Endoglin interacts with PI3K via GIPC to recruit and activate PI3K/Akt at the cell membrane. TGF-β1 attenuates, whereas BMP-9 enhances, endoglin/GIPC-mediated membrane scaffolding of PI3K/Akt to alter endothelial capillary tube stability in vitro, and GIPC mediates endoglin function during developmental angiogenesis in vivo.
Abstract
Endoglin (CD105) is an endothelial-specific transforming growth factor β (TGF-β) coreceptor essential for angiogenesis and vascular homeostasis. Although endoglin dysfunction contributes to numerous vascular conditions, the mechanism of endoglin action remains poorly understood. Here we report a novel mechanism in which endoglin and Gα-interacting protein C-terminus–interacting protein (GIPC)–mediated trafficking of phosphatidylinositol 3-kinase (PI3K) regulates endothelial signaling and function. We demonstrate that endoglin interacts with the PI3K subunits p110α and p85 via GIPC to recruit and activate PI3K and Akt at the cell membrane. Opposing ligand-induced effects are observed in which TGF-β1 attenuates, whereas bone morphogenetic protein-9 enhances, endoglin/GIPC-mediated membrane scaffolding of PI3K and Akt to alter endothelial capillary tube stability in vitro. Moreover, we employ the first transgenic zebrafish model for endoglin to demonstrate that GIPC is a critical component of endoglin function during developmental angiogenesis in vivo. These studies define a novel non-Smad function for endoglin and GIPC in regulating endothelial cell function during angiogenesis.
INTRODUCTION
Endoglin (CD105) is a transforming growth factor β (TGF-β) superfamily coreceptor required for angiogenesis and vascular maintenance. A critical role for endoglin in the vasculature is supported by several in vivo findings, including the embryonic lethal phenotype observed in endoglin-knockout mice due to impaired angiogenesis and vasculature of the yolk sac (Li et al., 1999) and endoglin mutations found in patients with human disease, in particular hereditary hemorrhagic telangiectasia (HHT1), an autosomal-dominant disorder characterized by arteriovenous malformations (AVMs) and hemorrhagic events in multiple tissues and organs (McAllister et al., 1995; Bourdeau et al., 1999; Mahmoud et al., 2010). Endoglin dysfunction contributes to many other conditions, including tumor-associated angiogenesis, inflammation, and pre-eclampsia (Fonsatti et al., 2003; Gilbert et al., 2009). Despite these advances in understanding endoglin biology, the underlying mechanisms of endoglin function during embryonic development and pathogenesis remain obscure.
Endoglin is a single polypeptide chain consisting of an extracellular ligand-binding domain, a single membrane-spanning segment, and a short cytoplasmic domain (Cheifetz et al., 1992; Lebrin et al., 2004). Endoglin exists as a disulfide-linked homodimer expressed primarily in vascular endothelial cells that serves as a high-affinity cell surface receptor for TGF-β1, TGF-β3, and bone morphogenetic proteins (BMPs) 2 and 7 in the context of heteromeric receptor complexes with type I serine/threonine activin–like kinase receptors (ALKs 1–7; Lastres et al., 1996; Shi and Massague, 2003; Bernabeu et al., 2007). Endoglin further associates with its signaling partner, ALK1, an endothelial-specific, BMP-responsive receptor that can contribute causal mutations in HHT2 (Johnson et al., 1996; Marchuk, 1998; Bernabeu et al., 2007). More recently, BMP-9 and -10 were characterized as direct ligands for endoglin and ALK1 (David et al., 2007, 2009). In endothelial cells, TGF-β1 and BMP-9/10 signal through ALK1 and Smad 1/5/8 to promote proangiogenic responses, including endothelial cell proliferation and migration (Lebrin et al., 2004). In contrast, ALK5 activates the TGF-β1–induced Smad 2/3 pathway, yielding an overall antiangiogenic response. Although the preponderance of data suggests that endoglin/ALK1–induced Smad 1/5/8 signaling yields proangiogenic responses, there also exists substantial contradicting evidence drawn from in vitro and in vivo models (David et al., 2009). We and others determined the role of endoglin and ALK1 in potentiating the Smad 1/5/8 pathway, the outcomes of which include the inhibition of proliferation, migration, and, in the case of ALK1, capillary sprout formation (Lee et al., 2008, 2009). More recently, studies using conditional ALK1-knockout mice demonstrated the antiangiogenic potential of ALK1 (Park et al., 2008).
We and others previously examined how endoglin binding partners alter endothelial cell signaling during angiogenesis (Conley et al., 2004; Sanz-Rodrigues et al., 2004; Lee and Blobe, 2007; Lee et al., 2008). The cytoplasmic domain of endoglin serves as a binding site for four adaptor proteins with distinct scaffolding/trafficking and cellular functions: zyxin (Conley et al., 2004), zyxin-related protein-1 (ZRP-1; Sanz-Rodrigues et al., 2004), β-arrestin2 (Lee and Blobe, 2007), and Gα-interacting protein C-terminus–interacting protein (GIPC; Lee et al., 2008). Endoglin, via its interaction with zyxin and ZRP-1, has been demonstrated to regulate focal adhesions and the actin cytoskeleton to inhibit endothelial cell migration (Conley et al., 2004; Sanz-Rodrigues et al., 2004). β-Arrestin2 has been demonstrated to internalize endoglin into endocytic vesicles and suppress TGF-β1–induced ERK activation and migration in endothelial cells (Lee and Blobe, 2007), whereas GIPC stabilizes endoglin on the cell surface to potentiate ALK1-induced Smad 1/5/8 signaling (Lee et al., 2008). Here we investigate how endoglin and its scaffolding partners might integrate other non-Smad signaling pathways to impact endothelial cell behavior and angiogenesis.
RESULTS
Endoglin exerts biphasic effects on endothelial capillary and tube formation
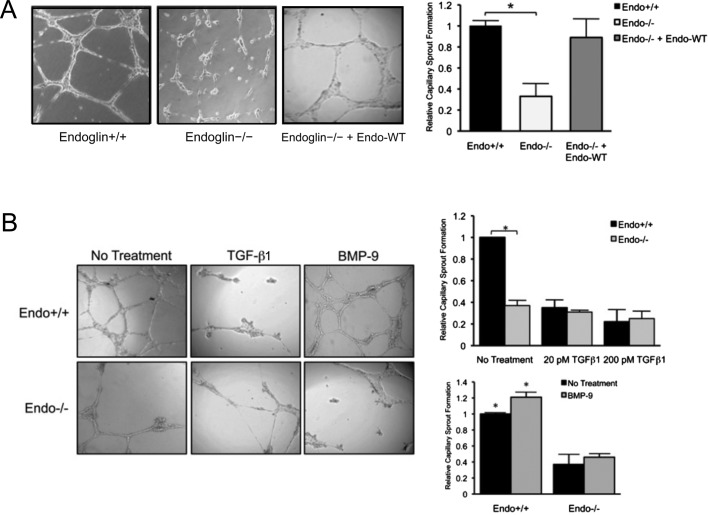
Although endoglin is a positive regulator of angiogenesis, the underlying molecular and cellular mechanisms for endoglin function are not fully understood (Lebrin et al., 2004; Pece-Barbara et al., 2005; Lee et al., 2008). We used Matrigel- and collagen-induced capillary-sprouting and tubulation assays to examine endoglin-mediated signaling and cellular properties. We compared control wild-type (Endo+/+) and endoglin-null (Endo−/−) murine embryonic endothelial cells (MEECs) that otherwise express all of the TGF-β signaling constituents, including ALK1, TβRII, and ALK5 receptors (Pece-Barbara et al., 2005). Consistent with previous in vitro findings (Fernandez et al., 2005; Jerkic et al., 2006; Davis et al., 2007; Ponce, 2009), Endo+/+ MEECs formed a significantly greater number of capillaries than did Endo−/− MEECs when plated on Matrigel (Figure 1A), and restoring endoglin expression rescued capillary formation in Endo−/− MEECs (Figure 1A). The Endo+/+ capillaries also formed adherens junctions, as assessed by VE-cadherin staining (Supplemental Figure S1A), and endothelial tubules when grown in three-dimensional collagen matrix (Supplemental Figure S1B). Similar effects were observed in primary human umbilical vein endothelial cells (HUVECs) and human microvascular endothelial cells (HMEC-1s), in which short hairpin RNA (shRNA)–mediated knockdown of endogenous endoglin (shEndo) resulted in a considerable loss of capillaries compared with the nontargeting control (NTC; Supplemental Figures S1, C and D, and S2A), supporting a general role for endoglin in promoting endothelial capillary formation.
