Abstract
AIM: To assess the clinicopathologic features and its relationship with prognosis of pseudomyxoma peritonei (PMP) in Chinese patients.
METHODS: The clinicopathologic features and follow-up data of 92 patients with PMP were reviewed and retrospectively analyzed. The cases were categorized into three groups: disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and peritoneal mucinous carcinomatosis with intermediate or discordant features (PMCA-I/D). The log-rank test was used to analyze survival for each group and various clinicopathological parameters. Multivariate Cox proportional-hazard models were constructed to determine the important factors associated with survival.
RESULTS: The median age at diagnosis was 51.9 years (range: 22-76 years). The median follow up was 124 mo. The 3-, 5- and 10-year survival rates were 74.0%, 67.4% and 49.1%, respectively. There were 49 (53.2%) patients with DPAM, 26 (28.3%) with PMCA-I and 17 (18.5%) with PMCA. Patients with DPAM, PMCA-I/D and PMCA exhibited statistically significant difference in survival (P = 0.001). The 3 year survival for DPAM, PMCAI/D and PMCA was 97.0%, 80.0% and 67.0%, respectively; the 5 year survival was 80.0%, 67.0% and 50.0%, respectively; and the 10 year survival was 65.0%, 28.0% and 14.0%, respectively. Survival rate was significantly lowest in patients < 40 age years of age (P = 0.011). Appendiceal tumor and extra-ovarian parenchymal organ involvement were significantly related to overall survival. Patients with appendiceal mucinous adenocarcinoma (MACA) showed the significantly poorer prognosis (P = 0.011). Multivariate analysis showed that pathological classification, age, appendiceal tumor were significant related to overall survival.
CONCLUSION: The clinical process “PMP” should be pathologically classified into DPAM, PMCA and PMCA-I/D. Pathological classification, age, appendiceal MACA are survival independent predictors in Chinese patients with PMP.
Keywords: Pseudomyxoma peritonei, Pathologic, Clinical, Classification, Prognosis
INTRODUCTION
Pseudomyxoma peritonei (PMP) is a rare clinical entity that is characterized by grossly disseminated intraperitoneal mucinous tumors, often with gelatinous ascites and usually secondary to an appendiceal mucinous tumor[1-8]. Occasionally, mucinous tumors in other sites are the culprit, such as colon[9,10], ovarian[11-13], pancrease[14] and so on[15]. Currently, the pathologic classification and biological behavior of PMP has been plagued with controversy and confusing terminology. There is considerable variability in the criteria and terminology used by different pathologists to diagnose patients with “PMP”. For lesions with the same morphology, the diagnosis may be described as “ruptured appendix mucinous adenoma with PMP” by some pathologists, or “well-differentiated mucinous adenocarcinoma (MACA)” by others. Inconsistent pathological diagnostic criteria and designations may lead to confusion and uncertainty in survival analyses and clinical treatment. In recent years, the classification and nomenclature of PMP has been discussed in some English literatures[16-23]; However the conclusion were not consistent completely. In addition currently, clinicians and pathologists still lack sufficient understanding of this disease in China. In 2007 we reported a clinicopathologic analysis of 40 patients with PMP[24], but the results was not very satisfied. Taking into account that the number of cases was small and the statistical results may be influenced, we added 52 new cases treated in our hospital in the past 4 years into the present analysis. We aimed to further clarify the nomenclature and prognostic factors of PMP, and to provide guidance for clinical treatment and prognosis decision.
MATERIALS AND METHODS
Patient selection
We reviewed the demographic and clinicopathological data of 101 patients with PMP who received treatment at our hospital. All of the pathological data from each patient were reviewed as far as possible, including lesions of peritoneal, appendix and other involved organs. All patients were followed up by letters and telephone calls. 9 patients (four males and five females) were excluded: suboptimal (R2/3) cytoreduction (n = 7), biopsy (n = 2). Eventually, 92 patients were selected for the present analysis. Some of these patients were reported previously.
Assessment of peritoneal lesions
The following items were evaluated for the peritoneal lesions[16,17]: Architecture was evaluated for (1) simple nonstratified strips or cluster of epithelium, or focal proliferative strips of cells with stratification; (2) extensive proliferative strips of cells wtih extensive stratification; and (3) individual signet ring cells, clusters of cells, or complex glands, arranged in a cribriform pattern consistant with caicinoma.
Cytologic atypia was assessed based on the most proliferative area and was graded as follows: (1) “minimal” cytologic atypia, histologically benign or mildly atypical; (2) “moderate” cytologic atypia, moderately enlarged hyperchromatic or vesicular nuclei with or without prominent nucleoli and some nuclear irregularity; and (3) “marked” cytologic atypia, enlarged, irregular, hyperchromatic or vesicular pleomorphic nuclei with prominent nucleoli or signet ring cells.
Mitotic activity was assessed by scanning multiple high-power fields (approximately 10 at × 400) in the most active areas according to the following system: Rare (0-2 mitotic figures), occasional (approximately 3-5 mitotic figures), and abundant (> 5 mitotic figures).
