Abstract
A multicenter meta-analysis including data from 9389 psoriasis patients and 9477 control subjects was performed to investigate the contribution of the deletion of genes LCE3C and LCE3B, involved in skin barrier defense, to psoriasis susceptibility in different populations. The study confirms that the deletion of LCE3C and LCE3B is a common genetic factor for susceptibility to psoriasis in European populations [OROverall = 1.21 (1.15–1.27)], and for the first time directly demonstrated the deletion's association with psoriasis in [Chinese OR = 1.27 (1.16–1.34); Mongolian OR = 2.08 (1.44–2.99)] populations. The analysis of the HLA-Cw6 locus showed significant differences in the epistatic interaction with the LCE3C and LCE3B deletion in at least some European populations, indicating epistatic effects between these two major genetic contributors to psoriasis. The study highlights the value of examining genetic risk factors in multiple populations to identify genetic interactions, and indicates the need of further studies to understand the interaction of the skin barrier and the immune system in susceptibility to psoriasis.
Introduction
Psoriasis is a common chronic inflammatory disease of the skin with a variable worldwide prevalence, being common in European descent individuals and less frequent in Asian ancestry populations (Bowcock et al, 2005). To date, several loci have been underlined as psoriasis risk susceptibility factors, with PSORS1, a Major Histocompatibility Complex (MHC) class I region on chromosome 6p21, being the locus with the largest effect identified to date (Nestle et al, 2009). Within PSORS1, the HLA-Cw06 allele has been pinpointed as the risk variant that confers the strongest susceptibility to psoriasis (Nair et al, 2006).
In a previous study we reported the association of the deletion of two late cornified envelope (LCE) genes, LCE3C and LCE3B, (LCE3C_LCE3B-del) with psoriasis in 1426 unrelated psoriatic patients and 1406 controls from four populations of European ancestry. The LCE3C_LCE3B-del involves a 32.2-kb deletion, removing genes LCE3C and LCE3B of the LCE cluster, which is part of the epidermal differentiation complex (EDC) on chromosome 1q21.3. Association with rs4112788, a tag single nucleotide polymorphism (SNP) for the biallelic LCE3C_LCE3B-del copy number variant (CNV), located 584 nucleotides downstream of LCE3D, was also found. Interaction analysis showed epistatic effects between the LCE3C_LCE3B-del and HLA-Cw06 allele only in the Dutch population (de Cid et al, 2009). At the time of publication, an independent genome wide association scan (GWAS) in a Chinese cohort also identified association of rs4112788 with the disease, indicating a major role of the LCE locus in psoriasis susceptibility (Zhang et al, 2009). Furthermore this locus was replicated in a German case control study of psoriasis vulgaris (Hüffmeier et al, 2010) and in a Spanish case control study of chronic plaque type psoriasis vulgaris (Coto et al, 2010). Since these initial studies in psoriasis, the LCE3C_LCE3B locus has been evaluated in other and psoriasis-related phenotypes. Hüffmeier found no association of this locus with susceptibility to psoriatic arthritis in German samples (Hüffmeier et al, 2010), while association with this phenotype has been detected in British and Irish (Bowes et al, 2010) and in Spanish (Docampo et al, unpublished) patients. Finally, Bergboer et al has found negative association of the LCE3C_LCE3B locus with atopic dermatitis (Bergboer et al, 2010).
The aim of this meta-analysis with individual patient data was to further investigate the contribution of LCE3C_LCE3B-del to psoriasis susceptibility and its possible interaction with the PSORS1 locus. Thirteen cohorts from twelve populations, nine of European ancestry [Finland, France, Germany, Ireland, Italy, Spain, The Netherlands, UK, and US (US-California: US-CA, and US-Michigan: USMI)], and three of Asiatic origin (China, Mongolia and Japan) were included in the study (See Supplementary Methods for sample description). Overall, 9389 psoriasis cases and 9477 control samples were analyzed for the association of LCE3C_LCE3B-del with psoriasis. Association of rs4112788 was also investigated in eleven of the 13 datasets included. A possible relationship between PSORS1 and LCE3C_LCE3B-del and its tag SNP was assessed through interaction analysis using directly typed HLA-Cw06 when available, or rs130076, a SNP in linkage disequilibrium (LD) with it (Asumalahti et al, 2002).