FIGURE 1:
Endoglin promotes Matrigel-induced endothelial capillary sprouting and tube morphogenesis. (A) Comparison of capillary sprouting for Endo+/+ and Endo−/− MEECs are shown, along with reconstituted expression of Endo-WT (human) in Endo−/− MEECs. Graph represents the quantification, normalized to Endo+/+, of 10 independent experiments performed in triplicate. Error bars, SEM (see Materials and Methods). *p < 0.01 (B) Effects of ligands on capillary sprouting. Exogenous TGF-β1 (20 or 200 pM) and BMP-9 (16.5 nM) were added for 16 h. Quantification of capillary sprouting for Endo+/+ and Endo−/− MEECs in the presence of each ligand is represented by graphs derived from six independent experiments performed in triplicate. *p< 0.05.
To test the role of endoglin ligands in capillary formation, we treated endothelial cells with TGF-β1 and BMP-9. Exogenous treatment of TGF-β1 significantly impaired, whereas BMP-9 moderately enhanced, capillary formation in wild-type endoglin–expressing cells, whereas Endo−/− MEECs failed to respond to either ligand (Figure 1B). TGF-β1 potently inhibited capillary formation even at subsaturating levels (20 pM), whereas Endo−/− MEECs had minimal response to exogenous TGF-β1 treatment (Figure 1B, graph). Of interest, BMP-9 had negligible impact on capillary formation in Endo−/− MEECs despite the presence of ALK1, TβRII, and ALK5 (Pece-Barbara et al., 2005), suggesting that endoglin is essential for mediating the effects of BMP-9. These patterns were also observed in HUVECs with and without shRNA-mediated knockdown of endogenous endoglin (shEndo; Supplemental Figure S2, B and C), confirming that ligand-induced effects are mediated by endoglin. To determine whether endoglin requires BMP-9 to promote capillary formation, we treated HUVECs plated on Matrigel with BMP-9–neutralizing antibody. Of note, BMP-9–neutralizing antibody significantly impaired the formation of capillary sprouts compared with no treatment or immunoglobulin G (IgG) control, suggesting that endoglin promotes capillary sprouts in large part through BMP-9 (Supplemental Figure S2D). Taken together, these results support a unique role for endoglin in exerting distinct effects on endothelial capillary formation in response to TGF-β1 and BMP-9 and support previous in vivo findings that indicate an essential role for endoglin during angiogenesis.
Endoglin regulates endothelial capillary sprout stabilization
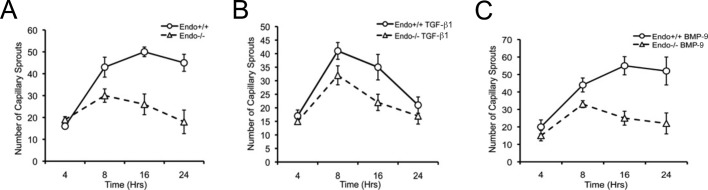
To determine whether endoglin deficiency renders endothelial cells incapable of capillary sprouting, we performed time-course experiments. Of interest, endothelial cells were able to sprout largely independent of endoglin expression (i.e., Endo+/+ vs. Endo−/− MEECs, wild-type HUVECs vs. shEndo-HUVECs) during the first 4–8 h of differentiation (Figure 2A and Supplemental Figure S2A). However, in Endo−/− MEECs there was significant regression of capillary sprouts evident in later stages, whereas Endo+/+ MEECs maintained their differentiated structures (Figure 2A; period 16–24 h). These outcomes were independent of cellular proliferation, given that results from our cell growth assays (Supplemental Figure S3A) are consistent with previous reports that Endo−/− MEECs proliferate faster than Endo+/+ MEECs (Pece-Barbara et al., 2005). Taken together, our data suggest that endoglin has a specific role in promoting the stability of endothelial capillaries.
FIGURE 2:
Endoglin modulates the stability of Matrigel-induced capillary sprouting and tube formation. (A) Comparison of capillary sprouting for Endo+/+ and Endo−/− MEECs at 0–4, 8, 16, and 24 h. Data derived from the quantification of the total number of capillary sprouts of a representative experiment from three independent experiments performed in triplicate. Error bars, SEM of total scored sprouting branches (see Materials and Methods) (B) Exogenous TGF-β1 (50 pM) treatment for indicated times. (C) Exogenous BMP-9 (16.5 nM) treatment for indicated times.
To investigate further the role of the TGF-β1 or BMP-9 in endothelial capillary stability, we monitored endothelial cells for their capillary formation in the presence of these ligands. Of interest, neither ligand altered the early stages of capillary formation in the Endo+/+ or Endo−/− MEECs (Figure 2B and C; period 0–8 h). However, after the initial capillary formation, there was a specific TGF-β1–induced loss of capillary structures in Endo+/+ cells, whereas Endo−/− cells regressed, as previously noted, irrespective of TGF-β1 treatment (Figure 2B; period 8–24 h). In contrast, BMP-9 selectively stabilized the capillaries formed in Endo+/+ MEECs (Figure 2C; period 16–24 h). These findings suggest that endoglin mediates differential effects of ligands on endothelial capillary stability rather than endothelial activation and capillary sprouting.
Endoglin mediates effects of TGF-β1 and BMP-9 on phosphatidylinositol 3-kinase/Akt activation
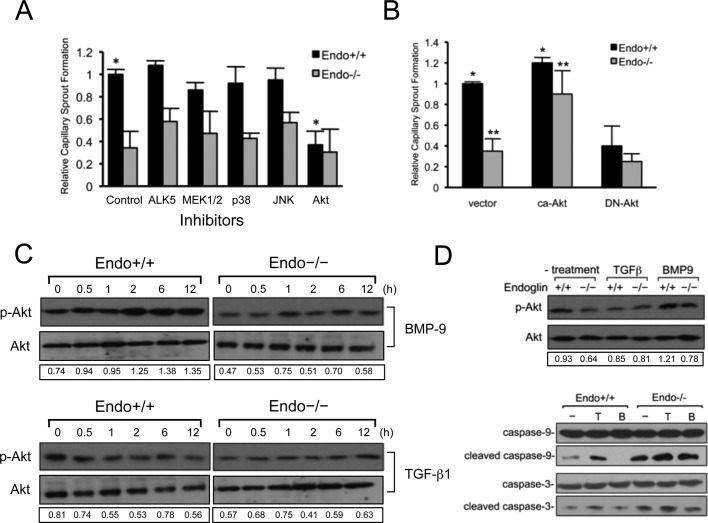
The precise signaling role for endoglin during angiogenesis remains unclear, given that both proangiogenic and antiangiogenic properties have been ascribed to Smad 1/5/8 and Smad 2/3 pathways on endothelial migration, proliferation, and capillary formation (Lebrin et al., 2004; Pece-Barbara et al., 2005; Lee et al., 2009). Because endoglin specifically regulates the stability of endothelial capillaries in our model systems, we sought to identify the signaling pathway mediating this effect. To this end, we used a panel of pharmacological inhibitors, including small molecules against mitogen-activated protein kinase (MAPKs; MEK/ERK, JNK, and p38), phosphatidylinositol 3-kinase (PI3K)/Akt, and ALK5. Inhibiting either the ALK5/Smad2/3 signaling axis or the MAPK pathways had minor impact on capillary formation and stability (Figure 3A). However, even partially blocking the PI3K/Akt signaling significantly impaired capillary stability in Endo+/+ MEECs while having no effect on Endo−/− MEECs (Figure 3A), suggesting a distinct role for the PI3K/Akt signaling pathway in capillary stability downstream of endoglin.
