Abstract
Alzheimer’s disease (AD) is a fatal neurological disorder that is a leading cause of death, with its prevalence increasing as the average life expectancy increases worldwide. There is an urgent need to develop new therapeutics for this disease. A newly described protein, the γ-secretase activating protein (GSAP) has been proposed to promote elevated amyloid-β production, an activity that seems to be inhibited using the well-establish cancer drug, imatinib (Gleevec). Despite much interest in this protein, there has been little biochemical characterization of GSAP. Here we report protocols for the recombinant bacterial expression and purification of this potentially important protein. GSAP is expressed into inclusion bodies, which can be solubilized using harsh detergents or urea; however, traditional methods of refolding were not successful at generating soluble forms of the protein that contained well-ordered and homogeneous tertiary structure. However, GSAP could be solubilized in detergent micelle solutions, where it was seen to be largely α-helical but to adopt only heterogeneous tertiary structure. Under these same conditions, GSAP did not associate with either imatinib or the 99 residue transmembrane C-terminal domain of the amyloid precursor protein. These results highlight the challenges that will be faced in attempts to manipulate and characterize this protein.
Alzheimer’s Disease (AD) is a devastating neurodegenerative disease that impacts millions of people worldwide at enormous personal and economic cost (1). Unfortunately, there is currently no cure or effective treatment, but researchers have made significant progress in characterizing the pathophysiology of AD (2). The most widely-accepted hypothesis for disease etiology revolves around the amyloid precursor protein (APP) (3). APP is cleaved by β-secretase to generate its 99 residue transmembrane C-terminus (C99), which is then cleaved by γ-secretase to produce amyloid beta (Aβ) peptides of different lengths. These peptides form neurotoxic oligomers that go on to deposit as neuritic plaques, the pathological markers of the disease.
Inhibition of the heterotetrameric γ-secretase to block cleavage of C99 would reduce Aβ production (4–6). Unfortunately, γ-secretase has numerous substrates and has not, so far, been an effective therapeutic target because of the important role that its cleavage of other substrates, particularly Notch, plays in cellular differentiation (7). As such, there is great interest in exploring how to prevent or modulate C99 cleavage without inhibiting cleavage of other γ-secretase substrates. This imperative resulted in the discovery of the γ-secretase activating protein (GSAP).
GSAP was first described by He et al. 2010 (8). A previous study had shown that the Abl kinase inhibitor imatinib decreases Aβ production, likely by inhibiting γ-secretase activity (9). The search for the imatinib target led to photo-labeling of the C-terminal domain of the uncharacterized Pigeon Homolog Protein (PION). The domain is proteolytically released from PION under cellular, the resulting protein being referred to as GSAP. GSAP appears to form a ternary complex with γ-secretase and C99, as determined through immunoprecipitation reactions and pull-down assays. Knockdown of GSAP through siRNA in N2a cells selectively lowered Aβ levels, and did not reduce the cleavage of other γ-secretase substrates. GSAP knockdown also reduced Aβ plaque burden in a mouse model of AD (8). These data suggest that GSAP may selectively promote Aβ production by promoting γ-secretase cleavage of C99, making GSAP a potential AD drug target. Since the initial discovery of GSAP, one additional research paper has been published, which characterized the immunohistochemical distribution of GSAP in the brains of AD patients (10). GSAP immunoreactivity was observed in four distinct morphological structures present in different regions of the brain in AD patients, one of these structures was largely unique to AD brains as compared to age-matched control brains. GSAP immunoreactivity was also detected in close proximity to presenilin (PS1, a component of γ-secretase) as well as in close association with Aβ-containing senile plaques. While recombinant expression and purification of GSAP was briefly mentioned in these reports, methods were not provided. This paper details the expression, purification, and characterization of GSAP.
Materials and Methods
Materials
BL21 (DE3) and Rosetta (DE3) competent cell lines and the pET32a vector were purchased from EMD Millipore (Darmstadt, Germany). The restriction enzymes NdeI, XhoI, BamI and NcoI were purchased from New England Biolabs (Ipswich, MA). The 15NH4Cl used to isotopically label GSAP was purchased from Cambridge Isotope Laboratories (Andover, MA). Ampicillin and MEM vitamin solution were purchased from Cellgro (Manassas, VA). Imatinib mesylate was purchased from Selleck Chemical Company (Houston, TX). Ni-NTA chromatography resin was purchased from Qiagen (Valencia, CA). The protease inhibitor P8849, Empigen BB detergent (n-dodecyl-N,N- dimethylglycine) and imidazole (≥99% titration grade) were purchased from Sigma- Aldrich (Saint Louis, MO). n-Dodecylphosphocholine (DPC), lyso- myristoylphosphatidylglycerol (LMPG) and isopropyl β-D-1-thiogalactopyranoside (IPTG) were purchased from Affymetrix/Anatrace (Maumee, OH).