Results and Discussion
Association analyses of the genotyping data confirmed that the deletion of both LCE3C and LCE3B genes is a susceptibility determinant for psoriasis in European ancestry populations, with a significantly higher frequency of the LCE3C_LCE3Bdel allele in psoriatic patients compared with control individuals. [OROverall = 1.21 (1.15–1.27), POverall = 4.58×10−13] (Table 1). As no significant evidence of heterogeneity between European ancestry populations was observed, a combined OR was calculated under a population fixed effects model. In addition, the estimation of an overall OR under a population random effects model –which better accommodates potential heterogeneity across populations of the genetic effects estimates due to genuine differences and/or different biases- was practically identical, which is a further indication of absence of significant heterogeneity (Lau et al, 1997; Ioannidis et al, 2007) (Table 1 and Figure 1). At the genotype level, analysis suggests a potential dosage effect with genotypes having two copies of genes LCE3C and LCE3B being a protective factor against the development of the disease in European ancestry populations (OROverall = 1.20 (1.15–1.28), POverall = 1.42×10−13) (Supplementary Table 1).
Table 1.
Association of LCE3C_LCE3B-del and its tag SNP rs4112788 with psoriasis in individuals of European and Asian ancestry
| Data set | Status | LCE3C_LCE3B CNV | HWE | OR (95% CI) (del vs. intact) |
P-value | rs4112788 | HWE | OR (95% CI) (C vs. T) |
P-value | r2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
LCE3C_ LCE3B-del |
Intact | Allele C | Allele T | |||||||||
| Spain | Control | 420 (55.0) | 344 (45.0) | 0.10 | 1.49 (1.15–1.93) | 0.00284 | 427 (55.9) | 337 (44.1) | 0.67 | 1.57 (1.21–2.05) | 0.00079 | 0.92 |
| Psor | 227 (64.5) | 125 (35.5) | 0.02 | 233 (66.6) | 117 (33.4) | 0.09 | ||||||
| Italy | Control | 516 (57.3) | 384 (42.7) | 0.03 | 1.30 (1.08–1.58) | 0.00603 | 510 (57.2) | 382 (42.8) | 0.08 | 1.39 (1.15–1.68) | 0.00079 | 0.93 |
| Psor | 573 (63.7) | 327 (36.3) | 0.48 | 583 (64.9) | 315 (35.1) | 1.00 | ||||||
| France | Control | 211 (64.3) | 117 (35.7) | 0.08 | 1.26 (0.90–1.77) | 0.1767 | 216 (65.1) | 116 (34.9) | 0.06 | 1.29 (0.99–1.95) | 0.1405 | 0.96 |
| Psor | 196 (69.5) | 86 (30.5) | 1.00 | 202 (70.6) | 84 (29.4) | 1.00 | ||||||
| The Netherlands | Control | 334 (59.6) | 226 (40.4) | 0.54 | 1.50 (1.14–1.96) | 0.00329 | 333 (59.9) | 223 (40.1) | 0.62 | 1.54 (1.18–2.02) | 0.00155 | 0.99 |
| Psor | 281 (68.9) | 127 (31.1) | 0.63 | 278 (68.8) | 126 (31.2) | 0.74 | ||||||
| Germany | Control | 1,215 (64.9) | 657 (35.1) | 0.67 | 1.31 (1.15–1.48) | 3.03e-05 | 1,151 (64.7) | 627 (35.3) | 0.56 | 1.22 (1.07–1.38) | 0.00229 | 0.94 |
| Psor | 1,899 (70.8) | 785 (29.2) | 0.43 | 1,799 (69.1) | 803 (30.9) | 0.47 | ||||||
| UK | Control | 1,370 (67.1) | 672 (32.9) | 0.94 | 1.16 (1.01–1.33) | 0.0306 | 1,303 (66.4) | 659 (33.4) | 0.57 | 1.12 (0.98–1.28) | 0.0872 | 0.86 |