FIGURE 3:
Endothelial capillary stability requires endoglin-dependent signaling to the PI3K/Akt pathway. (A) The effects of inhibitors to ALK5 (10 μM SB431542), MEK1/2 (30 μM PD98059), p38 (10 μM SB203580), JNK (30 μM SP600125), and PI3K (5 μM LY294002) pathways in Matrigel-induced capillary sprouting. Graph represents the normalized compilation of three independent experiments performed in triplicate. Cells were treated with the inhibitors for 12–16 h and biochemically tested for their efficacy of inhibition. Error bars, SEM. *p < 0.05. (B) Akt signaling functions downstream of endoglin. Shown are Endo−/− MEECs expressing vector, ca-Akt, or DN-Akt. Error bars, average SEM. *p < 0.05, **p < 0.03 (C) Biochemical analysis of Akt activation in response to TGF-β1 (50 pM) and BMP-9 (16.5 nM) using phospho-Akt antibody in Endo+/+ and Endo−/− MEECs under monolayer culture conditions. Data are representative of at least three independent experiments. Densitometric analysis was based on band intensities of phospho-Akt relative to total Akt. (D) Direct biochemical analysis of Akt activation under no treatment, TGF-β1 (50 pM), or BMP-9 (16.5 nM) (top two panels and a representative sprout formation) and caspase cleavage (bottom three panels) upon capillary formation for 16 h. Data are representative of at least three independent experiments. Densitometric analysis was based on band intensities of phospho-Akt relative to total Akt.
To gauge the contribution of endoglin-dependent PI3K/Akt signaling on capillary stability, we examined the effects of increasing or decreasing PI3K/Akt signaling through expression of constitutively active Akt (myristoylated-Akt; ca-Akt) or dominant-negative Akt (DN-Akt) in Endo+/+ and Endo−/− MEECs. ca-Akt expression restored capillary stability in Endo−/− MEECs while only modestly enhancing the ability of Endo+/+ MEECs to maintain capillary stability (Figure 3B). Conversely, DN-Akt expression sharply antagonized Endo+/+ capillary stability (Figure 3B), further supporting endoglin function in the regulation of PI3K/Akt signaling and capillary stability.
Although both TGF-β1 and BMP-9 are established ligands for endoglin signaling to the Smad 1/5/8 pathway, endoglin has not been demonstrated to couple to the PI3K/Akt signaling. To investigate whether endoglin mediates differential effects of TGF-β1 and BMP-9 through the PI3K/Akt signaling pathway, we examined Akt activation in response to its ligands in Endo+/+ and Endo−/− MEECs first under normal monolayer culture conditions (Figure 3C). Endoglin expression significantly elevated basal Akt activation, as determined by phospho-Akt levels, in Endo+/+ MEECs compared with Endo−/− MEECs (Figure 3C, left). Exogenous BMP-9 stimulation moderately enhanced Akt activation in a time-dependent manner, whereas TGF-β1 decreased Akt activity in Endo+/+ MEECs (Figure 3C, left). Exogenous ligand treatment had little effect on Akt activation in Endo−/− MEECs (Figure 3C, right). In addition, when Akt activation was assessed directly from Matrigel-induced endothelial capillaries, endoglin expression up-regulated basal Akt activation and TGF-β1 treatment decreased Akt activation, whereas BMP-9 increased Akt activation in Endo+/+ MEECs (Figure 3D) while having modest effects on Endo−/− MEECs (Figure 3D, top).
As a key mediator of antiapoptotic signaling, Akt promotes cell survival and growth in many cell contexts. Given that endoglin-dependent up-regulation of Akt signaling is consistent with elevated endoglin expression in highly proliferating endothelial cells, we examined whether the observed effect of endoglin and the opposing effects of TGF-β1 and BMP-9 on Akt activation differentially regulated apoptosis by monitoring caspase-3 and caspase-9 activation. Endoglin expression significantly decreased caspase-3 and caspase-9 cleavage in Endo+/+ MEECs compared with Endo−/− MEECs (Figure 3D, bottom), consistent with previous studies (Li et al., 2000). In the presence of exogenous TGF-β1, both cell types exhibited higher levels of cleaved caspase products, indicating apoptotic activity, whereas BMP-9 treatment slightly inhibited caspase cleavage. Taken together, these results indicate that endoglin may activate Akt signaling downstream of BMP-9 to inhibit apoptosis and stabilize endothelial capillaries while suppressing Akt signaling downstream of TGF-β1 to promote apoptosis and capillary regression.
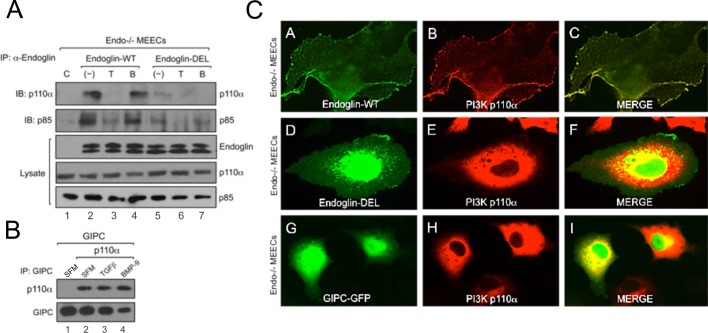
GIPC mediates endoglin-dependent regulation of PI3K/Akt signaling and endothelial capillary sprout and tube stabilization
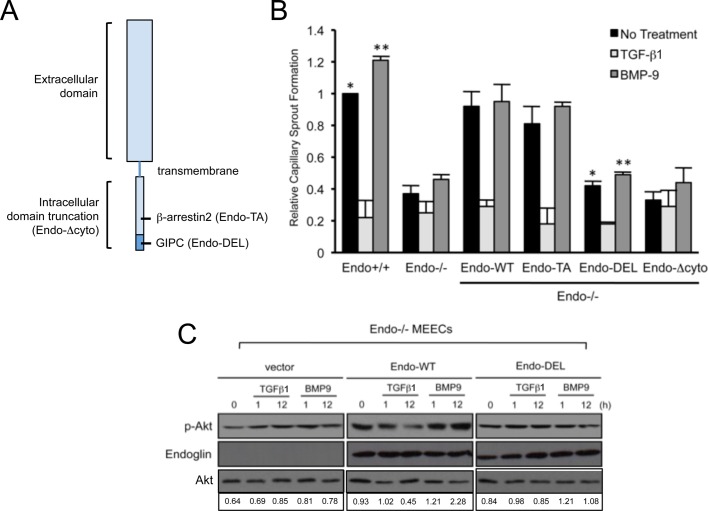
To determine how endoglin couples to the PI3K/Akt pathway to regulate capillary stability in a ligand-dependent manner, we restored the expression of endoglin wild type or mutants lacking specific functional domains in Endo−/− MEECs (Figure 4A) and assessed effects in our capillary assay (Figure 4B). Whereas restoring expression of wild type (Endo-WT) or endoglin point mutant unable to bind β-arrestin2 (Endo-TA) in Endo−/− MEECs largely rescued their capillary integrity to levels comparable to those with Endo+/+ MEECs, expression of endoglin unable to bind GIPC (Endo-DEL) or endoglin lacking the entire cytoplasmic domain (Endo-Δcyto) failed to promote capillary stability (Figure 4B). Cell surface biotinylation revealed that both Endo-DEL and Endo-Δcyto were expressed at the cell surface (Supplemental Figure S3C), suggesting that the cytoplasmic domain, and specifically the GIPC-binding site, was critical for mediating endoglin's effects on capillary stability.
FIGURE 4:
Endoglin requires GIPC interaction for Akt activation and capillary sprout stabilization. (A) Schematic of endoglin structure. Shown are regions of truncation for the entire intracellular domain (Endo-Δcyto) and mutations on binding sites for β-arrestin2 (Endo-TA; amino acid residue substitution Thr-650 to Ala) and GIPC (Endo-DEL; amino acid truncation SMA656–658). (B) Endo−/− MEEC assessed for capillary stability upon reconstituting expression of human endoglin, including the wild type (Endo-WT), a point mutant unable to bind β-arrestin2 (Endo-TA), a PDZ-motif truncation mutant unable to bind GIPC (Endo-DEL), or a mutant lacking the cytoplasmic domain (Endo-Δcyto). Graph represents three independent experiments performed in triplicate. Error bars, average SEM. *p < 0.01, **p < 0.01. (C) Biochemical analysis of Akt activation for the indicated times in human Endo-WT– and Endo-DEL– expressing Endo−/− MEECs in response to TGF-β1 (50 pM) and BMP-9 (16.5 nM). Data are representative of at least three independent experiments. Densitometric analysis was based on band intensities of phospho-Akt relative to total Akt.