Cloning and Construction of the vectors encoding His-tagged forms of GSAP
GSAP corresponds to the 121 residue (amino acids 733–854) C-terminus of the human pigeon homolog protein (PION). The GSAP gene (NM_017439.3) was purchased from GeneCopoeia (Rockville, MD). Two constructs were prepared, with either N- or C- terminal His purification tags (His6 and His10, respectively). To construct the C-terminally His10-tagged construct, the GSAP DNA was digested with NdeI and XhoI after PCR amplification, and was then was inserted into the pET-21b vector. The N-terminal His6- tagged construct was similarly engineered using NcoI and XhoI restriction enzymes and a pET-16a vector. Constructs were confirmed by DNA sequencing.
A second set of constructs was prepared to replicate as closely as possible the constructs used in He et al. 2010 (8). The first was a pET-32a vector encoding a fusion protein in which thioredoxin is linked to the N-terminus GSAP through an intervening His6 tag. As with the His6-tagged constructs expressing only GSAP, the thioredoxin fusion protein was constructed from the DNA digested with BamI and XhoI after PCR amplification of the GSAP gene and was then inserted into the pET-32a vector. A second construct, expressing only thioredoxin, was prepared as a control by inserting a stop codon just before the start of the GSAP coding region in a pET-32a vector. This was accomplished using standard site-directed mutagenesis methods (QuickChange, Agilent Technologies, Santa Clara, CA).
Expression of GSAP in E. coli
Vectors were transformed into E. coli BL21(DE3) cells, which were plated onto ampicillin LB-agar plates and then incubated overnight at 37°C. A single colony was used to inoculate a 5ml culture of LB media containing 100μg/ml of ampicillin. The starter culture was grown for eight hours at 37°C. A 1 L culture of M9 minimal media was prepared using 15NH4Cl for isotopic labeling. The medium for large-scale growth also included ampicillin, glucose, MEM vitamins, 0.1 mM CaCl2, and 1 mM MgSO4. Starter culture (1.2 ml) was added directly to the 1 L culture and the cells were grown at room temperature until the OD600 reached 0.8. Protein expression was induced using 1 mM IPTG, and the cells were harvested by centrifugation 24 hours after induction. Expression of the recombinant GSAP was confirmed by Western blotting using a monoclonal anti-5X His mouse antibody (Cell Signaling Technology, Danvers, MA).
Purification of N- and C-Terminally His6-Tagged GSAP
The harvested cells were weighed and lysed in 20 ml of lysis buffer (75 mM Tris, 300 mM NaCl, 0.2 mM EDTA, pH 7.8) per gram of cells. Also added to the following concentrations were 5 mM MgAcetate, 2 mg/ml of lysozyme, 0.2 mg/ml DNase and RNase, and 50 μl of protease inhibitor per gram of cells. The suspension was tumbled for 90 minutes at room temperature. Following tumbling, cells were further disrupted by five-minute probe sonication with a 50% duty cycle at approximately 57 watts using a Misonix (Farmingdale, NY) sonicator. The lysate was centrifuged at 20,000 rpm in a Beckman-Coulter (Indianapolis, IN) JA 25.5 rotor (approximately 48,000xg) and the pellet, which includes inclusion bodies, was retained. The inclusion bodies containing GSAP were solubilized in 20 ml of lysis buffer per original gram of cells using 3% Empigen (%v/v), a harsh detergent. This solution was tumbled at room temperature until a clear mixture was observed (approximately 4.5 hours). The sample was then centrifuged to remove any remaining insoluble particulates. Ni-NTA resin (1.2 ml per gram of cells) was equilibrated with buffer A (40 mM HEPES, 300 mM NaCl pH 7.8). The supernatant was tumbled with the resin for one hour at room temperature. The resin was loaded into a column and sequentially washed with buffer A containing 3% Empigen (%v/v), and buffer A containing 30 mM imidazole and 1.5% Empigen (%v/v), respectively, to elute all non-His10-tagged proteins from the resin. Empigen was then exchanged for the detergent n-dodecylphosphocholine (DPC) by re-equilibrating the column with 12 column volumes of 20 mM phosphate, pH 7.2, containing 0.5% DPC (%w/v). GSAP was eluted from the column with 250 mM imidazole containing 0.5% DPC (%w/v), pH 7.8. Purification was monitored by A280. After purification, 2 mM DTT was added to the sample to reduce disulfide bonds.
The purification process was monitored by SDS-PAGE gel electrophoresis. Electrophoresis experiments were carried out using an Invitrogen (Grand Island, NY) Novex-Mini Gel system and NuPAGE 4–12% Bis-Tris polyacrylamide gels and MES running buffer.
Purification of Thioredoxin-His6-GSAP Fusion Protein
The purification of Trx-His6- GSAP was alluded to but not described in the original paper (8), but is similar to the His10-GSAP purification strategy described above (personal communication). Trx-His6- GSAP expressing cells were grown, harvested, and lysed as above. After lysis, the supernatant was collected and bound to Ni-NTA resin equilibrated in 50 mM phosphate, 500 mM NaCl pH 7.8. The slurry tumbled for one hour at room temperature. The resin was rinsed with 50 mM phosphate, 500 mM NaCl pH 7.8 and then washed with 50 mM phosphate, 500 mM NaCl, 40 mM imidazole pH 7.8 to remove any remaining non-specifically bound protein. Trx-His6-GSAP was eluted from the column with 50 mM phosphate, 500 mM NaCl, 300 mM imidazole pH 7.8. The eluate was concentrated to a volume below 5 ml and filtered with a 0.2 μm filter. The sample was then subjected to size exclusion chromatography using a HiPrep™ Sephacryl™ S300 16/60 gel filtration column on an AKTAprime™-plus FPLC eluted with 20 mM HEPES, 200 mM NaCl, 1 mM EDTA pH 8.0.
