Sexual agglutinins move into the Chlamydomonas flagellum directly from a pool located in the PM (route 2 in Figure 1A; Hunnicutt et al., 1990). In neurons, there is evidence that the somatostatin receptor-3 (Sstr3) also enters the ciliary membrane via this PM route (Berberi et al., 2008; Jin et al., 2010). Smoothened—a component of the hedgehog signaling pathway—has been reported both to use route 1a (Wang et al., 2009) and route 2 (Milenkovic et al., 2009) to reach the ciliary membrane. It even appears that important sensory signaling molecules targeted to the ciliary membrane (polycystin-2) can move directly from the cis-Golgi, bypassing the rest of the Golgi, to the periciliary targeting domain (perhaps as shown in route 1b in Figure 1A; Hoffmeister et al., 2011). Aside from the route taken, there is more to learn about the informational aspects of protein targeting to the ciliary membrane, such as the involvement of specific soluble N-ethylmaleimide–sensitive factor attachment protein receptors and Rabs (Lim et al., 2011). Although there is clear evidence for ciliary targeting sequences within some membrane proteins (Pazour and Bloodgood, 2008; Nachury et al., 2010), it is also possible that PM retention signals might play a role in deciding which membrane proteins can enter the cilium (Francis et al., 2011).
FIGURE 1:
Pathways for delivery of components to (A) and removal from (B) the ciliary membrane.
Getting out
There are three possible fates for ciliary membrane components during bulk turnover, organelle resorption, and the trafficking of specific signaling molecules out of the cilium (Figure 1B). Although clathrin-dependent endocytosis clearly occurs in association with periciliary membrane domains located at the base of many motile and primary cilia (Molla-Herman et al., 2010; Ghossoub et al., 2011), it has not been possible to demonstrate the presence of ciliary membrane proteins associated with these structures (Molla-Herman et al., 2010). Although it has been known since the 1970s that membrane vesicles are released from the flagellar surface in Chlamydomonas; there is current interest in determining whether these vesicles represent a random sample of the flagellar membrane or are enriched in specific membrane components (Baldari and Rosenbaum, 2010). Future work needs to determine whether this ciliary membrane-shedding phenomenon, which may simply allow rapid changes in ciliary membrane composition or may be used for cell–cell signaling, is widespread and whether and how it is regulated. The third route for removal of material from the ciliary membrane involves direct movement into the adjacent PM. Although there are few observations addressing this route (route 2 in Figure 1B), mastigonemes associated with the flagella of fungal zoospores flow onto the general PM surface during flagellar resorption (Fuller and Reichle, 1965). Aside from the routes for bulk removal of membrane from the cilium, there is the question of how the cell removes specific proteins from the ciliary membrane, as occurs when Patched is removed from the ciliary membrane upon the initiation of hedgehog signaling and Smoothened is removed from the ciliary membrane at the termination of hedgehog signaling (Huangfu et al., 2003). The BBSome is necessary for export of signaling proteins from the Chlamydomonas flagellum (Lechtreck et al., 2009), as well as the export of the dopamine 1 receptor from cilia on cultured neurons (Domire et al., 2011). Polycystin-2 accumulates in cilia from a mouse with an IFT defect (Pazour et al., 2002) suggesting a possible role for retrograde IFT. Certain ciliary membrane signaling proteins are substrates for ubiquitin ligase, suggesting a role for proteasome destruction (Huang et al., 2009).