| Psor | 1,323 (70.3) | 559 (29.7) | 0.10 | 1,390 (68.9) | 626 (31.1) | 0.30 | ||||||
| Ireland | Control | 1,311 (69.2) | 583 (30.8) | 0.17 | 1.07 (0.89–1.29) | 0.459 | 1,335 (68.8) | 605 (31.2) | 0.33 | 1.18 (0.99–1.41) | 0.0695 | 0.94 |
| Psor | 554 (70.7) | 230 (29.3) | 0.90 | 624 (72.2) | 240 (27.8) | 0.47 | ||||||
| Finland | Control | 436 (65.1) | 234 (34.9) | 0.05 | 1.37 (1.01–1.87) | 0.046 | 433 (65.6) | 227 (34.3) | 0.09 | 1.37 (1.00–1.88) | 0.0512 | 0.98 |
| Psor | 194 (71.9) | 76 (28.1) | 0.39 | 188 (72.3) | 72 (27.7) | 0.19 | ||||||
| US-CA | Control | 378 (64.3) | 210 (37.7) | 0.04 | 1.36 (1.11–1.68) | 0.00378 | 379 (64.0) | 213 (36.0) | 0.13 | 1.37 (1.11–1.69) | 0.00339 | 0.95 |
| Psor | 847 (71.1) | 345 (28.9) | 0.09 | 835 (70.9) | 343 (29.1) | 0.06 | ||||||
| US-MI | Control | 2,478 (64.8) | 1,348 (35.2) | 0.52 | 1.09 (1.00–1.20) | 0.0584 | 2,472 (65.0) | 1332 (35.0) | 0.45 | 1.10 (1.00–1.20) | 0.0515 | 0.98 |
| Psor | 2,835 (66.8) | 1,411 (33.2) | 0.33 | 2,828 (67.0) | 1,390 (33.0) | 0.46 | ||||||
| Overall1 | Control | 6,028 (64.2) | 3,364 (35.8) | — | 1.21 (1.15–1.27)2 | 4.58e-132 | 6,087 (64.2) | 3,389 (35.8) | — | 1.21 (1.15–1.27)2 | 1.42e-122 | — |
| Psor | 5,902 (69.6) | 2,580 (30.4) | — | 1.21 (1.15–1.28)3 | 1.47e-123 | 6,132 (69.2) | 2,726 (30.8) | — | 1.21 (1.15–1.28)3 | 1.44e-123 | ||
| China | Control | 2,174 (57.3) | 1,620 (42.7) | 0.95 | 1.27 (1.16–1.34) | 1.70e-07 | 2,173 (57.6) | 1,601 (42.4) | 1.0 | 1.34 (1.21–1.46) | 6.42e-10 | 0.91 |
| Psor | 2,518 (63.1) | 1,472 (36.9) | 0.21 | 2,543 (64.4) | 1,403 (35.6) | 0.01 | ||||||
| Japan | Control | 631 (58.8) | 443 (41.2) | 0.79 | 1.17 (0.99–1.40) | 0.0638 | ND | ND | — | — | — | — |
| Psor | 689 (62.6) | 411 (37.4) | 1.00 | |||||||||
| Mongolia | Control | 166 (49.4) | 170 (50.6) | 0.36 | 2.08 (1.44–2.99) | 8.16e-05 | ND | ND | — | — | — | — |
| Psor | 134 (67.0) | 66 (33) | 0.82 | |||||||||
Abbreviations: CI, confidence interval; CNV, copy number variant; HWE, Hardy–Weinberg equilibrium; ND, no data available; OR, odds ratio; SNP, singlenucleotide polymorphism; US-CA, US-California data set; US-MI: US-Michigan data set.
1Overall analyses for the European ancestry populations are computed according to a logistic model in which population was introduced as a confounding variable (based on a 2fixed effects model and a 3random effects model) after no significant evidence of heterogeneity was detected according to the Woolf test on homogeneity of odds ratios (P=0.0763 for LCE3C_LCE3B CNV; P=0.0553 for rs4112788). Overall values for the Asian ancestry populations are not presented as the Woolf test on homogeneity of odds ratios showed statistical significant heterogeneity among them (P=0.02091). Predictive performance of allele C with LCE3C_LCE3B-del is presented for each population using the coefficient of determination measure (r2).