Circular Dichroism (CD) Spectroscopy
Protein samples were purified as described above and were exchanged into a 25 mM sodium phosphate buffer, pH 7.5, containing 0.5% DPC using a PD-10 desalting column (Bio-Rad). The sample and buffers were passed through a 0.2 μm filter before CD data collection. Far-UV CD data was collected from 190 to 260 nm on a Jasco (Easton, MD) J-810 CD spectropolarimeter. Data from five scans were averaged together and blank-corrected.
Solution NMR Spectroscopy
For NMR spectroscopy, the pH of GSAP was adjusted to 7.5 and D2O to 10% was added, followed by concentration through ultrafiltration using an Amicon Centrifugal Filter unit molecular weight cut-off 10,000 Da (Millipore, Billerica, MA). An HSQC spectrum was collected on a 600 MHz Bruker AVANCE III spectrometer at 298 K using TopSpin3 and a standard Bruker pulse sequence.
Titration of C99 by GSAP
Uniformly 15N-labeled C99 with a His6-containing purification tag at its C-terminus was expressed and purified as described in Beel et al. 2008 (11), with a few minor variations. Cultures of E. coli with an expression vector encoding C- terminally His6-tagged human C99 were grown at 37°C in minimal media with Cellgro MEM vitamins and induced at OD600= 0.8 using IPTG, at 18°C overnight. Cells were lysed and inclusion bodies were isolated and washed three times with lysis buffer followed by sonication and recentrifugation. Tagged C99 was then purified using Ni-NTA affinity chromatography into 0.05% LMPG micelles with 250 mM imidazole pH 7.8. After purification, U-15N-C99 was buffer-exchanged and centrifugally concentrated in Amicon concentrators to a final condition of 0.6 mM, with 2.5% LMPG and 100 mM imidazole. The pH was adjusted to 7.5 using glacial acetic acid and ammonium hydroxide. Unlabeled GSAP was prepared as described above using 0.05% LMPG as the detergent for the final purification steps. The purified protein was buffer-exchanged to reduce the imidazole concentration to 100 mM and concentrated to 0.45 mM. The pH was adjusted to 7.5 and LMPG was added to a final concentration of 2.5%.
For titrations, NMR samples were prepared with 0.1 mM C99 in each sample and increasing molar ratios GSAP up to 4:1 GSAP:C99, in 100 mM imidazole, 2.5% or 10% LMPG. 1H,15N-TROSY spectra were collected at 298 K for each sample to determine the effect of GSAP on the chemical shifts of the peaks in the C99 spectrum.
Titration of Imatinib by GSAP
GSAP was prepared as described, except that the final buffers were made using D2O. The sample was concentrated and passed over a PD-10 desalting column to remove all traces of imidazole. This sample contained 0.325 mM GSAP in 25 mM sodium phosphate in D2O, 1% DPC, pH 7.5 and served as a stock solution for the titration. Starting with a solution of 339 mM imatinib mesylate in DMSO, a 1 mM stock solution of the drug in 1% DPC (%w/v) in D2O was prepared. The NMR samples were prepared with 50 μM imatinib, 25 mM imidazole and increasing amounts of GSAP up to a four-fold molar excess. The imatinib 1-D 1H NMR peaks not obscured by detergent and protein were monitored for changes with increasing amounts of GSAP using a 600 MHz magnet at 298 K.
Results
Expression and Purification of GSAP
The GSAP domain of the PION (8) was cloned into pET vectors. Two constructs, one with an N-terminal (pET16) His6- and one with a C-terminal (pET21) His10- purification tag were cloned. Both constructs overexpressed well in different strains of E. coli (BL21(DE3) and Rosetta(DE3)). It was found that the N-terminally tagged construct was highly expressed, but was highly unstable and prone to aggregation. For this reason, the experiments here were conducted using the C-terminally tagged construct, which behaved more favorably.
Following expression, cell lysis, and centrifugation, GSAP was located primarily in inclusion bodies (IB), despite culturing the cells in minimal medium at room temperature, conditions sometimes found to promote folding of unstable or misfolding-prone recombinant proteins. Consequently, IB solubilization and protein refolding was necessary. Solubilization methods tested included dissolution of IB in 8M urea and 0.2% sodium dodecyl sulfate (SDS), both together and separately, followed by removal of the denaturant under different buffer and pH conditions, ranging from pH 5.5 to 7.8. Unfortunately, despite much effort all refolding attempts resulted in precipitation of GSAP. For further details on refolding attempts and outcomes, see Supplemental Table 1.