Assessment of appendiceal tumors
Appendiceal tumors were classified into two groups based upon their architectural complexity and degree of cytologic atypia. Tumors that demonstrated lowgrade cytologic atypia (nucleomegaly, nuclear stratification, rare mitotic figures and single cell necrosis) and minimal architectural complexity (villiform, flat epithelial proliferation and small papillary excrescences) were classified as low-grade appendiceal mucinous neoplasms (LAMNs). Appendiceal tumors were classified as MACAs if they demonstrated any of the following: destructive invasion of the appendiceal wall; high-grade cytologic atypia (extensive full-thickness nuclear stratification, vesicular nuclei, marked nuclear membrane irregularities, prominent nucleoli and brisk mitotic activity); or complex epithelial proliferation (complex papillary fronds and cribriform glandular spaces)[4].
Parenchymal organs invasion was defined as mucin and epithelium within the parenchymal of an organ. Involvement of only the peritoneal surface of an organ was not considered invasion.
Assignment of pathological category
We classified peritoneal lesions into three groups, disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and peritoneal mucinous carcinomatosis with intermediate or discordant features (PMCA-I/D)[1,3]. Cases were categoried as DPAM (Figure 1) if the peritoneal lesions were characterized by relatively scant strips of simple or focally proliferative mucinous epithelium with minimal to moderated cytologic atypia and no significant mitotic activity in abundant extracellular mucin, with or without appendiceal LAMN. Cases were categoried as PMCA (Figure 2), if the peritoneal lesions were characterized by more abundant proliferative mucinous epithelium, glands, nests, or individual cells, including signet ring cells, consistent with carcinoma and demonstrating marked mitotic activity, accompanied by MACAs of the appendix. Cases with PMCA-I/D (Figure 3) including those which the appendiceal tumor was a LAMN with significant atypia and those in which the peritoneal lesions displayed a spectrum of patterns from histologically benign to carcinomatous epithelium, were also characterized as DPAM if the peritoneal tumors displayed at least focal areas consistent with mucinous carcinoma similar to the other carcinoma cases, although some areas had the appearance of the peritoneal lesions seen in the DPAM cases.
Figure 1.
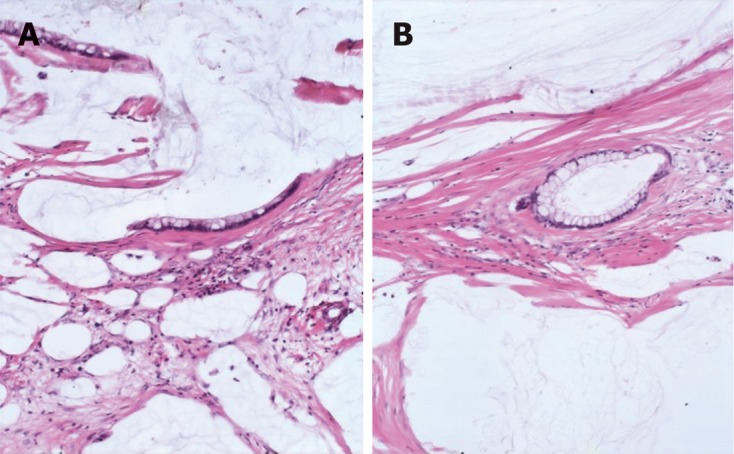
Peritoneal lesions consist of scant strips (A) and gland (B) of histologically bland mucinous epithelium associated with abundant extracellular mucin and fibrousis in disseminated peritoneal adenomucinosis (HE, × 40).
Figure 2.
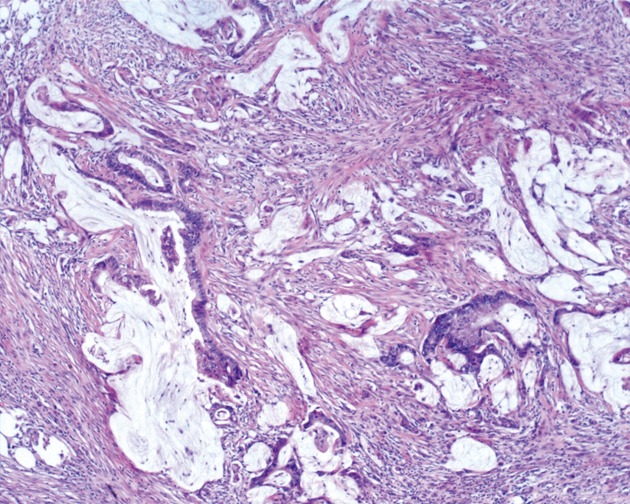
Markedly atypical epithelial fragments are suspended in pools of extracellular mucin in peritoneal mucinous carcinomatosis (HE, × 40).
Figure 3.
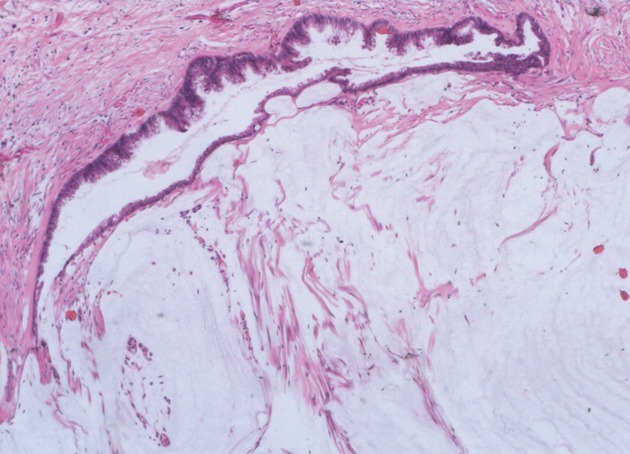
The mucinous epithelium displays nuclear stratification and cytologic atypia in peritoneal mucinous carcinomatosis-I/D (HE, × 40).