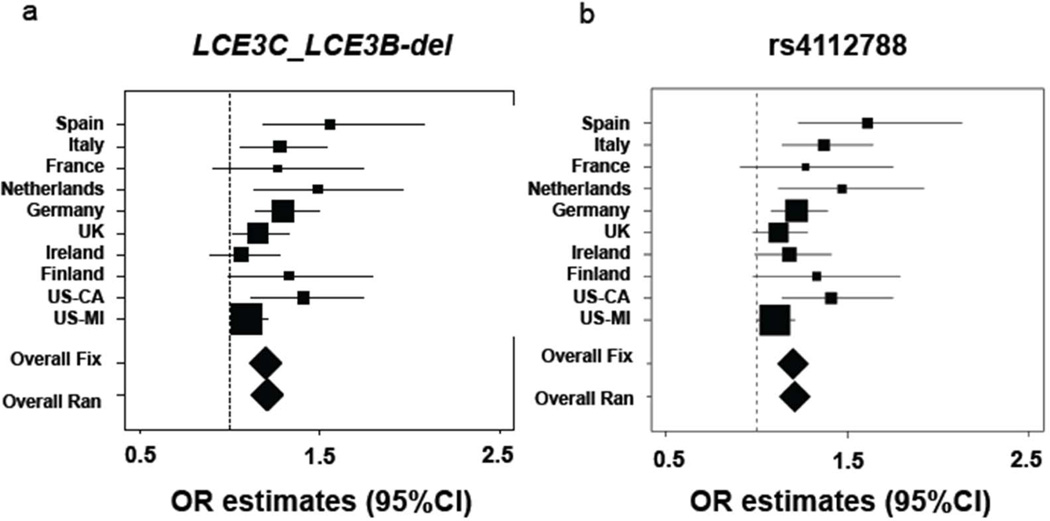
Figure 1. Meta-analysis of LCE3C_LCE3B-del and rs4112788 for association with psoriasis across populations of European ancestry.
Panel a shows the data for LCE3C_LCE3B-del and panel b for rs4112788. Squares show the point estimate of the odds ratio (OR) and its 95% confidence intervals (95% CIs) with regard to genotype frequencies. Diamonds show the summary effect by fixed (Overall Fix and random (Overall Ran) effects model. Different square sizes represent different weights of each population.
Genotyping of Asian population samples for the LCE3C_LCE3B-del confirmed that the strong genetic association with SNPs at the LCE3 genes detected in the Chinese population (Zhang et al, 2009) is due to the presence of the deletion of LCE3C and LCE3B. Genotyping for LCE3C_LCE3B-del in the other Asian populations further confirmed its presence in these populations. The deletion has the same sequence structure as that found in Caucasian populations. The detection of significant heterogeneity among the Asiatic population for LCE3C_LCE3B-del allelic frequencies impeded the estimation of the overall association of LCE3C_LCE3B-del with the psoriatic phenotype in Asian ancestry populations. The deletion is strongly associated with psoriasis in Chinese and Mongolian populations in regard of both the allelic (OR = 1.27 (1.16–1.34), P = 1.70×10−07; OR = 2.08 (1.44–2.99), P = 8.16×10−05, respectively) and the genotype level (OR = 1.28 (1.16–1.41), P = 1.41×10−07; OR = 2.04 (1.41–2.94), P = 9.38×10−05, respectively) (Supplementary Table 1). In Japanese population however, the higher frequency of the deleted allele among the psoriatic individuals compared with controls did not reach the level of significance (P = 0.063) (Table 1).
Analysis of rs4112788 showed association of allele C with the disease in the European ancestry populations [OROverall = 1.21 (1.15–1.27), POverall = 1.42×10−12] (Table 1, Figure 1) as well as in the Chinese population [OR = 1.34 (1.21–1.46), P = 6.42×10−10] (Table 1). At the genotype level, a statistically significant higher risk for psoriasis was observed in individuals homozygous for the C allele in populations with European ancestry (OROverall = 1.20 (1.15–1.27), POverall = 1.81×10−12) as well as in China (OROverall = 1.35 (1.22–1.47), POverall = 3.62×10−12). (Supplementary Table 2). The high coefficient of determination measure (r2) (over 0.85 in all populations) indicates that rs4112788 is a close proxy of the LCE3C_LCE3B-del allele, also in the Chinese population (Table 1). This is the first direct indication that the strong association of psoriasis with rs4112788, detected in the initial analysis in Chinese samples (Zhang et al, 2009) is also associated with the LCE3C_LCE3B-del allele.
Interestingly, we observed a significant negative correlation between the frequency of LCE3C_LCE3B-del among controls and the corresponding OR for psoriasis for the eight populations from Europe examined –the more common the risk allele, the smaller its effect on psoriasis risk (Supplementary Figure 1). Allele frequency, and the correlated effect strength, appears to follow an approximate north-south gradient pattern. Even though this observation could be due to sampling error, and additional European populations would need to be studied, a genuine significance of this phenomenon on the genetic predisposition to psoriasis cannot be ruled out.