NONUNIFORMITY OF THE CILIARY MEMBRANE
The ciliary membrane possesses a unique protein and lipid composition relative to other PM domains, but only in recent years has it become appreciated that both membrane proteins and lipids may be localized in a nonuniform manner within the plane of the ciliary membrane. Although the size and existence of lipid rafts within membrane domains are controversial, there are emerging hints that ciliary membranes (known to be enriched in sterols and sphingolipids) may possess lipid rafts (Tyler et al., 2009) or may even constitute one large lipid raft. Use of the dye Laurden suggested a high degree of lipid organization in the trypanosome ciliary membrane (Tyler et al., 2009) and identified a zone of particularly high lipid order at the base of the primary cilium in Madin–Darby canine kidney (MDCK) cells (Vieira et al., 2006). There are now numerous examples (many associated with calcium or cyclic nucleotide signaling) of an asymmetric distribution of sensory- and signaling-associated membrane proteins within the ciliary membrane (Flannery et al., 2006; Fujiu et al., 2009; Shah et al., 2009; Wilson et al., 2009; French et al., 2010; Lee et al., 2010). Although the functional significance of asymmetric localization in many of these cases is unclear (the examples cited include both proximal and distal localizations), it has been argued that signaling-associated calcium channels need to be kept away from the base of the cilium in order to avoid calcium-induced deciliation during any signaling process that increases intraciliary free calcium. Although lipid rafts have been invoked in at least one case of asymmetric protein localization (Tyler et al., 2009), tethering to the axoneme or to some morphologically indistinct cytoskeletal layer beneath the ciliary membrane could also contribute to asymmetric distribution of membrane proteins within the cilium.
MORPHOGENESIS OF THE CILIARY MEMBRANE
There are two principal pathways for the assembly of cilia, and they differ in the origin of the ciliary membrane (Ghossoub et al., 2011). In one case, a centriole/basal body migrates to and forms a functional coupling to the cell's PM, which becomes the ciliary membrane concomitant with controlled axonemal growth. In the other case, a cytoplasmic vesicular cap forms around the end of a centriole/basal body; this membrane vesicle expands to cover an axoneme growing within the cytoplasm. At some point, the vesicular membrane covering the nascent axoneme fuses with the PM, inserting the vesicular membrane into the PM, often leaving a deep invagination of the now PM around the base of the cilium (creating a ciliary pocket). Little is known about how a cell decides between these two modes of ciliogenesis and the molecular details of these alternative pathways, which are coupled to two different sources of basal bodies (Dawe et al., 2007), depending on whether the cell will possess a solitary primary cilium or be a multiciliated cell.
Evolution of cilia with specialized functions has also been associated in some cases with dramatic morphological change in the shape and configuration of the ciliary membrane (Silverman and Leroux, 2009). Some of the best examples of this poorly studied phenomenon are found in cilia specialized for light reception in mammals (photoreceptor outer segments); chemosensation and thermosensation in Caenorhabditis; and adhesion to the insect hindgut wall (Vickerman and Tetley, 1990). The ciliary membrane of the sensory neurons in the worm can assume a variety of unusual morphologies (including bipartite, fork-like, and wing shapes), which can be altered. For example, a single homeodomain transcription factor is sufficient to convert a typically shaped cilium into a ciliary morphology with numerous finger-like projections of the ciliary membrane (Satterlee et al., 2001). Alternations in the coupling of the two anterograde IFT motors (kinesin-2 and OSM3) in the worm may be responsible for the unusual membrane morphology in one class of sensory neurons (Mukhopadhyay et al., 2007); in addition, sensory input can result in alteration of the ciliary membrane structure of these cilia from a fork-like to a fan-like morphology (Mukhopadhyay et al., 2008). A mutation in BBS proteins (and presumably BBSome function) results in a dramatic change in the morphology of the membrane of the motile respiratory cilia in the mouse (Shah et al., 2008); the swelling of the ciliary membrane tip concomitant with accumulation of internal membrane vesicles looks strikingly like an intermediate stage in the early morphogenesis of rod outer segments. More attention needs to be given to understanding the morphogenesis of unusual ciliary membrane configurations.