To determine whether the deficiency of Endo-DEL to restore capillary stability correlated with effects on Akt signaling, we reconstituted Endo-WT and Endo-DEL expression in endoglin-null cells and compared the relative levels of Akt activation at the basal state and upon ligand treatment (Figure 4C). As controls, we first tested for TGF-β1– and BMP-9–induced activation of Smad 1/5/8 in Endo-WT– and Endo-DEL–expressing cells. Consistent with our previous report, Endo-WT expression resulted in greater TGF-β1– and BMP-9–induced Smad1/5/8 activation compared with Endo-DEL (Supplemental Figure S3B). In the case of Akt, Endo-WT expression elevated the level of basal Akt activation compared with control Endo−/− MEECs and rescued TGF-β1–mediated inhibition of Akt activity, as well as BMP-9–induced activation of Akt activity over time (Figure 4C). In contrast, restoration of Endo-DEL expression did not alter basal Akt activation and failed to rescue either TGF-β1–mediated inhibition of Akt activity or BMP-9–induced activation of Akt activity over time (Figure 4C), supporting a critical role for the GIPC-binding function of endoglin in mediating Akt signaling and capillary stability.
GIPC scaffolds PI3K to endoglin at the plasma membrane to regulate Akt activation
Given the supporting evidence for endoglin and GIPC in altering Akt signaling downstream, we examined whether endoglin interacts with PI3K. PI3K exists as a heterodimer comprising the catalytic (p110) and regulatory (p85) subunits. p85 contains both Src-homology domain 2 (SH2) and SH3, which couple numerous cell surface receptors, including tyrosine kinase receptors, to the PI3K/Akt signaling pathway (Kontos et al., 1998; Geering et al., 2007). We transiently expressed Endo-WT and Endo-DEL in Endo−/− MEECs and performed coimmunoprecipitation to detect possible interactions between endoglin and PI3K subunits. Whereas neither p110α nor p85 was coimmunoprecipitated with endoglin antibody in control Endo−/− MEECs (Figure 5A, lane 1), Endo-WT expression resulted in coprecipitation of endogenous p110α and p85 (Figure 5A, lane 2). In addition, whereas BMP-9 had no effect on the interaction of endoglin and PI3K subunits (Figure 5A, lane 4), TGF-β1 treatment significantly attenuated these interactions (Figure 5A, lane 3). In contrast, Endo-DEL failed to efficiently coprecipitate either p110α or p85 (Figure 5A, lanes 5–7), suggesting a critical role of GIPC in scaffolding the interaction between endoglin and PI3K. We tested whether GIPC associates with PI3K by coexpressing GIPC and p110α in Endo−/− MEECs. Of interest, immunoprecipitation of GIPC resulted in the coprecipitation of p110α independent of ligands or endoglin expression, suggesting that their interaction is constitutive (Figure 5B, lanes 2–4). Given that endoglin and p110α coprecipitate, we investigated whether they colocalize by immunofluorescence. In Endo−/− MEECs, the expression of green fluorescent protein (GFP)–tagged GIPC was diffusely localized in the cytoplasm and nucleus, whereas p110α expression was restricted to the cytoplasm (Figure 5C, panels G–I). The restored expression of Endo-WT in Endo−/− MEECs resulted in the translocation of p110α to the membrane, where it colocalized with endoglin (Figure 5C, panels A–C). In contrast, Endo-DEL failed to alter the native cytoplasmic localization of p110α (Figure 5C, panels D–E). Collectively, these data suggest a scaffolding mechanism by which endoglin and GIPC regulate the localization and activation of PI3K.
FIGURE 5:
GIPC scaffolds PI3K to plasma membrane in an endoglin-dependent manner. (A) Endo−/− MEECs expressing either human Endo-WT or Endo-DEL coimmunoprecipitated with endogenous p110α and p85 under no treatment, TGF-β1 (50 pM), or BMP-9 (16.5 nM) for 2 h. (B) Endo−/− MEECs expressing FLAG-tagged GIPC coimmunoprecipitated with p110α in serum-free media (SFM; lanes 1 and 2), TGF-β1 (lane 3, 50 pM), or BMP-9 (lane 4, 16.5 nM). (C) Representative images of Endo−/− MEECs expressing p110α with human Endo-WT (A–C), Endo-DEL (D–F), and GIPC-GFP (G–I).
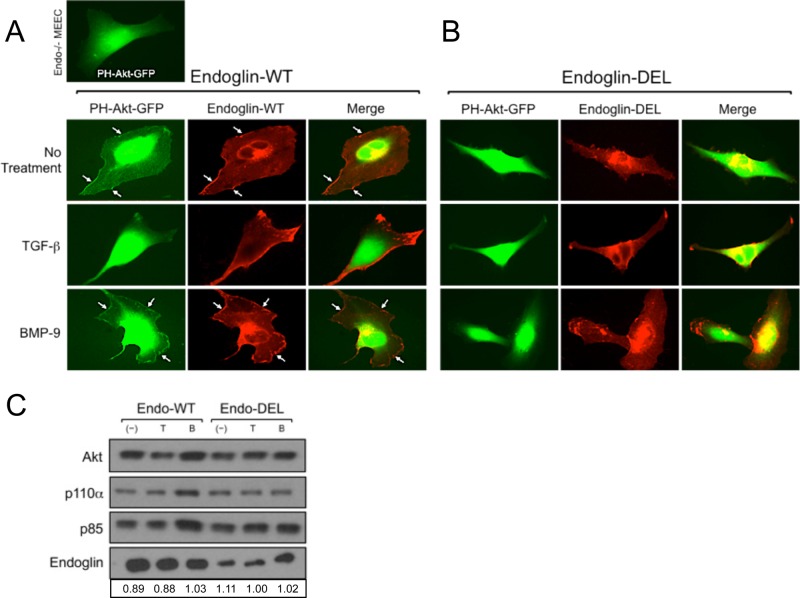
To test our hypothesis that endoglin/GIPC mediates the membrane trafficking of PI3K to regulate Akt activation, we followed the localization of a GFP-Akt pleckstrin homology domain fusion protein (PH-Akt-GFP) that targets second messengers phosphatidylinositol (4,5)-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate present in the plasma membrane (Kontos et al., 1998; Hellyer et al., 2001). In Endo−/− MEECs, PH-Akt-GFP expression displayed a diffuse cytoplasmic and nuclear distribution (Figure 6A, top left). On expression of Endo-WT in Endo−/− MEECs, a significant proportion of PH-Akt-GFP translocated to the plasma membrane, where it colocalized with endoglin (Figure 6A, top three panels). Consistent with our coimmunoprecipitation studies of endoglin and PI3K subunits, TGF-b1 stimulation reduced, whereas BMP-9 enhanced, the colocalization of endoglin with PH-Akt-GFP at the plasma membrane (Figure 6A, middle and lower three panels). Furthermore, expression of Endo-DEL in Endo−/− MEECs failed to recruit appreciable levels PH-Akt-GFP to the plasma membrane at baseline or with TGF-b1 or BMP-9 stimulation (Figure 6B), supporting an essential role for endoglin interacting with GIPC in Akt activation. To quantitatively assess the ability of endoglin to recruit endogenous Akt to the plasma membrane, we performed biochemical subcellular fractionation studies on Endo−/− MEECs expressing either Endo-WT or Endo-DEL upon no treatment or TGF-b1 or BMP-9 stimulation (Figure 6C). Overall, p110α, p85, and Akt were more abundant in the isolated plasma membrane fractions of Endo-WT–expressing cells than Endo-DEL–expressing cells (Figure 6C). BMP-9 treatment selectively resulted in greater recruitment of the PI3K/Akt components to the plasma membrane fraction in Endo-WT– compared with Endo-DEL–expressing cells (Figure 6C). The PI3K signaling components were still present in Endo-DEL membrane fractions, suggesting that either a basal level of the PI3K/Akt complex is present at the membrane, independent of endoglin expression, or endoglin can associate with PI3K at the membrane independent of GIPC binding. In either event, Endo-DEL expression did not significantly alter the level of PI3K/Akt at the membrane in response to either TGF-b1 or BMP-9 stimulation.