Direct typing of HLA-Cw06 in the Netherlands, Italy, Japan, Mongolia and US samples allowed the estimation of a potential interaction between the LCE3C_LCE3B deletion, or its tag SNP rs4112788, with PSORS1 locus. Apart from the already known interaction observed in the Dutch population alone, evidence for interaction was observed also in the US-MI dataset, but not in the Italian sample. The existence of heterogeneity among the cohorts with European ancestry prevented the analysis of the interaction in those cohorts as a whole. Evidence of interaction between either LCE3C_LCE3B-del or rs4112788 with HLACw06 was not observed in the Japan and Mongolia datasets (Table 2).
Table 2.
Genetic interaction analysis between HLACwo6 in PSORS1 and LCE3C_LCE3B-del and its rs4112788 tag SNP
| HLA-Cw061 | Epistasis2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive vs. negative |
rs4112788-HLA-Cw06 |
LCE3C_LCE3B- HLA-Cw06 |
||||||||
| Data set | OR | 95% CI | P-value | Group | OR | 95% CI | P-value | OR | 95% CI | P-value |
| The Netherlands | 3.45 | 2.27–5.25 | 2.974e-09 | + | 2.58 | 1.46–4.57 | 0.0160 | 2.60 | 1.47–459 | 0.0180 |
| − | 1.15 | 0.83–1.60 | 1.17 | 0.84–1.63 | ||||||
| Italy | 2.5 | 1.86–3.36 | 4.994e-10 | + | 1.22 | 0.45–1.75 | 0.57897 | 1.14 | 0.80–1.62 | 0.5445 |
| − | 1.38 | 1.09–1.76 | 1.30 | 1.03–1.64 | ||||||
| US-MI | 3.69 | 3.19–4.27 | 1.063e-75 | + | 1.44 | 1.19–1.75 | 0.00028 | 1.44 | 1.19–1.75 | 0.00027 |
| − | 0.95 | 0.85–1.06 | 0.95 | 0.85–1.06 | ||||||
| Japan | 9.25 | 3.94–21.7 | 6.854e-11 | + | ND | 1.09 | 0.23–5.08 | 0.9266 | ||
| − | ND | 1.17 | 0.98–1.39 | |||||||
| Mongolia | 34.39 | 16.48–1.7 | 1.902e-31 | + | ND | 1.46 | 0.82–2.59 | 0.1286 | ||
| − | ND | 3.67 | 1.30–10.37 | |||||||
Abbreviations: CI, confidence interval; ND, no data; OR, odds ratio; SNP, single-nucleotide polymorphism; US-MI, US-Michigan data set.
OR and 95% CI for psoriasis of directly typed HLA-Cw06 was analyzed using the carrier status definition for Cw06 allele.
Epistasis analysis performed by logistic regression models that included an interaction term (rs4112788-HLA-Cw06 or LCE3C_LCE3B-del-HLA-Cw06); P-values are derived from the log-likelihood ratio test between the model including both additive effects plus the interaction term against the model that only includes additive effects. Overall values for the European and Asian ancestry populations are not presented, as significant heterogeneity based on allelic frequencies was detected by population according to the Woolf test on homogeneity of ORs (P=0.0045 and P=0.0012, respectively).
In the remaining populations, interaction with PSORS1 was assessed through its proxy marker rs130076. No evidence for interaction was seen with LCE3C_LCE3B-del or its tag SNP in any of the populations interrogated (Supplementary Table 3). To investigate whether the association of rs130076 with psoriasis (Supplementary Table 4) is independent or secondary to HLA-Cw06, the effect of this SNP was analyzed in a stratified analysis that defined strata by carriage of HLA-Cw06 in the Italian dataset (since the Italian was the only population in which both rs130076 and HLA-Cw06 were genotyped). In the subset of samples that does not contain an HLA-Cw06 allele, rs130076 was no longer significantly associated with psoriasis [OR = 4.64 (2.74–7.84), P = 3.61×10−09 in HLA-Cw06 positive samples versus OR = 1.05 (0.73–1.50), P = 0.7938 in HLA-Cw06 negative samples]. This suggests that the association of this locus is dependent on HLA-Cw06, at least in the Italian population, and therefore interaction analysis between the LCE3C_LCE3B deletion (or its tag SNP) with PSORS1 might be interchangeably performed using either HLA-Cw06 or rs130076, although it may not be coincidental that significant interaction was detected in 2 of the 5 datasets with HLA-Cw06 typing, but in none of the eight datasets with rs130076 typing. The existence of a potential epistasis found only in the Dutch and US-MI datasets but in none of the remaining datasets in which HLA-Cw06 was typed, might be due to population-specific effects, different genetic backgrounds or varying environmental exposures among datasets. The fact that no interaction was observed between LCE3C_LCE3B-del and HLA-Cw06 in the Chinese dataset is probably due to the fact that despite HLA-Cw6 is a major risk allele for psoriasis in the Chinese population, it does not explain by itself the full linkage evidence of the PSORS1 locus in that population (Fan et al, 2008).