COLLABORATION BETWEEN THE AXONEME AND THE CILIARY MEMBRANE
It is naive to consider the ciliary membrane in isolation from its partner, the axoneme. There must be cross-talk between these structures, much of which occurs in the compartment between the membrane and the axoneme. A variety of structural links connect the ciliary membrane to the sides of the axonemal doublets, as well as to the ends of the outer doublet and central pair microtubules (Dentler, 1990). The major structural occupant of the compartment between the ciliary membrane and the axoneme are large protein complexes that bidirectionally move along the length of the cilium in the process of IFT (Kozminski et al., 1993). An interesting question is whether the membrane is an essential component of the mechanism of IFT transport within the cilium; the IFT raft/train is a protein scaffold that must simultaneously bind cargo as well as motor proteins that allow it to “walk” along the doublet microtubule surface. The issue of whether the membrane plays any functional role in the mechanism of IFT is separate from the question of whether membrane proteins are cargos for IFT. Furthermore, we must make a distinction between the role of IFT in getting membrane proteins into cilia (across the membrane diffusion barrier at the level of the transition zone) versus moving membrane proteins around within the ciliary membrane domain itself. Although some ciliary membrane proteins are dependent upon IFT (or at least IFT motors) for entry into cilia or for directed movements within the ciliary membrane (Qin et al., 2005; Follit et al., 2006; Wang et al., 2006), others are not (Pan and Snell, 2002; Pazour et al., 2002; Tull et al., 2004; Bae et al., 2006). The ability to reactivate IFT in a model system lacking an intact membrane would be one test of the necessity for the ciliary membrane; repeated attempts in several labs have met with failure, although this might reflect the lability of the IFT trains. A dramatic example of the collaboration of the axoneme and membrane is whole-cell gliding motility in Chlamydomonas, which requires force transduction at the flagellar membrane surface driven by microtubule-associated motors (Laib et al., 2009) acting to move a transmembrane adhesion protein through the membrane; recent results suggest that gliding motility may be dependent on IFT (Shih et al., 2012).
Another important question that is only recently being explored is how ciliary membrane and axoneme assembly and disassembly are coordinated during ciliary growth and resorption. Normally, these processes appear to be tightly coordinated temporally in an as-yet-unknown manner. Inhibitors of Golgi function can inhibit ciliogenesis and alter the length of existing cilia, suggesting that a limitation in membrane components may affect the process of axonemal growth (Haller and Fabry, 1998; Dentler, 2011). Alterations in the lipid composition of the flagellar membrane can affect both the flagellar membrane and the axoneme (Tull et al., 2004). A breakdown in the ciliary membrane diffusion barrier also compromises ciliogenesis (Hu et al., 2010). In Leishmania, targeting of a membrane protein with lipid anchors to the flagellar membrane can occur independent of IFT or even the presence of an axoneme (Tull et al., 2004); it would be interesting to explore other means for uncoupling membrane assembly from axonemal assembly. Clearly, we have much to learn about the cross-talk between the ciliary membrane and the axoneme.
CILIARY MEMBRANE TARGETING AND THE DEVELOPMENT OF THERAPEUTICS FOR CILIOPATHIES
One of the more exciting areas in ciliary membrane research today involves the discovery and exploitation of small molecules that induce or inhibit targeting of clinically significant signaling proteins to the ciliary membrane. For example, small-molecule agonists and antagonists of hedgehog signaling have been identified that can induce or inhibit the targeting of Smoothened to the ciliary membrane. One small molecule antagonist of hedgehog signaling, cyclopamine, stimulates the accumulation of Smoothened in the ciliary membrane (Wang et al., 2009) and has been shown to inhibit tumor growth in certain models (Gould and Missailidis, 2011). Clearly, this is just the beginning of a wave of translational research involving the cilium.
CONCLUSION
During the past decade, a veritable flood of new information about the cilium has moved it into a very central position in vertebrate cells as a focal point for both the reception of sensory input from the environment and as a central processor for important signaling pathways, such as the hedgehog pathway. The message from this all too brief, noncomprehensive survey is that, although enormous progress is being made in understanding many of the important questions surrounding the ciliary membrane, as many new questions are being raised as are being answered. One thing is for certain—the era of neglect of the ciliary membrane is over, and we will never return to the hegemony of the axoneme!
Acknowledgments
Keith Kozminski and Roger Sloboda provided very helpful advice during the preparation of this essay.