Statistical analysis
Retrospective analysis was performed in combination with follow-up data. Survival time was calculated from the date of first visit to our hospital to the date of death from PMP. Surviving patients were censored as of the last date on which they were known to be still alive. Survival estimates were calculated by using the Kaplan-Meier method. The log-rank test was used to analyze survival among the patients with between different pathological category, gender, age, operations times, appendix tumor, the architecture, cytologic atypia and mitotic activity of the peritoneal lesions, and organ involvement. Multivariate Cox proportional-hazard models were constructed to determine the important factors associated with survival. All analyses were performed using SPSS13.0. The significance level α was set at 0.05.
RESULTS
Clinicopathologic characteristics
Characteristics of clinicopathology are shown in Table 1. A total of 92 patients, 45 male, 47 female, median age 51.9 years (range: 22-76 years) were identified. The most common presenting symptoms were abdominal distension and enlargement (94.6%, 87/92), abdominal mass (67.4%, 62/92), abdominal pain (62.0%, 57/92) and weight loss (16.3%, 15/92).
Table 1.
Clinicopathologic finding of 92 cases of pseudomyxoma peritonei n (%)
| Index | DPAM | PMCA-I/D | PMCA | Total |
| No. of cases | 49 (53.2) | 26 (28.3) | 17 (18.5) | 92 (100.0) |
| Gender | ||||
| Male | 27 | 10 | 10 | 45 (48.9) |
| Female | 22 | 16 | 7 | 47 (51.1) |
| Age (range, yr) | 55.2 (22-79) | 51.9 (24-76) | 50.9 (31-75) | 53.4 (22-79) |
| Operative times | ||||
| ≤ 2 | 34 | 18 | 12 | 64 (69.6) |
| > 2 | 15 | 8 | 5 | 28 (30.4) |
| Appendix | ||||
| UR | 1 | 0 | 1 | 2 (2.2) |
| UP | 6 | 4 | 0 | 10 (10.9) |
| Appendicitis | 1 | 1 | 1 | 3 (3.1) |
| LAMN | 31 | 11 | 6 | 48 (52.2) |
| MACA | 10 | 10 | 9 | 29 (31.5) |
| Architecture | ||||
| Simple | 15 | 0 | 0 | 15 (16.3) |
| FP | 20 | 16 | 1 | 37 (40.2) |
| EP | 4 | 10 | 4 | 18 (19.6) |
| Carcinomatous | 0 | 0 | 12 | 12 (13.1) |
| Cytologic atypia | ||||
| Minimal | 45 | 12 | 1 | 58 (63.0) |
| Moderate | 4 | 14 | 4 | 22 (23.9) |
| Marked | 0 | 0 | 12 | 12 (13.1) |
| Mitosis | ||||
| Rare | 48 | 13 | 1 | 52 (56.5) |
| Occasional | 1 | 10 | 7 | 18 (19.6) |
| Abundant | 0 | 3 | 9 | 12 (13.1) |
| Organs involvment | 2 | 3 | 10 | 15 (16.3) |
| Small intestine | 11 | 1 | 52,3 | 7 (7.6) |
| Colon | 0 | 1 | 33 | 4 (4.3) |
| Rectum | 0 | 0 | 14 | 1 (1.1) |
| Urinary bladder | 11 | 0 | 0 | 1 (1.1) |
| Liver | 1 | 0 | 0 | 1 (1.1) |
| Uterus | 0 | 1 | 14 | 2 (2.2) |
| Fallopian tube | 0 | 0 | 1 | 1 (1.1) |
| Pleura | 0 | 0 | 22 | 2 (2.2) |
| Ovarian involvment (n = 47) | 13 | 12 | 7 | 32 (68.1) |
1,2,3,4Represents one case had two organ involvement. LAMN: Low-grade appendiceal mucinous neoplasm; MACA: Mucinous adenocarcinoma; UP: Unknown pathology; UR: Unresected; FP: Focally proliferation; EP: Extensively proliferation; DPAM: Disseminated peritoneal adenomucinosis; PMCA-I/D: Peritoneal mucinous carcinomatosis with intermediate or discordant features.
During surgery, a large volume of jelly-like mucous substances was seen in the abdomen. Multiple mucous lesions could be observed on the surface of the peritoneum and visceral organs, and complete cytoreduction (R0 61, R1 31) was performed. Ninety patients underwent appendectomy. Among them 48 (52.2%) with LAMN, 29 (31.5%) with MACA, 10 (10.9%) with unknown pathologic diagnosis, and 3 (3.1%) with appendicitis. All the 3 patients with appendicitis had undergone appendectomy at other hospitals before they presented at our hospital, and the appendiceal slides were not available, no mucinous tumor in other organs were found.
Patients received an average of 2.1 operations (range: 1-6 operations), and 30.4% (28/92) patients underwent 3 or more operations. 63.0% (58/92) patients had hyperthermic intraperitoneal chemotherapy.
In all the patients, the intra-abdominal masses consisted of multiple nodules or grape-like masses; most of which had a smooth and shiny surface. Upon sectioning, the nodules were full of a jelly-like mucous substance. Microscopically some mucous glandular structures were floating in a large number of mucous lakes, whose epithelium showed different architecture, cytologic atypia and mitotic activity. Ninety-two patients were designated into three groups, DPAM in 53.2% (49/92), PMCA-I/D in 28.3% (26/92), and PMCA in 18.5% (17/92). There was a slight preponderance of women (16/26) in the PMCA-I/D group, but there were more men than women in the DPAM and the PMCA group. The number of operations was not significantly different among the three groups.