Additional experiments were carried out in an effort to refold the protein, this time with GSAP immobilized by binding to Ni-NTA resin through its His10 tag. Inclusion bodies were solubilized with 8M urea and 0.2% SDS and incubated with Ni-NTA resin. The first on-column refolding test involved the stepwise removal of SDS and urea from the solution bathing the resin. After complete removal of denaturant, an attempt was made to elute the protein from the column with 250 mM imidazole, pH 7.8. However, GSAP did not elute, indicating insolubility in a denaturant-free and detergent-free elution buffer. Based on the knowledge that GSAP can be solubilized using a harsh detergent (SDS), additional attempts were made to refold GSAP in the presence of a milder detergent.
Inclusion bodies were solubilized using the harsh zwitterionic detergent Empigen and GSAP was then associated with the nickel resin. The detergent present in the solution that bathes the Ni-NTA-bound GSAP was then switched from Empigen to one of several detergents: DPC, lyso-myristoylphosphatidylglycerol (LMPG) or decylmaltoside (DM), followed by attempted elution using 250 mM imidazole in that same detergent solution. It was found that GSAP could not be eluted in DM detergent micelle solutions, but did elute when either DPC or LMPG solutions were used. Both of these detergents have previously been widely used as membrane mimetics in studies of membrane proteins (12). Figure 1 shows an SDS-PAGE gel that documents protein purification. The elution fraction has only two bands, which have been confirmed by mass spectrometry to be the monomer and dimer forms of GSAP. The dimer band is likely due to the presence of the single cysteine at amino acid position 32, as this band is absent in the presence of a reducing agent. The total yield of purified protein was approximately 15 mg per liter of culture.
Figure 1.
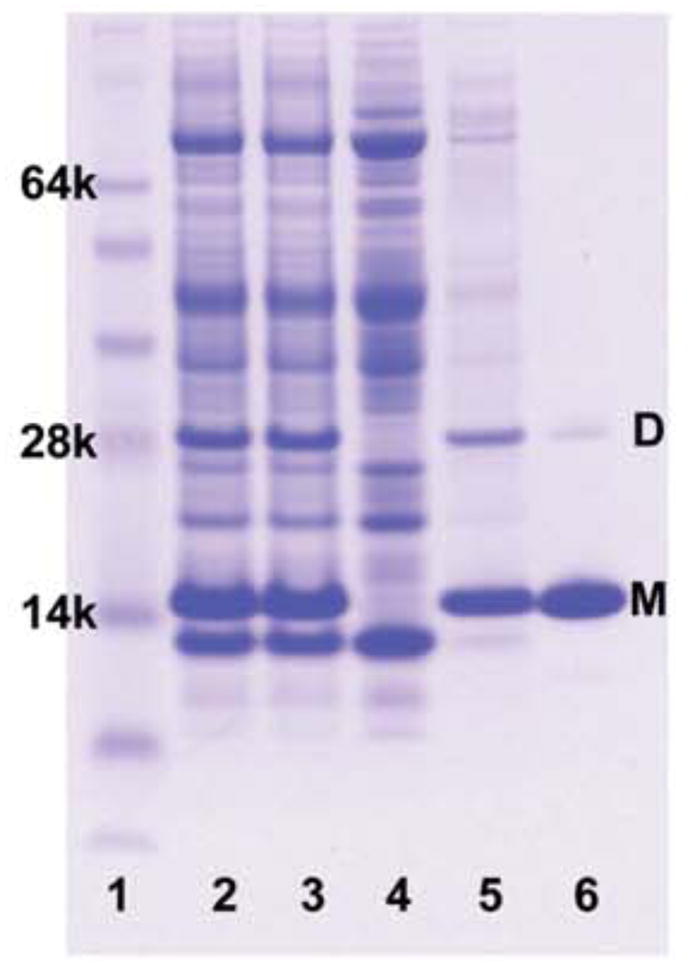
SDS-PAGE documentation of GSAP purification. A 4–12% polyacrylamide gel was stained with Coomassie R-250 brilliant blue, with lanes: 1) SeeBlue Plus 2 protein molecular weight markers. 2) Whole cell lysate solubilized with 4M urea and SDS. 3) Sonicated cell lysate further solubilized with 4M urea and SDS. 4) Cellular supernatant after centrifugation. 5) Insoluble inclusion bodies solubilized with SDS. 6) Purified protein fraction after elution with 250mM imidazole elution plus 0.5% DPC. All samples were first mixed with an SDS loading buffer prior to loading on the gel. (M) represents monomeric GSAP and (D) represents dimeric GSAP, as confirmed by mass spectrometry.
These results indicate that GSAP is highly prone to form insoluble aggregates. Despite extensive testing of refolding conditions we found the protein could be solubilized only in the presence of detergents or denaturing agents.
We next tested a fusion protein form of GSAP. In previous work (8), recombinant GSAP was expressed as a fusion protein with thioredoxin, a widely used fusion partner for enhancing the solubility and stability of partner proteins (13, 14). We therefore constructed and tested a thioredoxin-His6-GSAP-thioredoxin fusion protein. We found that the fusion protein also expressed primarily into inclusion bodies. A small fraction that expressed in soluble form in the supernatant was associated with Ni-NTA resin, but could not then be eluted from the column in the absence of a harsh detergent, such as SDS. The amount of protein that was purified without detergent was negligible.