FIGURE 6:
Endoglin–GIPC interaction regulates Akt trafficking and activation. (A) Endo-WT expression with PH-Akt-GFP in Endo−/− MEECs under no treatment or TGF-β1 (50 pM) or BMP-9 (16.5 nM) for 2 h. (B) Endo-DEL expression with PH-Akt-GFP in Endo−/− MEECs under no treatment or TGF-β1 (50 pM) or BMP-9 (16.5 nM) for 2 h. (C) Subcellular fractionation followed by plasma membrane isolation of Endo−/− MEECs expressing human Endo-WT or Endo-DEL under no treatment or TGF-β1 (50 pM) or BMP-9 (16.5 nM) for 4 h. Shown are transiently expressing human Endo-WT or Endo-DEL, along with endogenous levels of Akt, p110α, and p85 present in the isolated plasma membrane fraction. Densitometric analysis was based on band intensities of Akt relative to either Endo-WT or Endo-DEL in each lane.
The interaction between endoglin and GIPC is critical during developmental angiogenesis
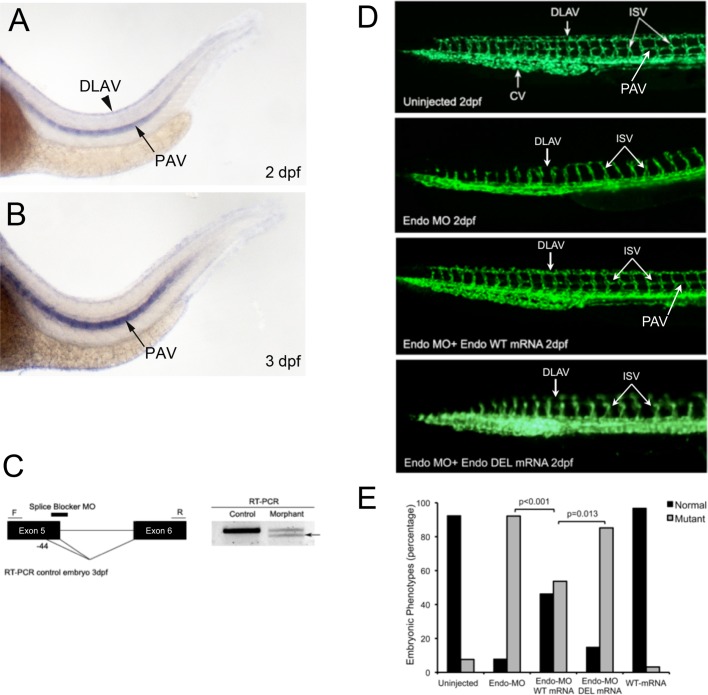
A critical role for GIPC in angiogenesis has been well established in vitro and in vivo (Wang et al., 2006). Morpholino-mediated knockdown of GIPC in zebrafish results in an embryonic lethal phenotype due to severe defects in angiogenesis, as determined by disrupted formation of dorsal longitudinal anastomotic vessel (DLAV) and failure of individual angioblast sprouts from the aorta to form intersegmental vessels (ISVs; Wang et al., 2006). A similar phenotype is observed during morpholino-mediated knockdown of ALK1 during zebrafish embryogenesis (Roman et al., 2002; Gu et al., 2006). To examine the function of endoglin and its ability to bind GIPC, we examined potential endoglin orthologues in the zebrafish genome. Because ENDOGLIN was not annotated in the zebrafish genome during our initial investigation, we identified a potential medaka (Oryzias latipes) orthologue for ENDOGLIN in the Ensembl database (http://uswest.ensembl.org/index.html; Ensembl Gene ID ENSORLG00000016288, transcript ID ENSORLT000000203389 ENSORLT00000020389; protein ID ENSORLP00000020388). Considering that medaka and zebrafish are both teleost fish, we next blasted the zebrafish genome using the protein sequence of the medaka orthologue. We identified and cloned three consecutive exons and introns with high homology to medaka protein sequence located on chromosome 3 in zebrafish, now annotated in the Ensembl database (Ensembl Gene ID ENSDARG00000088795, transcript ID ENSDART00000125008; protein ID ENSDARP00000108319). As expected, in situ hybridization revealed robust expression of this endoglin-like orthologue in the DLAV and the parachordal vessel (PAV) during zebrafish development (Figure 7, A and B). We designed a splice-blocker morpholino (Endo-MO) targeting the second exon–intron junction we cloned, which corresponds to the actual fifth exon–intron junction of the recently annotated zebrafish endoglin-like orthologue (Figure 7C). To define the role of GIPC in mediating endoglin function during capillary formation in vivo, we used the transgenic Fli1–enhanced GFP (EGFP) zebrafish and first monitored the effects of endoglin-like orthologue expression on the endothelial cells and the overall vasculature. Fli1-driven expression of GFP begins early during embryonic development, with angiogenesis evident within the first 24 h, as monitored via fluorescence microscopy. We injected the Endo-MO to knock down endogenous endoglin-like orthologue expression in Fli1-EGFP embryos and observed significant defects: the DLAV and the PAV were partially or completely absent, consistent with the endoglin-like orthologue expression profile, and ISV failed to sprout or grew only up to the horizontal myoseptum half-way up the trunk at 2 d postfertilization (dpf; Figure 7D, top two panels of uninjected vs. middle two panels of Endo-MO). The injection of wild-type human endoglin mRNA along with Endo-MO into Fli1-EGFP transgenic embryos effectively rescued the phenotype, as evidenced by the normal presence of the DLAV, the PAV, and ISVs that have grown all the way to the dorsal trunk at 2 dpf (Figure 7D, bottom two panels). To test whether the interaction with GIPC is critical for endoglin function in promoting angiogenesis in vivo, we injected embryos with Endo-MO and human endoglin mRNA bearing the deletion of the PDZ motif (Endo-DEL). Consistent with our in vitro findings, Endo-DEL mRNA completely failed to rescue the observed defects in angiogenesis induced by Endo-MO (Figure 7, D–E; Endo-MO vs. Endo-MO + DEL mRNA). Taken together, our in vivo data support a pivotal role for GIPC in mediating endoglin-like orthologue function during angiogenesis.
FIGURE 7:
Endoglin/GIPC interaction is required for angiogenesis in Fli1-EGFP zebrafish embryos. (A, B) Side views of endoglin expression in control embryos at 2 and 3 dpf, respectively. Black arrowheads (A) show robust and specific expression of endoglin at 2 dpf in the DLAV. Black arrows (A, B) show robust and strong expression of endoglin in the PAV, ventral vessel lateral to the notochord, adjacent to the myotome, at the level of the horizontal myoseptum (C) The MO targeting splice site is complementary to the fifth exon–intron boundary. RT-PCR was performed to confirm MO-targeting effects. Sequencing of the abnormal shorter transcript (black arrow) confirms that the natural splicing site is disrupted by the MO and a new cryptic splicing site is used in morphants, leading to the generation of an abnormal truncated transcript lacking the last 44 base pairs from exon 5. (D) Images of Fli1-EGFP–expressing control embryos, embryos injected with Endo-MO alone, or embryos rescued with human Endo mRNA at 48 hpf, as visualized by fluorescence microscopy. The Endo-MO–injected embryos display impaired sprouting of ISVs, an absence of the DLAV and the PTA, and a reduction of the caudal vein (CV) compared with controls. (E) Scoring of normal vs. mutant embryos based on the phenotype of Fli1-EGFP–expressing vasculature. Endo-WT mRNA rescues the MO phenotype, whereas Endo-DEL mRNA cannot. Each experimental condition is quantified based on the phenotype of at least 85 embryos.