The disease was accompanied by ovarian mucinous tumors in 68.1% (32/47) female patients. In 15 cases (17.5%), a total of 19 other intra-abdominal parenchymal organs were involved (Table 1). In the PMCA group, the lesions penetrated the diaphragm and involved the pleura in two cases (2.2%).
Outcome and overall survival
Among the 92 patients, 15 died of intestinal obstruction, 5 died of septicemia secondary to postoperative wound infection, and 2 died of complications of tumor invasion into the thoracic cavity. Fifty-six patients were still alive. The longest survival was 350 mo. Fourteen patients were lost to follow-up at 1 to 146 mo after surgery. The median survival of the whole patient population was 124 mo (range: 1-350 mo). Using the Kaplan Meier survival curve method, the overall 3-, 5-, and 10-year survival rates were 74.0%, 67.4% and 49.1%, respectively.
Survival analysis
The survival time was compared among three groups, 16.3% patients (8/49) died in the DPAM group with the median survival time 312.9 mo. Twenty-three point one percent of patients (6/26) died in the PMCA-I/D group with the median survival time 84.0 mo, and 47.1% patients (8/17) died in the PMCA group with the median survival time 31.7 mo. The 3 year survival for DPAM, PMCA-I/D and PMCA was 97.0%, 80.0% and 67.0%, respectively; the 5 year survival was 80.0%, 67.0% and 50.0%, respectively; and the 10 year survival was 65.0%, 28.0% and 14.0%, respectively. A significant difference among three groups in overall survival was seen (P = 0.001) (Figure 4A). Pairwise comparison showed the survival characteristics are significantly different between the DPAM and PMCA-I/D groups (P = 0.005), the PMCA-I/D and PMCA groups (P = 0.003). The prognosis was best of DPAM and worst of PMCA.
Figure 4.
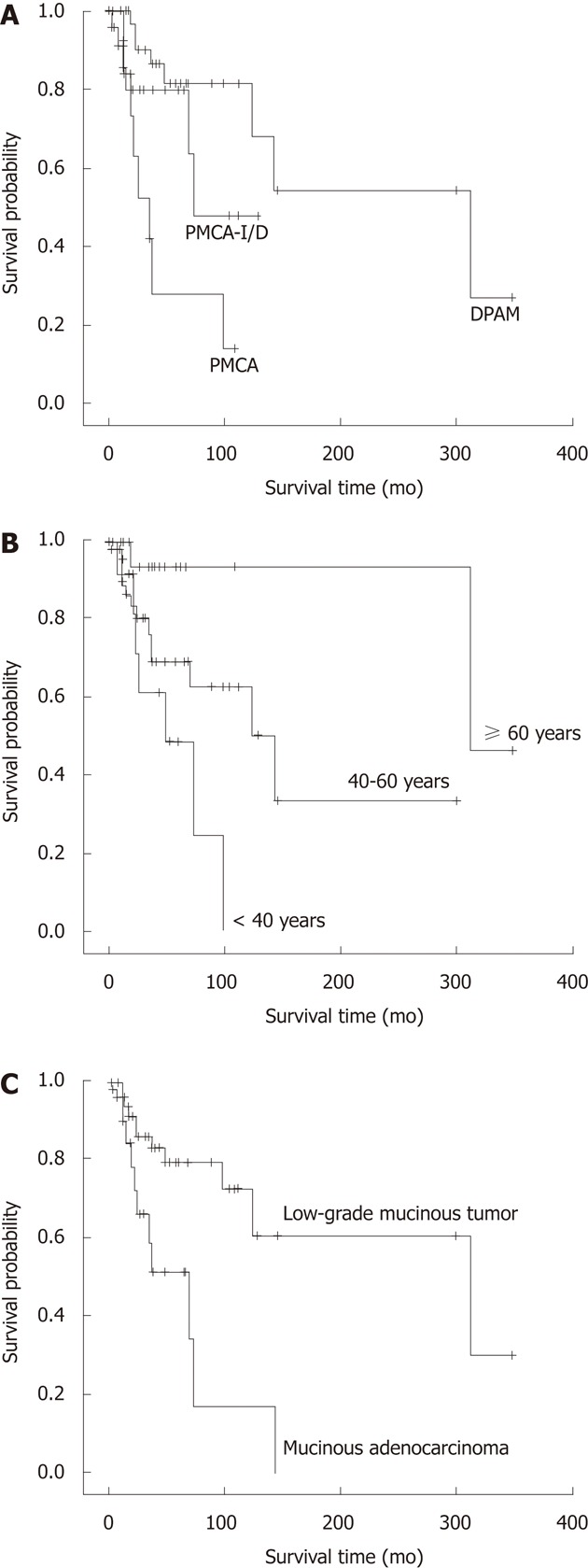
Survival related to histological classification, age and appendiceal mucinous adenocarcinoma. A: Overall survival of the three histologic groups: Disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis with intermediate or discordant features (PMCA-I/D) and peritoneal mucinous carcinomatosis (PMCA) (P = 0.001); B: Overall survival of the three age groups: < 40, 40-59 and ≥ 60 years (P = 0.011); C: Overall survival of the two appendiceal tumor groups: low-grade appendiceal mucinous neoplasm and mucinous adenocarcinoma (P = 0.008).