We also attempted to generate soluble GSAP without a fusion partner in the commercial competent cell line SoluBL21™ (AMS Biotechnology, El Toro, CA). This cell line has been modified to enhance the solubility of difficult proteins and to allow for soluble expression where no soluble expression is seen in standard competent cell lines. When expressed in SoluBL21 cells, GSAP was initially soluble based on detection of a GSAP band on an SDS-PAGE gel in the supernatant of the cell lysate after centrifugation. However, after binding to the nickel resin followed by elution of all impurities in a low concentration imidazole buffer, GSAP failed to elute from the nickel resin in the presence of 250 mM imidazole. Application of SDS to the resin released the protein. This suggests that while expression conditions can be found that initially produce a soluble form of GSAP, the protein is highly susceptible to aggregation. This result implies that a propensity of GSAP to aggregate is an intrinsic property of this protein.
Characterization of Solubilized GSAP
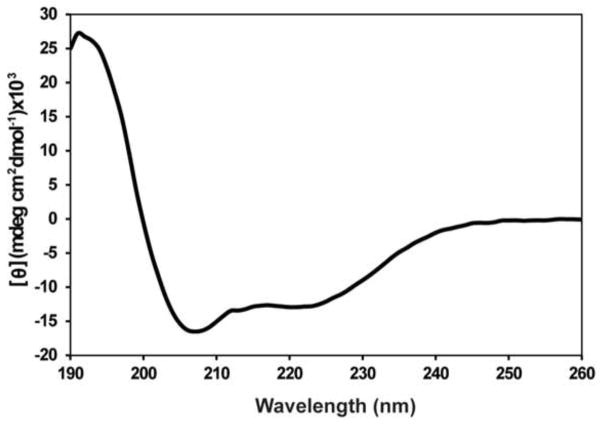
The properties of GSAP were examined in detergent-containing solutions in which the protein was soluble. Secondary structure predictions indicate that GSAP is largely α-helical with stretches of random coil or unstructured loops between helices. Near-UV CD spectroscopy in the 250–310 nm range (25 mM NaPO4 pH 7.5) revealed a flat spectrum, providing no evidence for stable tertiary structure (data not shown). The far-UV CD spectrum collected under the same sample conditions shows a pattern consistent with mostly helical secondary structure (Figure 2). Analysis of this spectrum using the secondary structure prediction server K2D3 suggests that GSAP is largely (ca. 92%) helical (15).
Figure 2.
Estimation of secondary structure from far-UV CD spectroscopic data. This spectrum indicates that GSAP is largely α-helical with approximately 92% helicity based on analysis using the K2D3 secondary structure prediction server. Data represent an average of 5 scans.
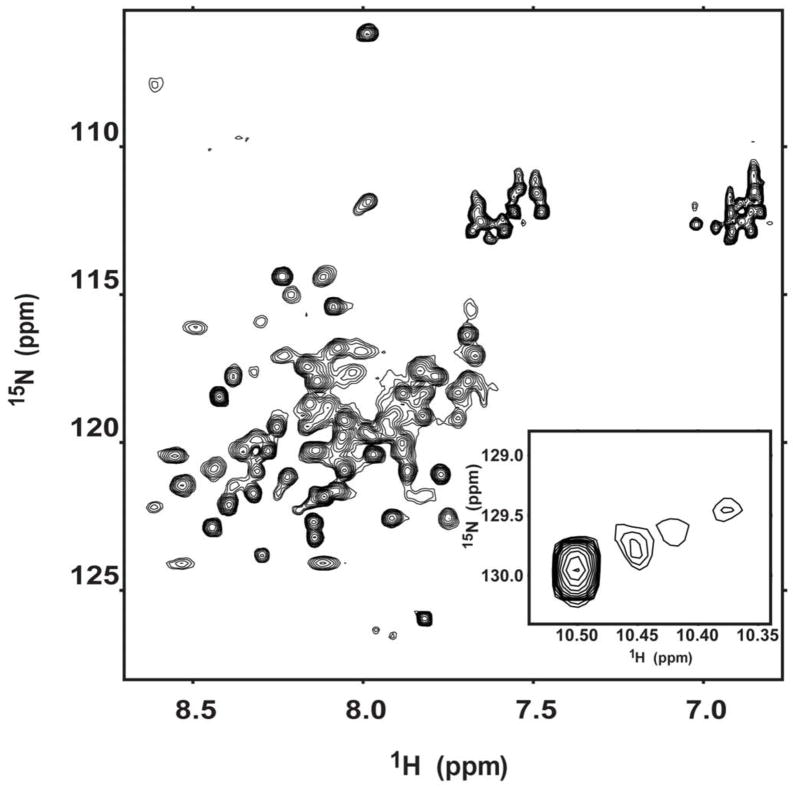
Two-dimensional 1H,15N-HSQC NMR spectra are routinely used to provide general insight into protein structure. All NMR spectra of GSAP-containing samples were collected at pH 7.5 because the protein precipitated when reduced to a neutral or acidic pH. The HSQC spectrum of 15N-GSAP in DPC micelles is shown in Figure 3 and is poorly dispersed, showing only a fraction of the expected 126 backbone amide peaks. This spectrum is consistent with GSAP being largely α-helical but lacking well-defined tertiary structure, suggestive of a conformationally heterogeneous globular protein. The fact that there is only a single tryptophan in the protein, but 4 peaks are observed in the indole side chain resonance region of the spectrum is consistent with the protein populating multiple conformations that are only slowly exchanging on the NMR time scale.