DISCUSSION
Endoglin–GIPC complex exerts two distinct PI3K/Akt signaling and functional outcomes
Here we defined a new functional role for endoglin during angiogenesis. Our work demonstrates in multiple endothelial cell types that endoglin is required for stabilization of capillary sprouts and tubules. We also identified PI3K/Akt signaling as a novel endoglin target in regulating endothelial capillary stability and cell survival. Previous studies showed that TGF-β superfamily receptors can signal through non-Smad mechanisms, including the MAPKs (ERK, JNK, and p38) and PI3K/Akt pathways (Derynck and Zhang, 2003; Yi et al., 2005; Taniai et al., 2009; Pardali et al., 2010). Indeed, endoglin also has an important role in non-Smad signaling to the Ras/MAPK and PI3K/Akt in many cell types (Lee and Blobe, 2007; Fujita et al., 2010; Santibanez et al., 2010). Given that Ras is an upstream activator of PI3K/Akt, it remains to be determined whether regulation of Ras signaling is the major mechanism by which endoglin modulates Akt activity (Santibanez et al., 2010).
Our data indicate that GIPC is a critical mediator in coupling endoglin to the PI3K subunits, p110α and p85, and Akt at the plasma membrane. The scaffolding function of GIPC appears to be also critical for differential response to TGF-β1 and BMP-9, as indicated by the distinct phenotypes between Endo-WT– and Endo-DEL–expressing Endo−/− MEECs in our capillary-sprouting/tubulation assays, as well as in studies involving PI3K interaction and Akt activation. The opposing effects of TGF-β and BMP-9 on PI3K/Akt trafficking and activation are functionally relevant, as this provides an important mechanism by which endoglin governs endothelial differentiation into capillaries and tubules. Given that there is already an elevated basal level of Akt activation in endoglin-expressing cells, with only a modest enhancement by exogenous BMP-9 (Figures 1B, 3C, and 4C), it is a distinct possibility that basal autocrine BMP-9 signaling accounts for the elevated basal level of Akt activation. Although BMP-9 can also bind ALK1, we observed no ALK1-induced enhancement of capillary stability or Akt activity in endoglin-null systems (Figures 1B and 3C). This finding is consistent with our previous report that increased ALK1 signaling antagonizes endothelial capillary sprouting (Lee et al., 2009). On the basis of these results, we propose a model (Supplemental Figure S3D) in which endoglin binds BMP-9 independent of ALK1 and ALK5 to preferentially promote PI3K/Akt activation after membrane recruitment. In contrast, TGF-β preferentially promotes either endoglin/ALK1–induced Smad1/5/8 and/or ALK5/Smad2/3 activation.
Although the possibility of Smad 1/5/8 and PI3K/Akt signaling cannot be ruled out, our biochemical analyses of endoglin-dependent Akt activation, pharmacological inhibition of Akt (Figure 3A), and rescue experiments with ca-Akt (Figure 3B) all place endoglin as a novel upstream regulator of Akt signaling. The association between endoglin and PI3K subunits p110α and p85 is likely indirect, since endoglin does not possess any phosphorylated tyrosine in the context of a YXXM motif, a consensus sequence typically targeted by SH2 of p85 (Geering et al., 2007). Instead, our data suggest that endoglin associates with PI3K primarily at the plasma membrane through GIPC, which binds to p110α irrespective of TGF-β1 or BMP-9 but fails to alter their localization independent of endoglin (Figure 5C, panels G–I). This scaffolding property appears to be unique to GIPC and not another endoglin binding partner, β-arrestin2, since the expression of Endo-TA phenocopies wild-type endoglin in our capillary-sprouting assays (Figure 4B). Given that the site of interaction with β-arrestin2 is only six amino acids proximal to the site of GIPC interaction, it is unlikely that both GIPC and β-arrestin2 would be able to interact with endoglin simultaneously. Although we demonstrated here that the interaction of endoglin with β-arrestin2 is not required for the effects of endoglin on capillary assays, we previously demonstrated that the interaction of endoglin with β-arrestin2 resulted in their cointernalization in endocytic vesicles and suppression of ERK activation (Lee and Blobe, 2007). This is consistent with our findings in which ERK inhibition had little impact on capillary sprouting (Figure 3A). It is unlikely that the anchoring of endoglin and PI3K to the plasma membrane is the only mechanism by which GIPC controls Akt activity and capillary stability downstream, given that our previous immunofluorescence and current cell surface biotinylation studies (Figure 3C) showed that Endo-TA and Endo-DEL mutants are still able to localize at the membrane to a similar degree despite the loss of internalization and cell surface retention signals (Lee and Blobe, 2007; Lee et al., 2008). The potential involvement of other mechanisms, including zyxin and ZRP-1, cannot be ruled out, considering that these adaptor proteins also bind endoglin's cytoplasmic domain (Conley et al., 2004; Sanz-Rodrigues et al., 2004). How endoglin achieves the balance of endothelial capillary stability through GIPC and zyxin/ZRP-1 remains to be investigated.
Integral role of GIPC in endoglin function during angiogenesis in vivo
Previous in vivo studies using endoglin-knockout mice determined that although early vasculogenesis is unaffected, endoglin expression is specifically required for proper angiogenesis and development of the yolk sac (Li et al., 1999). Our Fli1-EGFP transgenic zebrafish model recapitulates this phenotype and further provides mechanistic insight, in that function of the endoglin-like zebrafish orthologue requires its interaction with GIPC during crucial stages of angiogenesis. In particular, given that GIPC expression is required for vascular endothelial growth factor/neuropilin-1 signaling and angiogenesis (Wang et al., 2006), our use of an endoglin mutant unable to bind GIPC enabled us to specifically gauge the effects of their interaction on angiogenesis without compromising GIPC expression. We therefore provide, for the first time, direct evidence that the endoglin-like zebrafish orthologue functions, in large part, through GIPC to regulate angiogenesis in vivo.
We note that the zebrafish endoglin-like orthologue does not have the human C-terminal PDZ domain postulated to mediate interaction with GIPC (Lee et al., 2008). Indeed, the endoglin-like zebrafish orthologue shares little homology to the otherwise highly conserved cytoplasmic domain of endoglin. The most closely related gene for endoglin in humans is for the TGF-β superfamily coreceptor, the type III TGF-β receptor (TGFBR3). The zebrafish orthologue for TGFBR3 is annotated on chromosome 6, 20632844-20810939, in Ensembl and shares 48% homology with the human TGFBR3. Thus the endoglin-like orthologue on chromosome 3 is unlikely the TGFBR3 zebrafish orthologue. In addition, the vascular expression pattern of the endoglin-like zebrafish orthologue (Figure 7, A and B), the vascular phenotype of morpholino-induced silencing of this endoglin-like zebrafish orthologue (Figure 7D), and the ability of wild-type human ENDOGLIN mRNA to rescue the zebrafish vascular phenotype (Figure 7D) suggest conservation of function, possibly through a different amino acid sequence. The partial phenotypic rescue of human ENDOGLIN might be indicative of a lower-affinity interaction between human ENDOGLIN and zebrafish GIPC.
Endoglin dysfunction in human disease
Here we describe a specific role for endoglin in promoting and maintaining the integrity of capillaries and tubes. Endoglin appears to function by promoting endothelial cell survival through activation of the PI3K/Akt pathway, as well as through maintenance of adherens junctions. These findings, in part, explain in vivo observations as to why endoglin haploinsufficiency in patients with HHT1 and endoglin heterozygotic mice share similar characteristic features, including AVMs and hemorrhagic events in multiple tissues and organs due to fragile vasculatures (Bourdeau et al., 1999). It is likely that endoglin possesses the dual function of promoting capillary stability and facilitating TGF-β–induced apoptosis as a means of tight regulation of vascular remodeling during angiogenesis. The contribution of the PI3K/Akt pathway to the pathogenesis of HHT remains to be explored.
In summary, we characterized a new facet of endoglin signaling and biology. Our studies suggest that endoglin functions as either a positive or negative regulator of angiogenesis by modulating the integrity of endothelial capillaries during angiogenesis, a function that is likely essential during vascular remodeling. Our data also suggest that the balance of TGF-β1 and BMP-9 in the angiogenic microenvironment might function to regulate disparate functions of endoglin during this process. The molecular bases for such effects include a novel signaling role for endoglin in engaging the PI3K/Akt pathway and its downstream cell survival mechanisms. Finally, our studies provide the first in vivo evidence that normal endoglin function requires its interaction with GIPC during angiogenesis. These novel insights may aid in generating more effective therapeutic approaches for endoglin-associated vascular disorders, including HHT, pre-eclampsia, and cancer-associated angiogenesis.