Abbreviations used:
- IFT
intraflagellar transport
- PM
plasma membrane
- TEM
transmission electron microscopy
- MDCK
Madin–Darby canine kidney
Footnotes
1For brevity, when speaking of cilia and flagella or ciliary and flagellar membranes, I use the terms cilia and ciliary membranes, respectively.
REFERENCES
- Bae Y-K, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- Baldari CT, Rosenbaum JL. Intraflagellar transport: it's not just for cilia anymore. Curr Opin Cell Biol. 2010;22:76–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberi NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood RA. Ciliary and Flagellar Membranes. New York: Plenum Press; 1990. [Google Scholar]
- Bloodgood RA. From central to rudimentary to primary: the history of an underappreciated organelle whose time has come: the primary cilium. Methods Cell Biol. 2009;94:3–52. doi: 10.1016/S0091-679X(08)94001-2. [DOI] [PubMed] [Google Scholar]
- Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- Bonhivers M, Nowacki S, Landrein N, Robinson DR. Biogenesis of the trypanosome endo-exocytotic organelle is cytoskeleton mediated. PLoS Biol. 2008;6:e105. doi: 10.1371/journal.pbio.0060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol. 2011;14:61–72. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- Craige B, Tsao C-C, Diener DR, Hou Y, Lechtreck K-F, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki PG, Shah JV. The ciliary transition zone: from morphology and molecules to medicine. Trends Cell Biol. 2012;22:201–210. doi: 10.1016/j.tcb.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe HR, Farr H, Gull K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J Cell Sci. 2007;120:7–15. doi: 10.1242/jcs.03305. [DOI] [PubMed] [Google Scholar]
- Dentler WL. Linkages between microtubules and membranes in cilia and flagella. In: Bloodgood RA, editor. Ciliary and Flagellar Membranes. New York: Plenum Press; 1990. pp. 31–64. [Google Scholar]
- Dentler WL. The plasma membrane is a pool for some, but not all flagellar membrane proteins. Mol Biol Cell. 2011;22:4705. [Google Scholar]
- Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci. 2011;68:2951–2960. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MC, Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- Flannery RJ, French DA, Kleene SJ. Clustering of cyclic-nucleotide-gated channels in olfactory cilia. Biophys J. 2006;91:179–188. doi: 10.1529/biophysj.105.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SS, Sfakianos J, Lo B, Mellman I. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol. 2011;193:219–233. doi: 10.1083/jcb.201009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French DA, Badamdorj D, Kleene SJ. Spatial distribution of calcium-gated chloride channels in olfactory cilia. PLoS ONE. 2010;5:e15676. doi: 10.1371/journal.pone.0015676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiu K, Nakayama Y, Yanagisawa A, Sokabe M, Yoshimura K. Chlamydomonas CAV2 encodes a voltage-dependent calcium channel required for the flagellar waveform conversion. Curr Biol. 2009;19:133–139. doi: 10.1016/j.cub.2008.11.068. [DOI] [PubMed] [Google Scholar]
- Fuller MS, Reichle R. The zoospore and early development of Rhizidiomyces apophysatus. Mycologia. 1965;57:946–961. [Google Scholar]
- Garcia-Gonzalo FR, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghossoub R, Molla-Herman A, Bastin P, Benmerah A. The ciliary pocket: a once-forgotten membrane domain at the base of cilia. Biol Cell. 2011;103:131–144. doi: 10.1042/BC20100128. [DOI] [PubMed] [Google Scholar]
- Gibbons IA. Discovery of dynein and its properties: a personal account. In: King SM, editor. Dyneins. Structure, Biology and Disease. New York: Academic Press; 2011. pp. 3–87. [Google Scholar]
- Gould A, Missailidis S. Targeting the hedgehog pathway: the development of cyclopamine and the development of anti-cancer drugs targeting the hedgehog pathway. Mini Rev Med Chem. 2011;11:200–213. doi: 10.2174/138955711795049871. [DOI] [PubMed] [Google Scholar]
- Haller K, Fabry S. Brefeldin A affects synthesis and integrity of a eukaryotic flagellum. Biochem Biophys Res Commun. 1998;242:597–601. doi: 10.1006/bbrc.1997.8015. [DOI] [PubMed] [Google Scholar]
- Hoffman-Berling H. Geisselmodelle and adenosintriphosphate. Biochim Biophys Acta. 1955;16:146–154. doi: 10.1016/0006-3002(55)90192-x. [DOI] [PubMed] [Google Scholar]