Figure 3.
600 MHz 1H,15N-HSQC NMR spectrum of GSAP. The sample contains ca. 300 μM uniformly 15N-labeled GSAP in 0.5% DPC, pH 7.5, 2 mM DTT and 10% D2O at 298 K. The protein precipitated when the pH was reduced to neutral or acidic values. The inset to the main graphic highlights the tryptophan indole NH peaks from the single tryptophan in the protein.
GSAP has previously been shown to bind both the small molecule kinase inhibitor imatinib and the 99 residue transmembrane C-terminal domain of the amyloid precursor protein (C99), which serves as the substrate for γ-secretase cleavage to produce the amyloid-β polypeptides(8). However, in neither case is it clear whether binary GSAP-imatinib or GASP-C99 complexes are formed, or whether they form complexes only in the presence of a tertiary partner such as γ-secretase. We therefore tested whether recombinant GSAP can form binary complexes with either imatinib or GSAP.
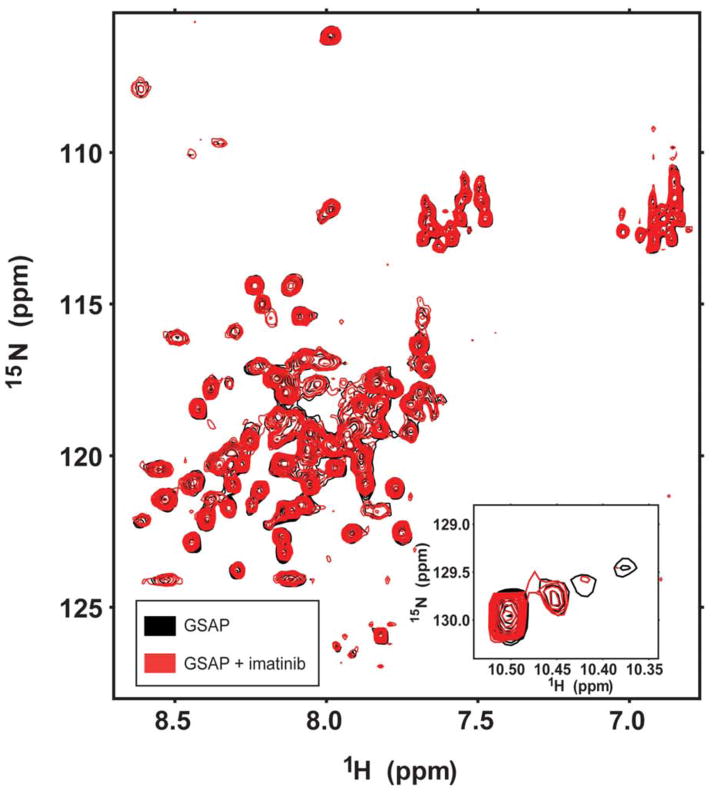
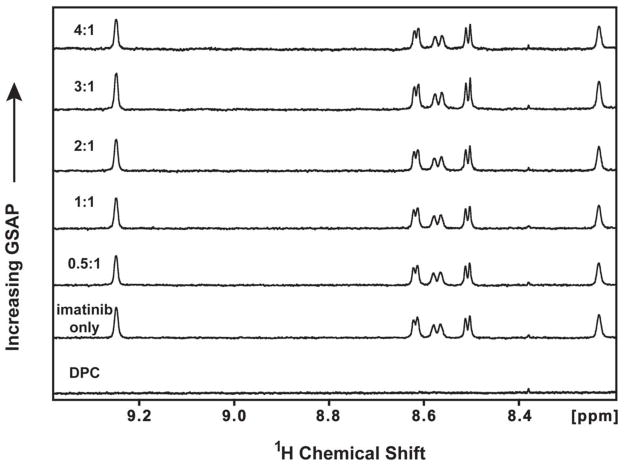
1H,15N-HSQC NMR spectra of 150 μM isotopically-labeled GSAP were collected in the absence and presence of a 1:1 molar ratio of imatinib. No shifts in GSAP resonances were seen upon addition of imatinib, as shown in Figure 4. In the reverse experiment, 1D NMR spectra were taken of 50 μM imatinib upon titration with increasing amounts of GSAP. Addition of GSAP to a 4X molar excess relative to the drug did not significantly affect the imatinib peaks (Figure 5). The results suggest that affinity between GSAP and imatinib under the tested conditions is weak or non-existent.
Figure 4.
Attempt to detect interaction of GSAP with imatinib. An overlay is shown of 600 MHz HSQC NMR spectra of U-15N-GSAP and no imatinib (black) and of GSAP in the presence of 1:1 molar ratio of imatinib (red). These samples contained 0.15 mM GSAP in 0.5% DPC, pH 7.5, 2 mM DTT, and 10%D2O at 298 K. The inset to the main graphic highlights the tryptophan indole NH peaks from the single tryptophan in the protein.
Figure 5.