MATERIALS AND METHODS
Endothelial cell culture and protein expression
Endothelial cells were cultured as described previously (Lee et al., 2008). Ectopic expression in MEECs was achieved by nucleofection using Amaxa (Kit L; Lonza, Basel, Switzerland) as reported previously (Lee et al., 2008). HMEC-1s were infected with adenovirus encoding nontargeting vector control or shRNA targeting endoglin sequence for 48–72 h before experimental procedures. Silencing endogenous GIPC was achieved by nucleofection of MEECs with shRNA vector targeting mouse GIPC (a generous gift from Jeffrey Rathmell, Duke University, Durham, NC) for 48 h before experiments. Endoglin constructs (Endo-WT, Endo-TA, Endo-DEL) were hemagglutinin (HA) tagged and have been reported previously (Lee and Blobe, 2007; Lee et al., 2008). ca-Akt and DN-Akt expression vectors were generous gifts (Frederick Quelle, University of Iowa, Iowa City, IA). PH-Akt-GFP fusion construct cloned into pcDNA 3 vector was a generous gift from John Koland (University of Iowa).
Biochemical analyses
Akt activation was assessed by immunoblotting with phosphospecific and total Akt antibodies (Cell Signaling, Beverly, MA). Caspase-3 and -9 cleavage was detected using their respective antibodies (Cell Signaling). Endoglin detection in cell lysates was assessed by immunoblotting with anti-HA antibody. p110α and p85 were detected using total p110α and p85 antibodies (Cell Signaling). Subcellular fractionation and plasma membrane isolation was performed as previously described (Murata et al., 2003). Briefly, Endo−/− MEECs were manually homogenized in 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.4, 10 mM NaF, 1 mM EDTA, and protease inhibitor cocktail [Sigma-Aldrich, St. Louis, MO]) at 4°C and then centrifuged at 19,000 × g for 20 min. The resulting pellet was resuspended in HEPES buffer and layered onto 1.12 M sucrose gradient and centrifuged at 100,000 × g for 1 h. The resulting white band was extracted and resuspended in HEPES buffer and then centrifuged again at 40,000 × g for 20 min. The pellet from this second spin was resuspended in HEPES buffer for Bradford protein concentration analysis.
Coimmunoprecipitation
MEECs expressing either Endo-WT or Endo-DEL were solubilized with lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 10 mM NaF, 10% glycerol [wt/vol], 2 mM EDTA, 1 mM Na3VO4, protease inhibitor cocktail [Sigma-Aldrich]). On centrifugation (20 min at 14,000 rpm), supernatants were collected and incubated for 4 h with endoglin antibody P3D1 (Developmental Studies Hybridoma Bank, University of Iowa). Protein agarose-A was subsequently added and incubated for additional 2 h before washing and final collection in 2× sample buffer.
Thiazolyl blue tetrazolium bromide assay
MEECs plated at 100,000 cells were treated with thiazolyl blue tetrazolium bromide (MTT) at various time points (0, 6, 12, and 24 h) for 2 h. The MTT metabolic product (formazan) was extracted in dimethyl sulfoxide, and the optical density was read at 570 nm.
Fluorescence microscopy
Endothelial tight cell–cell junctions were probed with VE-cadherin (Abcam, Cambridge, MA) for 15 min before fixation with 4% paraformaldehyde. Cells were then washed, incubated with Alexa 568–conjugated secondary antibody (Invitrogen, Carlsbad, CA). HMEC-1s infected with NTV or endoglin-shRNA adenovirus were visualized by recombinant red fluorescent protein fluorescence. Images were digitally acquired using a fluorescence microscope (Nikon, Melville, NY). Fluorescence intensity was evaluated using ImageJ (National Institutes of Health, Bethesda, MD) software and data analyzed by normalizing to either Endo+/+ MEECs or HMEC-1s infected with NTV adenovirus. The standard immunofluorescence protocol of 4% paraformaldehyde fixation and Triton X-100 permeabilization method was used to image the colocalization of endoglin (P3D1 antibody; Developmental Studies Hybridoma Bank, University of Iowa) and p110α (Cell Signaling) localization. PH-Akt-GFP localization was monitored 24 h postnucleofection in Endo−/− MEECs after a 10-h serum starvation.
Cell surface biotinylation assay
Cell surface biotinylation was performed according to manufacturer's protocol (Cell Surface Protein Isolation Kit; Pierce, Thermo Fisher Scientific, Rockford, IL). Briefly, Endo−/− MEECs transfected with endoglin constructs were washed with phosphate-buffered saline and treated with EZ-Link Sulfo-NHS-SS-Biotin on ice for 30 min. On quenching of the reaction, cells were lysed with lysis buffer (supplemented with protease inhibitor cocktail [Sigma-Aldrich]) and centrifuged at 10,000 × g for 2 min, and the resulting supernatant was incubated with NeutrAvidin agarose resin for 60 min at 4°C. The resin was washed twice before elution with elution buffer containing 50 mM dithiothreitol. Cell surface biotinylated endoglin was detected by immunoblotting with P3D1 antibody.
Endothelial capillary-sprouting and tube morphogenesis assays
In the Matrigel-based capillary-sprouting assays, Matrigel (BD Biosciences, San Diego, CA) was plated in 12-well plates and allowed to polymerize at 37°C in 5% CO2 incubator for 30 min. MEECs or HMEC-1s were plated (1.5 × 105 cells/ml) in MCDB-131 media (without serum or endothelial cell growth supplements [ECGS]) in the presence or absence of TGF-β1 (50 pM) or BMP-9 (16.5 nM) for the indicated times. BMP-9 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was coincubated (1:200 dilution) in Matrigel capillary-sprouting assays for 16–24 h. In collagen-based three-dimensional capillary tube assays, 12-well plates were coated with collagen type I at 1 mg/ml (BD Biosciences) and allowed to polymerize at 37°C in 5% CO2 incubator for 30 min. MEECs (2.5 × 105 cells/ml) were plated in regular growth media (15% fetal bovine serum and ECGS) for 1 h for cell adherence. A second layer of collagen was overlaid and allowed to polymerize. The cells are assessed for tube formation and regression after 36–48 h. In each assay, digital images (10× magnification) of random fields were acquired, and capillary-sprouting branches were counted in each field. Each experimental condition was normalized as percentage compared with Endo+/+ MEECs or NTV for HMEC-1s and HUVECs.
Morpholino in vivo analysis of gene expression and embryo manipulations
Zebrafish (Danio rerio) embryos were raised and fish were maintained as described (Westerfield, 1995). Embryos at 24 h postfertilization (hpf) were raised in 0.2 mM 1-phenyl-2-thio-urea (Sigma-Aldrich) to prevent pigment formation and allowed to develop until 48 hpf. Splice blocker morpholino (MO) against endoglin (Endo-MO) (TAGTAGAGAACTTACCCGCACAGGC) corresponding to exon 5 of the recently annotated zebrafish endoglin (chromosome 3: 6273243-6307324, transcript ID: ENSDART00000125008) was designed by and obtained from Gene Tools (Philomath, OR). For Endo-MO efficacy experiments, reverse transcription (RT)-PCR was performed using gene-specific primers in exons immediately flanking the targeted region on 72 hpf RNA isolated from control and Endo-MO–injected embryos (5 ng of MO and/or 100 pg of RNA into Fli1-EGFP zebrafish embryos at the one- to two-cell stage). Injected embryos were scored at 48 hpf and classified into two groups—normal and mutant—on the basis of the relative angiogenesis defects compared with age-matched controls from the same clutch. For RNA rescue experiments, End-WT and Endo-DEL mRNA were transcribed in vitro using the SP6 Message Machine Kit (Ambion, Austin, TX). All the experiments were repeated three times, and we ran a t test to determine the significance of the phenotypic rescue. Whole-mount RNA in situ hybridizations were carried out as described (Zaghloul et al., 2010).