Association analysis of age and survival time using age as a continuous variable showed that age was associated with the survival time; the younger the patient, the shorter the survival (P = 0.020). The mean age was 51.9 years. So we separated the patients into three age groups, < 40 years, 40-59 years and ≥ 60 years, to analyze. The results showed that the survival was significantly different among the three groups (P = 0.011) (Figure 4B). The prognosis was poorest in patients < 40 years, in whom the 3- and 5-year survival rates were 83.0% and 48.0%, respectively. The longest survival time was 96 mo (the survival rate was 24.0%).
Because of its poor reliability and the limited number of cases we combined the 3 patients with “appendicitis” and 2 patients without appendectomy into the “pathology-unknown” group and excluded them for analysis. Survival time was significantly different between patients with LAMN and MACA (P = 0.008). The prognosis of patients with MACA of the appendix was poorer than those with LAMN (Figure 4C).
In addition, ovarian involvement did not appear to influence the female patients’ outcome (P = 0.897). But extra-ovarian parenchymal organ involvement were significantly associated with the survival time of patients (P = 0.005). Gender and operation times were not associated with the survival (P = 0.547 and 0.692, respectively).
Stepwise multivariate Cox proportional-hazard regression analysis indicated that age (P = 0.046), pathological classification (P = 0.012) and appendiceal MACA (P = 0.029) were significant related to survival and predicted for death (Table 2).
Table 2.
Stepwise multivariate Cox proportional hazards regression analysis
| Variable | β | Se (β) | Wald, χ2 | P | RR | 95% CI |
| Age | -0.943 | 0.473 | 3.984 | 0.046 | 0.39 | 0.154, 0.983 |
| Pathologic classification | 0.778 | 0.311 | 6.255 | 0.012 | 2.18 | 1.183, 4.006 |
| Appendiceal tumor | 0.723 | 0.331 | 4.765 | 0.029 | 2.06 | 1.077, 3.941 |
RR: Risk ratio; CI: Confidence interval.
DISCUSSION
PMP is a rare clinical disease characterized by a large amount of gelatinous mucus in the abdominal cavity, and wide peritoneal and omental dissemination. Its incidence is approximately 2/10 000 patients undergoing laparotomy[25]. Clinically, it is characterized by non-remitting abdominal pain, abdominal swelling, and mucoid ascites. Ultimately, the tumor occupies most space in the abdominal cavity, showing manifestations of “jelly belly” syndrome. It is very difficult to have a definite diagnosis before surgery, and most cases are diagnosed after laparotomy. Pathological examination shows that the abdominal tumor consists of a large amount of gelatinous mucinous substance, some of which may have an appearance of multiple mucinous nodules. It can be seen under the microscope that varying amounts of the mucinous epithelial component is floating in the mucous lake, and in most cases, the morphology of the epithelial component is benign. In recent years, most scholars believe that PMP has originated from appendiceal mucinous disease[7,8] and occasionally, mucinous tumors of other places have been implicated[9-15].
Naming and pathological diagnosis of PMP
As the chemical nature of the gelatinous substance of PMP is different from that of mucous protein, it is called pseudo-mucous protein, and accordingly, the condition was named “PMP”. After it was first proposed in 1884, the name of “PMP” has been in use to date. However, strictly speaking, PMP reflects only the clinical process and characteristics of the disease, and cannot be used for pathological diagnosis. Therefore, its pathological classification and naming have long been debated. Some scholars believe that it can be diagnosed as cancer as long as the epithelial component is found outside of the appendiceal wall; however, some believe that in some cases, the survival time of patients is very long although PMP tends to be characterized by multiple recurrences; therefore, it should be considered benign or a low-grade malignancy. Pathological diagnosis directly affects the postoperative treatment. The difference in the pathological diagnosis of PMP results in confusion and uncertainty in clinical treatment.
In recent years, investigators have obtained different results. In 1995, Ronnett et al[16] analyzed the pathological conditions in 109 cases of PMP, and they first proposed that PMP should be divided into three categories: DPAM, PMCA-I/D and PMCA. Since then, they followed that group of patients for many years, and carried out a prognostic analysis in 2001. They grouped patients with PMCA-I/D and PMCA together and compared them with the DPAM group. They found that the 5- and 10-year survival rates (26% and 9%) were significantly worse than that of the DPAM group (75% and 68%)[17]. Miner et al[22] thought that PMP should be divided into low-grade and high-grade peritoneal MACA. However, in 2006 Bradley et al[20] analyzed 101 cases of PMP and found that the survival rate was not significantly different between the DPAM and PMCA-I/D groups. Therefore, they combined the two groups into low-grade mucinous carcinoma peritonei (MCP-L), and those patients classified into PMCA according to Ronnett et al[16,17] were renamed high grade mucinous carcinoma peritonei (MCP-H). The prognosis of MCP-L was significantly better than that of MCP-H with the 3 - and 5-year survival rates 72.8% and 62.5%, 37.7% and 37.7%, respectively[20]. However, the recent study of Bruin et al[9] supported Ronnett’s conclusion that the prognosis was not significantly different in PMCA-I/D and PMCA, and therefore, these two groups should be combined.