Titration of imatinib by GSAP, as monitored by 600 MHz 1D 1H NMR. The aromatic regions of the 1D 1H NMR spectra of 50 μM imatinib are shown as a function of increasing GSAP concentrations: 0 μM, 25 μM, 50 μM, 100 μM, 200 μM. Spectra were acquired in the presence of 1% DPC and 100% D2O at pH 7.5 and 298 K and normalized to an internal standard. The listed ratios are the GSAP:imatinib mole to mole ratios.
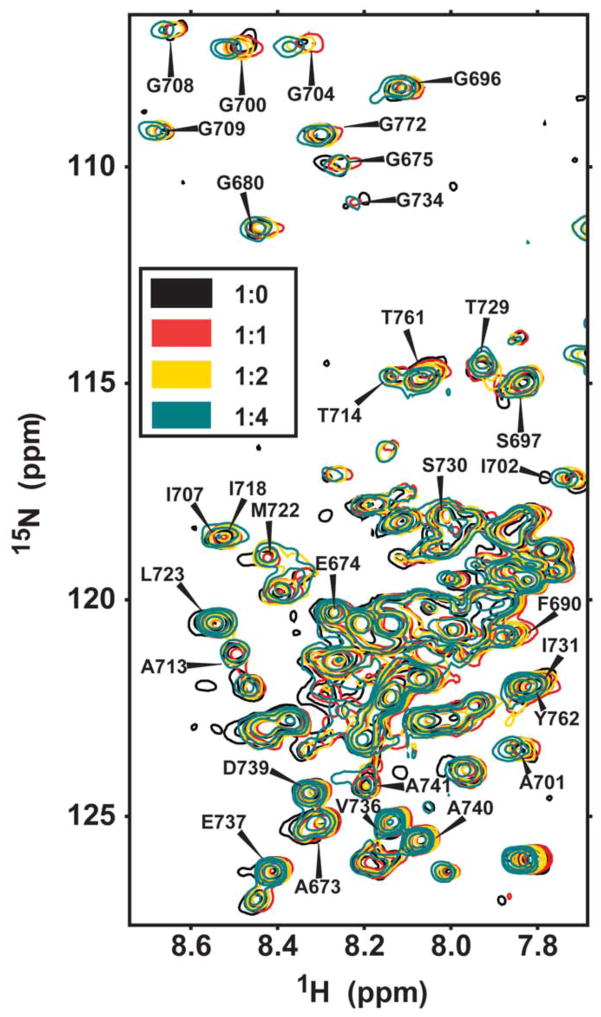
To test for complex formation between C99 and GSAP, 1H,15N-HSQC NMR was used to monitor titration of uniformly 15N-labeled C99 by increasing molar ratios of unlabeled GSAP. These experiments were initially carried out in 2.5% (w/v) LMPG micelles. Under these conditions only modest and non-saturable changes were seen in backbone amide 1H,15N peak positions (Figure 6), consistent with non-specific or weak interactions between these two proteins under the conditions of this experiment. Indeed, while the GSAP interaction domain on C99 was proposed to be localized to residues 725–735 in the juxtamembrane cytosolic domain (8), peaks from this domain were no more likely to undergo large shifts in response to GSAP than peaks found in the transmembrane domain (residues 700–723) for from the extracellular domain of C99 (residues 672–699), the latter of which is located on the other side of the membrane from GSAP under physiological conditions. An additional titration was completed in which the same protein concentrations were used but the detergent concentration was increased to 10% LMPG. Under these conditions little to no chemical shift changes were seen for C99 peaks upon titration by GSAP. The fact that the GSAP-induced changes in the spectrum seen at 2.5% LMPG in Figure 6 can be eliminated by increasing the micelle concentration (at fixed protein) suggests that GSAP has some affinity for the micelle surface that leads to non-specific interaction between GSAP and C99 when both are confined to the same micelle, an interaction that can be minimized by simply adding excess (C99-free) micelles, to which GSAP will redistribute. These results indicate that any binding of GSAP to C99 in LMPG micelles is either non-specific or very weak. These results do not, of course, rule out the possibility that GSAP and C99 do specifically and avidly interact, but only when both are bound to γ-secretase.
Figure 6.
Titration of U-15N-C99 by unlabeled GSAP, as monitored by 600 MHz 1H,15N-TROSY NMR. The samples contained 2.5% LMPG, pH 7.5, at 298 K. The listed ratios are the C99:GSAP mole-to-mole ratios.
Discussion
The notion that GSAP represents a protein that can be targeted by an already-approved drug to reduce production of the amyloid-β polypeptides is extremely appealing. Accordingly, there is a compelling impetus to conduct biochemical and biophysical studies of the structure and interactions of this protein. Unfortunately, based on the work presented here, working with GSAP is likely to be challenging. It appears to be insoluble in many conditions and seems to be conformationally heterogeneous under detergent micellar conditions in which it is soluble. These observations hold regardless of the nature of the protein construct, regardless of the E. coli expression strain, and regardless of the refolding methods and final solution composition. While we cannot rule out the possibility that a refolding method and/or solution conditions may ultimately be found in which GSAP is both soluble and well folded, we were not able to identify any such conditions despite considerable effort.