In summary, we have confirmed that the deletion of genes LCE3C and LCE3B is a common genetic factor for susceptibility to psoriasis in European populations, and for the first time directly demonstrated the deletion's association with psoriasis in some Asian groups. Interestingly, we detected significant differences in the epistatic interaction of the deletion with HLA-Cw6, with a positive interaction in the Dutch and US Michigan samples but no interaction with other European cohorts. This study highlights the value of examining genetic risk factors in multiple populations, and suggests further studies in experimental models of disease are needed to understand the interaction of the skin barrier and the immune system in susceptibility to psoriasis.
Materials and Methods
Genotyping
Typing of the LCE3C_LCE3B-del CNV was performed by direct PCR using a four primers or three primers assay as previously described (deCid et al, 2009) allowing the simultaneous detection of intact and deleted alleles. Genotyping rates for LCE3C_LCE3B-del ranged from 92.5% to 100% in all European ancestry populations and from 97.3% to 100% in Asian populations. In regard to rs4112788, genotyping rates ranged from 93.7% to 99.6% in European ancestry populations, while it reached 99.2% in Chinese. SNP assays in Spain, Netherlands, Italy and US California were genotyped as previously described (deCid et al, 2009). In the Ireland dataset, SNPs genotyping was performed using competitive allele specific PCR at Kbiosciences, Hoddesdon, Herts, UK, and in Finnish data set using matrix-assisted laser desorption/ionisation time-of-light masspectrometry (Sequenom, San Diego CA, US). In the remaining populations, SNPs genotyping was conducted using TaqMan® assays (Applied Biosystems). HLA allele discrimination in sample collections from The Netherlands and Italy were performed as described (de Cid et al, 2009). In Japanese and Mongolian cohorts HLA typing was conducted with LABType® SSO typing test (ONE LAMBDA, INC.) and LABScan™ 100 flow analyzer. HLA-Cw06 genotypes in the US Michigan sample were determined by genotyping 7 SNPs in exons 2 and 3 of the HLA-C gene, as previously described (Nair et al, 2006).
Statistical analysis
LCE3C_LCE3B–del and SNP association analysis. Logistic regression models assessed the genetic effect of the LCE3C_LCE3B–del and SNPs on psoriasis risk. Calculations for genotype frequency differences were performed by regression analysis for co-dominant, dominant, recessive and log-additive models. The best genetic model was selected using the Akaike information criteria (AIC). Heterogeneity among populations was assessed by the Woolf-test that evaluates the homogeneity of odds ratios. Overall values were calculated when the homogeneity assumption among populations was plausible, and were adjusted by population according to a logistic model that introduces population as a confounding variable. Potential interaction between LCE3C_LCE3B–del or rs4112788 and HLA-Cw06 or rs130076 was evaluated from the log-likelihood ratio test between a model that includes both the additive effect and the interaction term against a model that only includes additive effects.