Referring to the study of Ronnett et al[16,17] in 2007, we divided 40 cases of PMP into three groups, DPAM, PMCA-I/D and PMCA, and compared them[24]. The pair-wise comparison showed no significant differences between the PMCA-I/D and PMCA groups. Therefore, PMCA-I/D and PMCA were combined into one group, and re-analysis showed the 3-, 5- and 10-year survival rates were 58.9%, 47.1% and 35.3%, which were significantly different from those in the DPAM group.
Taking into account small cases for the previous analysis the statistical results may be influenced, now we added 52 new cases treated in the past 4 years into the analysis and the survival rate of the three groups were compared. There were 49 (53.2%) patients with DPAM, 26 (28.3%) with PMCA-I and 17 (18.5%) with PMCA. Patients with DPAM, PMCA-I/D and PMCA exhibited statistically significant difference in survival. The 3 year survival for DPAM, PMCAI/D and PMCA was 97.0%, 80.0% and 67.0%, respectively; the 5 year survival was 80.0%, 67.0% and 50.0%, respectively; and the 10 year survival was 65.0%, 28.0% and 14.0%, respectively. But Pair-wise comparison showed the survival characteristics are significantly different between the DPAM and PMCA-I/D groups, the PMCA-I/D and PMCA groups. The prognosis was best in the DPAM group and worst in the PMCA group. The results supported the morphological classification of PMP into three categories, and they should be named DPAM, PMCA-I/D and PMCA, respectively to further guide postoperative clinical treatment.
Biological behaviors, prognosis and treatment of PMP
Although the progression of PMP is slow, and the pathological morphology is benign or low-grade malignancy in the majority of cases, its biological behavior is malignant and it can not be removed completely during surgery because of its extensiveness and invasiveness. Relapse and adhesions may occur easily, and the patient may have cachexia of chronic disease. Postoperative recurrence occurs in 60% to 76% of patients, and Multiple surgical resections are often required. Although this disease may progress slowly, it is often fatal. Common causes of death are systemic infection secondary to wound infection, bowel obstruction, hernia and pleural pseudomyxoma caused by tumor passing through the diaphragm[26,27].
It is reported that the median survival time of patients with PMP is 5.9 years, and the 3-, 5- and 10-year survival rates were 81.1%-83%, 50.0%-81%, and 18.2%-32%, respectively. Prognostic factors of PMP are the location, primary tumor and the histopathological grade of the peritoneal lesions[22,27]. In this study the survival time ranged from 1 to 350 mo with the median 124 mo. The 3-year, 5-year and 10-year survival rates were 74.0%, 67.4% and 49.1%, respectively. The 10-year survival rate was higher than that reported in the literature. Thirty point four percent of (28/92) patients underwent 3 or more operations. Fifteen died of intestinal obstruction, 5 died of septicemia secondary to postoperative wound infection, and 2 died of complications of tumor invasion into the thoracic cavity. Among the clinicopathological characteristics we found age, the pathological classification of peritoneal tumor, appendiceal tumor, extra-ovarian parenchymal organ involvement were significantly related to the survival time. Stepwise multivariate Cox proportional-hazard regression analysis indicated that pathological classification, age, appendiceal MACA are survival independent predictors.
It has been reported that prognosis of PMP is related to age. The prognosis of patients > 50 years was significantly poorer than that of patients < 50 years[13]; However, the statistical analysis of this study showed that the younger the patient, the worse the prognosis. The prognosis of patients < 40 years was significantly worse than that of patients > 40 years.
Complete cytoreduction, supplemented by hyperthermic intraperitoneal and systemic chemotherapy was considered as the gold standard treatment of PMP. Complete cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy could prolongs long-term survival[28-36].
On conclusion we suggest that the name of PMP should be avoided as far as possible in the pathological diagnosis, and DPAM, PMCA-I/D, and PMCA should be used to indicate the degree of differentiation of the tumors. Therefore, additionally, the appendix should be explored as far as possible during gross examination, and sampling and serial sections should be made in order to determine and report the severity of the appendiceal lesions. Attention should be paid to examine the involvement of parenchymal organs in order to provide more information for clinical treatment. For patients with PMP, clinicians should determine the prognosis according to age on the basis of a complete pathology report.
ACKNOWLEDGMENTS
We want to thank Professor. Li ZW in the Institute of Reproductive and Child Health of Peking University for his help on statistical analysis.
COMMENTS
Background
Pseudomyxoma peritonei (PMP) is a rare clinical entity that is characterized by grossly disseminated intraperitoneal mucinous tumors, often with gelatinous ascites and usually secondary to an appendiceal mucinous tumor. Occasionally, mucinous tumors in other sites are the culprit, such as colon, ovarian, pancrease and so on. Currently, the pathologic classification and biological behavior of PMP has been plagued with controversy and confusing terminology. There is considerable variability in the criteria and terminology used by different pathologists to diagnose patients with “PMP” for lesions with the same morphology.
Research frontiers
In recent years, the classification and prognosis of PMP has been discussed in some English literatures. However the conclusions were not consistent completely. In addition currently, clinicians and pathologists still lack sufficient understanding of this disease in China. In this study, the authors demonstrate pathological classification, age(< 40 years), appendiceal appendiceal mucinous adenocarcinoma (MACA) are survival independent predictors of Chinese patients with PMP. They think the clinical process “PMP” should be pathologically classified into disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis with intermediate or discordant features (PMCA-I/D), and peritoneal mucinous carcinomatosis (PMCA).