Under conditions in which GSAP is solubilized by the presence of DPC micelles it was seen to be a mostly α-helical protein, but was conformationally heterogeneous. It was also observed that under micellar conditions GSAP does not undergo specific association with either imatinib or the C99 domain of the amyloid precursor protein. It does not appear that the conformationally heterogeneous form of GSAP can be induced to adopt stable tertiary structure by interaction with either of these potential binding partners.
Despite the failure in this work to observe formation of well-ordered tertiary structure by GSAP or complex formation with either imatinib or C99 titrations, our results are not definitively negative. We cannot rule out the possibility that either an unidentified refolding pathway or folding-favorable final solution conditions exist that we have not yet discovered. In native mammalian cells it is possible that chaperones might facilitate a different folding outcome than what we have observed working with purified protein. We also cannot rule out the possibility that GSAP is subject to an unidentified post-translational modification under mammalian cellular conditions that is required for folding or solubility in detergent-free solutions. While GSAP was not seen to form binary complexes with either imatinib or C99, it may do so under mammalian cellular conditions, perhaps as a result of ternary complex formation with an additional binding partner such as γ-secretase.
Our results should not be taken to imply a challenge of the data or interpretations regarding the GSAP protein as presented in previous work (8, 9). The previous studies were carried out primarily using cell-based methods involving model mammalian cell lines. However, for those considering the pursuit of biophysical studies on this protein, our results suggest that work with recombinant GSAP may prove difficult. We were unable to find conditions in which this protein is water soluble to an appreciable degree unless detergent micelles were used to facilitate solubilization, presumably by stabilizing a hydrophobic surface on GSAP that otherwise drives aggregation. When solubilized, GSAP was found to be mostly helical, though it did not adopt a stable and homogeneous tertiary structure. Nevertheless, we cannot rule out the possibility that further exploration of expression, purification, and protein refolding methods may eventually lead to a form of purified GSAP that is soluble, folded, and competent to bind imatinib and/or C99.
Supplementary Material
Acknowledgments
This work was supported by US NIH grant PO1 GM080513. We thank Dr. Paul Greengard and Arvys Proteins for providing us with additional details regarding the GSAP-containing fusion protein expression vector previously used to prepare the protein (8).
Abbreviations
- Aβ
amyloid-beta peptide
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- CD
circular dichroism
- DM
n-decylmaltoside
- DPC
n-dodecylphosphocholine
- DTT
dithiothreitol
- GSAP
gamma-secretase activating protein
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- LMPG
lyso-myristoylphosphatidylglycerol
- Ni-NTA
Ni(II) complex with nitrilotriacetic acid-derivatized agarose beads
- NMR
nuclear magnetic resonance
- PAGE
polyacrylamide gel electrophoresis
- PCR
polymerase chain reaction
- PION
pigeon homolog protein
- SDS
sodium dodecylsulfate
Footnotes
This study was supported by US NIH grant PO1 GM080513.
Supporting Information Available
Two supporting tables. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Alzheimer's Association. 2012 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The Amyloid Hypothesis of Alzheimer's Disease: Progress and Problems on the Road to Therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Esler WP, Kimberly WT, Ostaszewski BL, Ye W, Diehl TS, Selkoe DJ, Wolfe MS. Activity-dependent isolation of the presenilin–γ-secretase complex reveals nicastrin and a substrate. Proceedings of the National Academy of Sciences. 2002;99:2720. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. -Secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proceedings of the National Academy of Sciences. 2003;100:6382. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 7.Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nature reviews Molecular cell biology. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 8.He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P. Gamma-secretase activating protein is a therapeutic target for Alzheimer's disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netzer WJ, Dou F, Cai D, Veach D, Jean S, Li Y, Bornmann WG, Clarkson B, Xu H, Greengard P. Gleevec inhibits beta-amyloid production but not Notch cleavage. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12444–12449. doi: 10.1073/pnas.1534745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh J, Tabunoki H, Ishida T, Saito Y, Arima K. Immunohistochemical characterization of gamma-secretase activating protein expression in Alzheimer's disease brains. Neuropathology and applied neurobiology. 2011 doi: 10.1111/j.1365-2990.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- 11.Beel AJ, Mobley CK, Kim HJ, Tian F, Hadziselimovic A, Jap B, Prestegard JH, Sanders CR. Structural studies of the transmembrane C-terminal domain of the amyloid precursor protein (APP): does APP function as a cholesterol sensor? Biochemistry. 2008;47:9428–9446. doi: 10.1021/bi800993c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders CR, Sonnichsen F. Solution NMR of membrane proteins: practice and challenges. Magnetic resonance in chemistry : MRC. 2006;44(Spec No):S24–40. doi: 10.1002/mrc.1816. [DOI] [PubMed] [Google Scholar]
- 13.LaVallie ER, DiBlasio EA, Kovacic S, Grant KL, Schendel PF, McCoy JM. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology (N Y) 1993;11:187–193. doi: 10.1038/nbt0293-187. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen HP, Mortensen KK. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microbial cell factories. 2005;4(1) doi: 10.1186/1475-2859-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis-Jeune C, Andrade-Navarro MA, Perez-Iratxeta C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins. 2011 doi: 10.1002/prot.23188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.