- Hoffmeister H, Babinger K, Gurster S, Cedzich A, Meese C, Schadendorf K, Osten L, de Vries U, Rascle A, Witzgall R. Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J Cell Biol. 2011;192:631–645. doi: 10.1083/jcb.201007050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Diener DR, Rosenbaum JL. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J Cell Biol. 2009;186:601–613. doi: 10.1083/jcb.200903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signaling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Hunnicutt GR, Kosfiszer MG, Snell WJ. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol. 1990;111:1605–1616. doi: 10.1083/jcb.111.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Agular, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C, Salmi Y. The physiological basis of taxes in Paramecium. Annu Rev Physiol. 1982;44:519–534. doi: 10.1146/annurev.ph.44.030182.002511. [DOI] [PubMed] [Google Scholar]
- Laib JA, Martin JA, Bloodgood RA, Guilford WH. The reciprocal coordination and mechanics of molecular motors in living cells. Proc Natl Acad Sci USA. 2009;106:3190–3195. doi: 10.1073/pnas.0809849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN (= NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE. 2010;8:e11012. doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YS, Chua CEL, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell. 2011;103:209–221. doi: 10.1042/BC20100150. [DOI] [PubMed] [Google Scholar]
- Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla-Herman A, et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Lu Y, Qin H, Lanjuin A, Shaham S, Sengupta P. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 2007;26:2966–2980. doi: 10.1038/sj.emboj.7601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Lu Y, Shaham S, Sengupta P. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev Cell. 2008;14:762–774. doi: 10.1016/j.devcel.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Kinesin-II is required for flagellar sensory transduction during fertilization in Chlamydomonas. Mol Biol Cell. 2002;13:1417–1426. doi: 10.1091/mbc.01-11-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus towards the rod outer segment: ultrastructural, immunochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985;26:1386–1404. [PubMed] [Google Scholar]
- Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–149. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Sang L, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlee JS, Sasakura H, Kuhara A, Berkeley M, Mori I, Sengupta P. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron. 2001;31:943–956. doi: 10.1016/s0896-6273(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Shah AS, et al. Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia. Proc Natl Acad Sci USA. 2008;105:3380–3385. doi: 10.1073/pnas.0712327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih S-M, Bilyard T, Engel BD, Marshall WF, Yildiz A. Intraflagellar transport powers surface motility in Chlamydomonas. Biophys J. 2012;102:379a. [Google Scholar]
- Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–316. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Tull D, Vince JE, Callaghan JM, Naderer T, Spurck T, McFadden GI, Currie G, Ferguson K, Bacic A, McConville MJ. SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellum membrane in Leishmania. Mol Biol Cell. 2004;15:4775–4786. doi: 10.1091/mbc.E04-06-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler KM, Fridberg A, Toriello KM, Olson CL, Cleslak JA, Hazlett TL, Engman DM. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122:859–866. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman K, Tetley L. Flagellar surfaces of parasitic protozoa and their role in attachment. In: Bloodgood RA, editor. Ciliary and Flagellar Membranes. New York: Plenum Press; 1990. pp. 267–304. [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WLC, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin–Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Z, Walsh CT, McMahon AP. Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc Natl Acad Sci USA. 2009;106:2623–2628. doi: 10.1073/pnas.0812110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, Chen M-H, Chuang P-T. Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium. PLoS ONE. 2009;4:e5182. doi: 10.1371/journal.pone.0005182. [DOI] [PMC free article] [PubMed] [Google Scholar]