Innovations and breakthroughs
Recent reports have hignlighted the classification and prognosis of PMP. But the conclusions were not consistent completely. Unfortunately, few reports have observed the classification and prognosis of PMP in Chinese patients. And currently, clinicians and pathologists still lack sufficient understanding of this disease in China. This study is the first large sample study on the pathological classification and prognosis of PMP in Chinese patients.
Applications
By understanding the survival predictors in Chinese patients with PMP, the name of PMP should be avoided as far as possible in the pathological diagnosis, and DPAM, PMCA-I/D and PMCA should be used to indicate the degree of differentiation of the tumors. Therefore, additionally, the appendix should be explored as far as possible during gross examination, and sampling and serial sections should be made in order to determine and report the severity of the appendiceal lesions. Attention should be paid to examine the involvement of parenchymal organs in order to provide more information for clinical treatment. For patients with PMP, clinicians should determine the prognosis according to age on the basis of a complete pathology report.
Terminology
PMP is a disease with a large volume of extensively implanted gelatinous mucous substance on the surface of the peritoneum. The terminology is came from the jelly-like substance, whose chemical properties are different from those of the mucous proteins and called pseudo-mucin.
Peer review
This is a nice review of the histologic features of “PMP” in Chinese patients and and suggest that the clinical process “PMP” should be pathologically classified into DPAM, PMCA and PMCA-I/D. Pathological classification, age and appendiceal MACA are survival independent predictors in Chinese patients with PMP, nicely addresses the challenges with the pathologic diagnosis.
Footnotes
Peer reviewer: Alyssa M Krasinskas, MD, Assistant Professor, Department of Pathology, University of Pittsburgh Medical Center, Presbyterian Hospital, A610, 200 Lothrop Street, Pittsburgh, PA 15213-2546, United States
S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Ronnett BM, Shmookler BM, Diener-West M, Sugarbaker PH, Kurman RJ. Immunohistochemical evidence supporting the appendiceal origin of pseudomyxoma peritonei in women. Int J Gynecol Pathol. 1997;16:1–9. doi: 10.1097/00004347-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Dong Y, Li T, Zou W, Liang Y. [Pseudomyxoma peritonei: report of 11 cases with a literature review] Zhonghua Binglixue Zazhi. 2002;31:522–525. [PubMed] [Google Scholar]
- 3.Galani E, Marx GM, Steer CB, Culora G, Harper PG. Pseudomyxoma peritonei: the ‘controversial’ disease. Int J Gynecol Cancer. 2003;13:413–418. doi: 10.1046/j.1525-1438.2003.13318.x. [DOI] [PubMed] [Google Scholar]
- 4.Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089–1103. doi: 10.1097/00000478-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Smeenk RM, Verwaal VJ, Zoetmulder FA. Pseudomyxoma peritonei. Cancer Treat Rev. 2007;33:138–145. doi: 10.1016/j.ctrv.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Gatalica Z, Loggie B. COX-2 expression in pseudomyxoma peritonei. Cancer Lett. 2006;244:86–90. doi: 10.1016/j.canlet.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Guo AT, Song X, Wei LX, Zhao P. Histological origin of pseudomyxoma peritonei in Chinese women: clinicopathology and immunohistochemistry. World J Gastroenterol. 2011;17:3531–3537. doi: 10.3748/wjg.v17.i30.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojimahara T, Nakahara K, Shoji T, Sugiyama T, Takano T, Yaegashi N, Yokoyama Y, Mizunuma H, Tase R, Satou H, et al. Identifying prognostic factors in Japanese women with pseudomyxoma peritonei: a retrospective clinico-pathological study of the Tohoku Gynecologic Cancer Unit. Tohoku J Exp Med. 2011;223:91–96. doi: 10.1620/tjem.223.91. [DOI] [PubMed] [Google Scholar]
- 9.Bruin SC, Verwaal VJ, Vincent A, van’t Veer LJ, van Velthuysen ML. A clinicopathologic analysis of peritoneal metastases of colorectal and appendiceal origin. Ann Surg Oncol. 2010;17:2330–2340. doi: 10.1245/s10434-010-0984-5. [DOI] [PubMed] [Google Scholar]
- 10.Evers DJ, Verwaal VJ. Indication for oophorectomy during cytoreduction for intraperitoneal metastatic spread of colorectal or appendiceal origin. Br J Surg. 2011;98:287–292. doi: 10.1002/bjs.7303. [DOI] [PubMed] [Google Scholar]
- 11.Shen DH, Ng TY, Khoo US, Xue WC, Cheung AN. Pseudomyxoma peritonei--a heterogenous disease. Int J Gynaecol Obstet. 1998;62:173–182. doi: 10.1016/s0020-7292(98)00095-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am J Surg Pathol. 2000;24:1447–1464. doi: 10.1097/00000478-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Lee JK, Song SH, Kim I, Lee KH, Kim BG, Kim JW, Kim YT, Park SY, Cha MS, Kang SB. Retrospective multicenter study of a clinicopathologic analysis of pseudomyxoma peritonei associated with ovarian tumors (KGOG 3005) Int J Gynecol Cancer. 2008;18:916–920. doi: 10.1111/j.1525-1438.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- 14.Imaoka H, Yamao K, Salem AA, Mizuno N, Takahashi K, Sawaki A, Isaka T, Okamoto Y, Yanagisawa A, Shimizu Y. Pseudomyxoma peritonei caused by acute pancreatitis in intraductal papillary mucinous carcinoma of the pancreas. Pancreas. 2006;32:223–224. doi: 10.1097/01.mpa.0000194611.62723.51. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi M, Matsuzaki K, Yoshida S, Nishitani H, Uehara H. Localized pseudomyxoma peritonei in the female pelvis simulating ovarian carcinomatous peritonitis. J Comput Assist Tomogr. 2003;27:622–625. doi: 10.1097/00004728-200307000-00031. [DOI] [PubMed] [Google Scholar]