Supplementary Material
Acknowledgments
We thank all psoriasis patients and their families for their participation in this study. We acknowledge our collaborating partners who contributed to the meta-analysis (see Supplementary Note). Funding for this study was provided by: The Spanish Ministry of Science and Innovation (grant SAF 2008-00357) and the “Generalitat de Catalunya” Departments of Health and Universities and Innovation (E.R.-M., G.E, X.E.); General Program of National Natural Science Foundation of China (30771196, 30800990) (X-J.Z); The medical Research Council grant G0000934 and the Wellcome Trust grant 068545/Z/02. We also acknowledge support from the UK Medical Research Council (R.C.T. and J.N.B., grant G0601387) and the British Skin Foundation (F.C. grant 1006); Grant-in-Aid for Scientific Research(C) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Tokai University School of Medicine Research Aid (A.O., T.M., A.O., H.I.); ADIPSO (Italian Association for the Defence of Psoriasis Patients) (E.G., G.N.); The Interdisciplinary Centre for Clinical Research (IZKF B32/A8) of University of Erlangen-Nuremberg supported U.H., H.T. and A.R. W.L. is supported by a grant of the Dermatology Fundation; J.K. and K.K. were supported by Academy of Finland and Sigrid Juselius Foundation. We also acknowledge funding from Centre National de Génotypage (R.dC, J.F.). Research for the US-Michigan study was supported by grants R01AR42742, R01AR050511 and R01AR054966 from the National Institutes of Health, and by the Ann Arbor Veteran Affairs Hospital. A.B. is supported by NIH grant R01 AR050266 (A.M.B.). A.W.R. acknowledges funding from the Irish Health Research Board and Science Foundation, Ireland.
Abbreviations used
- MHC
Major Histocompatibility Complex
- LCE
Late Cornified Envelope
- LCE3C_LCE3B-del
deletion of LCE3C and LCE3B genes
- EDC
Epidermal Differentiation Complex
- GWAS
Genome Wide Association Scan
- LD
Linkage Disequilibrium
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Asumalahti K, Veal C, Laitinen T, et al. Coding haplotype analysis supports HCR as the putative susceptibility gene for psoriasis at the MHC PSORS1 locus. Hum Mol Genet. 2002;11:589–597. doi: 10.1093/hmg/11.5.589. [DOI] [PubMed] [Google Scholar]
- Bergboer JG, Zeeuwen PL, Irvine AD, et al. Deletion of Late Cornified Envelope 3B and 3C genes is not associated with atopic dermatitis. J Invest Dermatol. 2010;130:2057–2061. doi: 10.1038/jid.2010.88. [DOI] [PubMed] [Google Scholar]
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93–122. doi: 10.1146/annurev.genom.6.080604.162324. [DOI] [PubMed] [Google Scholar]
- Bowes J, Flynn E, Ho P, et al. Variants in linkage disequilibrium with the late cornified envelope gene cluster deletion are associated with susceptibility to psoriatic arthritis. Ann Rheum Dis. 2010 Jul 19; doi: 10.1136/ard.2010.130575. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coto E, Santos-Juanes J, Coto-Segura P, et al. Mutation analysis of the LCE3B/LCE3C genes in Psoriasis. BMC Med Genet. 2010;11:45. doi: 10.1186/1471-2350-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cid R, Riveira-Munoz E, Zeeuwen PL, et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet. 2009;41:211–215. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo E, Rabionet R, Riveira-Muñoz E, et al. Deletion of the late cornified envelope genes, LCE3C and LCE3B, is associated with rheumatoid arthritis. Arthritis Rheum. 2010;62:1246–1251. doi: 10.1002/art.27381. [DOI] [PubMed] [Google Scholar]
- Fan X, Yang S, Huang W, et al. Fine mapping of the psoriasis susceptibility locus PSORS1 supports HLA-C as the susceptibility gene in the Han Chinese population. PLoS Genet. 2008;4:e1000038. doi: 10.1371/journal.pgen.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüffmeier U, Bergboer JG, Becker T, et al. Replication of LCE3C-LCE3B CNV as a risk factor for psoriasis and analysis of interaction with other genetic risk factors. J Invest Dermatol. 2010;130:979–984. doi: 10.1038/jid.2009.385. [DOI] [PubMed] [Google Scholar]
- Hüffmeier U, Estivill X, Riveira-Munoz E, et al. Deletion of LCE3C and LCE3B genes at PSORS4 does not contribute to susceptibility to psoriatic arthritis in German patients. Ann Rheum Dis. 2010;69:876–878. doi: 10.1136/ard.2009.108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Patsopoulos NA, Evangelou E. Heterogeneity in meta-analyses of genome-wide association investigations. PLoS ONE. 2007;9:e841. doi: 10.1371/journal.pone.0000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Inter Med. 1997;9:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- Nair RP, Stuart P, Nistor I, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78:827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Huang W, Yang S, et al. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat Genet. 2009;41:205–210. doi: 10.1038/ng.310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.