Supplementary Material
Acknowledgments
We thank Frederick Quelle and John Koland for providing the Akt constructs. We also thank Jeffrey Rathmell for the GIPC shRNA vector. The Fli1:EGFP transgenic zebrafish line was a generous gift from Kenneth Poss (Duke University). This work was supported by National Institute of Health Grants F32CA124139 and K99HL103791 (to N.Y.L), F32CA1361252 (to C.E.G), R01CA105255 (to G.C.B), and AHA-11POST7160006 (to C.G) and seed funding from the Center of Human Disease Modeling. N.K. is a Distinguished Brumley Professor.
Abbreviations used:
- ALK
activin-like kinase receptor
- AVM
arteriovenous malformation
- BMP
bone morphogenetic protein
- GIPC
Gα-interacting protein C-terminus
- HHT
hereditary hemorrhagic telangiectasia
- HMEC-1
human microvascular endothelial cells
- HUVECs
human umbilical vein endothelial cells
- MEEC
murine embryonic endothelial cells
- PI3K
phosphatidylinositol 3-kinase
- shRNA
short hairpin RNA
- TGF-β
transforming growth factor β
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-12-0993) on May 16, 2012.
REFERENCES
- Bernabeu C, Conley BA, Vary CP. Novel biochemical pathways of endoglin in vascular cell physiology. J Cell Biochem. 2007;102:1375–1388. doi: 10.1002/jcb.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104:1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz S, Bellon T, Cales C, Vera SC, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- Conley B, Koleva R, Simth J, Kacer D, Zhang D, Bernabeu C, Vary CP. Endoglin controls cell migration and composition of focal adhesions: function of the cytosolic domain. J Biol Chem. 2004;279:27440–27449. doi: 10.1074/jbc.M312561200. [DOI] [PubMed] [Google Scholar]
- David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- Davis GE, Koh W, Stratman AN. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res C Embryo Today. 2007;81:270–285. doi: 10.1002/bdrc.20107. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, Sanz-Rodriguez F, Zarrabeitia R, Pérez-Molino A, Hebbel RP, Nguyen J, Bernabéu C, Botella LM. Blood outgrowth endothelial cells from hereditary haemorrhagic telangiectasia patients reveal abnormalities compatible with vascular lesions. Cardiovasc Res. 2005;68:235–248. doi: 10.1016/j.cardiores.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003;22:6557–6563. doi: 10.1038/sj.onc.1206813. [DOI] [PubMed] [Google Scholar]
- Fujita D, Tanabe A, Kamegai H, Ohmichi M. Role of extracellular signal-regulated kinase and AKT cascades in regulating hypoxia-induced angiogenic factors produced by a trophoblast-derived cell line. J Endocrinol. 2010;206:131–140. doi: 10.1677/JOE-10-0027. [DOI] [PubMed] [Google Scholar]
- Geering B, Cutillas PR, Vanhaesebroeck B. Regulation of class IA PI3Ks: is there a role for monomeric PI3K subunits. Biochem Soc Trans. 2007;35:199–203. doi: 10.1042/BST0350199. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Zhang L, Zhao X, Gao X, Ning Y, Meng A, Chen YG. Functional analysis of mutations in the kinase domain of the TGF-beta receptor ALK1 reveals different mechanisms for induction of hereditary hemorrhagic telangiectasia. Blood. 2006;107:1951–1954. doi: 10.1182/blood-2005-05-1834. [DOI] [PubMed] [Google Scholar]
- Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–42161. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- Jerkic M, Rodriguez-Barbero A, Prieto M, Letarte M, Lopez-Novoa JM. Reduced angiogenic responses in adult endoglin heterozygous mice. Cardiovasc Res. 2006;69:845–854. doi: 10.1016/j.cardiores.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Diamond A, Jackson CE, Attisano L, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- Kontos CD, Stauffer TP, Yang WP, York JD, Huang L, Blanar MA, Meyer T, Peters KG. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;18:4131–4140. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres P, Letamendia A, Almendro N, Raab U, Lopez LA, Langa C, Fabra A, Letarte M, Bernabeu C. Endoglin modulates cellular responses to TGF-beta 1. J Cell Biol. 1996;133:1109–1121. doi: 10.1083/jcb.133.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrin F, Goumans MJ, Jonker L, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Blobe GC. The interaction of endoglin with beta-arrestin2 regulates transforming growth factor-beta-mediated ERK activation and migration in endothelial cells. J Biol Chem. 2007;282:21507–21517. doi: 10.1074/jbc.M700176200. [DOI] [PubMed] [Google Scholar]
- Lee NY, Haney JC, Sogani J, Blobe GC. Casein kinase 2beta as a novel enhancer of activin-like receptor-1 signaling. FASEB J. 2009;23:3712–3721. doi: 10.1096/fj.09-131607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Ray B, How T, Blobe GC. Endoglin promotes transforming growth factor beta-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J Biol Chem. 2008;283:32527–32533. doi: 10.1074/jbc.M803059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Hampson I N, Hampson L, Kumar P, Bernabeu C, Kumar S. CD105 antagonizes the inhibitory signaling of transforming growth factor beta1 on human vascular endothelial cells. FASEB J. 2000;14:55–64. doi: 10.1096/fasebj.14.1.55. [DOI] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, Fruttiger M, Arthur HM. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res. 2010;106:1425–1433. doi: 10.1161/CIRCRESAHA.109.211037. [DOI] [PubMed] [Google Scholar]
- Marchuk DA. Genetic abnormalities in hereditary hemorrhagic telangiectasia. Curr Opin Hematol. 1998;5:332–338. doi: 10.1097/00062752-199809000-00005. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Baldwin MA, Thukkani AK, Gallione CJ, Berg JN, Porteous ME, Guttmacher AE, Marchuk DA. Six novel mutations in the endoglin gene in hereditary hemorrhagic telangiectasia type 1 suggest a dominant-negative effect of receptor function. Hum Mol Genet. 1995;4:1983–1985. doi: 10.1093/hmg/4.10.1983. [DOI] [PubMed] [Google Scholar]
- Murata H, Hresko RC, Mueckler M. Reconstitution of phosphoinositide 3-kinase-dependent insulin signaling in a cell-free system. J Biol Chem. 2003;278:21607–21614. doi: 10.1074/jbc.M302934200. [DOI] [PubMed] [Google Scholar]
- Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-β family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Park SO, et al. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece-Barbara N, Vera S, Karthirkamanthamby K, Liebner S, Di Guglielmo GM, Dejana E, Wrana JL, Letarte M. Endoglin null endothelial cells proliferate faster and are more responsive to transforming growth factor beta1 with higher affinity receptors and an activated Alk1 pathway. J Biol Chem. 2005;280:27800–27808. doi: 10.1074/jbc.M503471200. [DOI] [PubMed] [Google Scholar]
- Ponce ML. Tube formation: an in vitro Matrigel angiogenesis assay. Methods Mol Biol. 2009;467:183–188. doi: 10.1007/978-1-59745-241-0_10. [DOI] [PubMed] [Google Scholar]
- Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Weinstein BM. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129:3009–3019. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- Santibanez J, Perez-Gomez E, Vary C, Bernabeu C. The TGF-β co-receptor endoglin modulates the expression and transforming potential of H-Ras. Carcinogenesis. 2010;31:2145–2154. doi: 10.1093/carcin/bgq199. [DOI] [PubMed] [Google Scholar]
- Sanz-Rodrigues F, Guerrero-Esteo M, Botella L, Banville D, Vary C, Bernabeu C. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. J Biol Chem. 2004;279:32858–32868. doi: 10.1074/jbc.M400843200. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Taniai E, Kawai M, Dewa Y, Nishimura J, Harada T, Mitsumori K, Shibutani M. Crosstalk between PTEN/Akt2 and TGFbeta signaling involving EGF receptor down-regulation during the tumor promotion process from the early stage in a rat two-stage hepatocarcinogenesis model. Cancer Sci. 2009;100:813–820. doi: 10.1111/j.1349-7006.2009.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mukhopadhyay D, Xu X. C-terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene: University of Oregon Press; 1995. [Google Scholar]
- Yi JY, Shin I, Arteaga CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:10870–10876. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]
- Zaghloul NA, et al. Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet-Biedl syndrome. Proc Natl Acad Sci USA. 2010;107:10602–10607. doi: 10.1073/pnas.1000219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.