- 16.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Ronnett BM, Yan H, Kurman RJ, Shmookler BM, Wu L, Sugarbaker PH. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92:85–91. doi: 10.1002/1097-0142(20010701)92:1<85::aid-cncr1295>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Jackson SL, Fleming RA, Loggie BW, Geisinger KR. Gelatinous ascites: a cytohistologic study of pseudomyxoma peritonei in 67 patients. Mod Pathol. 2001;14:664–671. doi: 10.1038/modpathol.3880370. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed F, Gething S, Haiba M, Brun EA, Sugarbaker PH. Clinically aggressive pseudomyxoma peritonei: a variant of a histologically indolent process. J Surg Oncol. 2004;86:10–15. doi: 10.1002/jso.20038. [DOI] [PubMed] [Google Scholar]
- 20.Bradley RF, Stewart JH, Russell GB, Levine EA, Geisinger KR. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–559. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 21.van Ruth S, Acherman YI, van de Vijver MJ, Hart AA, Verwaal VJ, Zoetmulder FA. Pseudomyxoma peritonei: a review of 62 cases. Eur J Surg Oncol. 2003;29:682–688. doi: 10.1016/s0748-7983(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 22.Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241:300–308. doi: 10.1097/01.sla.0000152015.76731.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Parsa V, Adsay V, Heilbrun LK, Smith D, Shields AF, Weaver D, Philip PA, El-Rayes BF. Clinicopathological analysis of primary epithelial appendiceal neoplasms. Med Oncol. 2010;27:1073–1078. doi: 10.1007/s12032-009-9337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo AT, Wei LX, Song X. [Histologic classification and prognostic implication of pseudomyxoma peritonei] Zhonghua Binglixue Zazhi. 2007;36:474–479. [PubMed] [Google Scholar]
- 25.Sherer DM, Abulafia O, Eliakim R. Pseudomyxoma peritonei: a review of current literature. Gynecol Obstet Invest. 2001;51:73–80. doi: 10.1159/000052897. [DOI] [PubMed] [Google Scholar]
- 26.Lee BY, Kim HS, Lee SH, Moon HS, Cho SM, Lee KH, Song KS, Min KO, Seo EJ, Lee JM. Pseudomyxoma peritonei: extraperitoneal spread to the pleural cavity and lung. J Thorac Imaging. 2004;19:123–126. doi: 10.1097/00005382-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Wang CY, Gu MJ, Wang SX, Ma D. Analysis of the clinical pathologic characteristic and prognosis with pseudomyxoma peritone. Xiandai Fuchanke Jinzhan. 2002;11:268–270. [Google Scholar]
- 28.Baratti D, Kusamura S, Nonaka D, Langer M, Andreola S, Favaro M, Gavazzi C, Laterza B, Deraco M. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15:526–534. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- 29.Baratti D, Kusamura S, Nonaka D, Cabras AD, Laterza B, Deraco M. Pseudomyxoma peritonei: biological features are the dominant prognostic determinants after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2009;249:243–249. doi: 10.1097/SLA.0b013e31818eec64. [DOI] [PubMed] [Google Scholar]
- 30.Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FA. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104–109. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elias D, Honoré C, Ciuchendéa R, Billard V, Raynard B, Lo Dico R, Dromain C, Duvillard P, Goéré D. Peritoneal pseudomyxoma: results of a systematic policy of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2008;95:1164–1171. doi: 10.1002/bjs.6235. [DOI] [PubMed] [Google Scholar]
- 32.Elias D, Gilly F, Quenet F, Bereder JM, Sidéris L, Mansvelt B, Lorimier G, Glehen O. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36:456–462. doi: 10.1016/j.ejso.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Chua TC, Yan TD, Smigielski ME, Zhu KJ, Ng KM, Zhao J, Morris DL. Long-term survival in patients with pseudomyxoma peritonei treated with cytoreductive surgery and perioperative intraperitoneal chemotherapy: 10 years of experience from a single institution. Ann Surg Oncol. 2009;16:1903–1911. doi: 10.1245/s10434-009-0341-8. [DOI] [PubMed] [Google Scholar]
- 34.Cioppa T, Vaira M, Bing C, D’Amico S, Bruscino A, De Simone M. Cytoreduction and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis from pseudomyxoma peritonei. World J Gastroenterol. 2008;14:6817–6823. doi: 10.3748/wjg.14.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Järvinen P, Järvinen HJ, Lepistö A. Survival of patients with pseudomyxoma peritonei treated by serial debulking. Colorectal Dis. 2010;12:868–872. doi: 10.1111/j.1463-1318.2009.01947.x. [DOI] [PubMed] [Google Scholar]
- 36.Arjona-Sánchez Á, Muñoz-Casares FC, Rufián-Peña S, Díaz-Nieto R, Casado-Adam Á, Rubio-Pérez MJ, Ortega-Salas R. Pseudomyxoma peritonei treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: results from a single centre. Clin Transl Oncol. 2011;13:261–267. doi: 10.1007/s12094-011-0651-8. [DOI] [PubMed] [Google Scholar]


