Abstract
Sensitivity to commercial teat dips (nonoxinol-9 iodine complex and chlorhexidine digluconate) of 56 Staphylococcus (S.) aureus strains isolated from quarter milk samples of various German dairy herds treated with different teat dipping schemes was investigated in this study. The minimum inhibitory concentration was determined using a broth macrodilution method according to the German Veterinary Association guidelines. The main objective of the current study was to induce in vitro resistance induction of S. aureus to chemical disinfectants. Ten different strains were repeatedly passed ten times in growth media with sub-lethal concentrations of disinfectants. Nine strains showed a significant reduction in susceptibility to the nonoxinol-9 iodine complex but only one strain developed resistance to chlorhexidine digluconate. Stability of the acquired resistance was observed in all S. aureus strains adapted to the nonoxinol-9 iodine complex and chlorhexidine digluconate. In contrast, simultaneous resistance to different antibiotics was not observed in any of the ten investigated S. aureus strains. However, the isolates exhibited a high degree of resistance to penicillin G. Based on these results, resistance of S. aureus to chemical disinfectants may be more likely to develop if the chemicals are used at concentrations lower than that required for an optimal biocidal effect.
Keywords: antibiotics, bovine mastitis, resistance induction, Staphylococcus aureus, teat dips
Introduction
Bovine mastitis is an inflammatory reaction primarily caused by different pathogens that gain entry into the teat canal and mammary gland [3]. This represents one of the most costly diseases in the dairy industry worldwide with estimated losses of about 2 billion dollars per year in the United States alone [12]. These substantial economic losses are incurred by several factors such as rejected milk, reduced milk quality, drug costs, veterinary expenses, early culling, and increased laboratory costs [12]. Most cases of bovine mastitis are due to various types of bacteria. Bacteria of the genus Staphylococcus are one of the most common pathogens that cause mastitis worldwide. The genus Staphylococcus is divided according to the coagulase test into coagulase-negative (CNS) and coagulase-positive (CPS) species. Historically, CNS has often been considered to be minor important pathogens that cause intramammary infections (IMI). In contrast, recent studies on mastitis prevalence have found that CNS may be of major importance in some countries [3,26].
Staphylococcus (S.) aureus is among the CPS isolated from cases of bovine mastitis. This pathogen is considered to be one of the most common causes of bovine mastitis in different areas of the world and responsible for 25~30% of all IMI in the United States [35]. Mastitis caused by S. aureus is most frequently subclinical; however, major rates of clinical mastitis incidence are associated with this microorganism. S. aureus is regarded as a contagious mastitis pathogen because it is commonly spread from infected to non-infected cows during milking [33]. Although S. aureus is not difficult to cultivate and easily identified, there is still need for a rapid and sensitive DNA-based assay specific for detecting S. aureus [30]. Most recent studies used polymerase chain reaction (PCR) techniques to identify S. aureus and, in some cases, for genotyping [10,18]. In general, more rapid identification of bacteria using matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS) can be an important method for diagnosing infections [31].
Bovine S. aureus mastitis can be prevented and controlled by the use of effective post-milking teat germicides, antibiotic application on all quarters when drying off, culling animals with chronic infections, treatment of clinical mastitis during lactation, and proper use of functioning milking machines [3]. Post-milking teat disinfection is considered to be one of the most effective procedures for reducing the rate of subclinical and clinical cases of mastitis during lactation. More than ten different active compounds have been used in teat disinfectants worldwide over the last 20 years. The National Mastitis Council (NMC) (USA) reviewed and summarized nearly all the scientific literature on teat disinfectants published since 1980, and found that more than 10 different active ingredients as iodine and chlorhexidine have been used in teat disinfectants throughout the world [26]. Despite the universal acceptance of teat dipping as a method of mastitis control, a number of limitations are associated with most teat dips currently available. The most significant restriction is that teat dips do not provide equal protection against the huge amount of bacteria that cause bovine mastitis. Furthermore, prolonged in vitro exposure to germicidal teat dips has been shown to enhance the resistance of some bacteria to chemical disinfectants [22]. Several passages of bacterial isolates with sub-lethal concentrations of disinfectants were found to either induce resistance or select for resistant variants [36].
Some laboratory studies suggested that the development of resistances against biocides and antibiotics are linked whereas other studies failed to identify an association [19,29]. Microorganisms are limitlessly adaptable and have already demonstrated different mechanisms of resistance to different biocides; the concern is that these mechanisms may give cross-resistance to clinically important antibiotics. Several numbers of studies have been achieved to assess whether environmental and/or clinical strains that show decreased susceptibility to different types of biocides also display resistance to various types of antibiotics [29].
The current study was thus performed to evaluate the in vitro efficacy of two teat disinfectants (nonoxinol-9 iodine complex and chlorhexidine digluconate) and the ability of these compounds to induce resistance in identified strains of S. aureus. We also evaluated the antibiotic resistance patterns of S. aureus and CNS isolated from cases of bovine mastitis. Finally, we assessed the probability of cross-resistance in S. aureus to teat disinfectants and different types of antibiotics commonly used to treat bovine mastitis.
Materials and Methods
Milk samples, animals and bacterial strains
Quarter milk samples were collected from six dairy herds with a high prevalence of S. aureus in the federal state of Brandenburg, Germany using standard procedures described by the NMC [19]. From each herd, 32 cows in different stages of lactation and different ages were chosen for sampling. The animals were divided into three groups according to teat dipping schemes. Teats of the first group were dipped with a post-milking teat disinfectant (nonoxinol-9 iodine complex), the teats of second group were dipped in chlorhexidine digluconate, and the third group did not undergo any treatment (a negative control group).
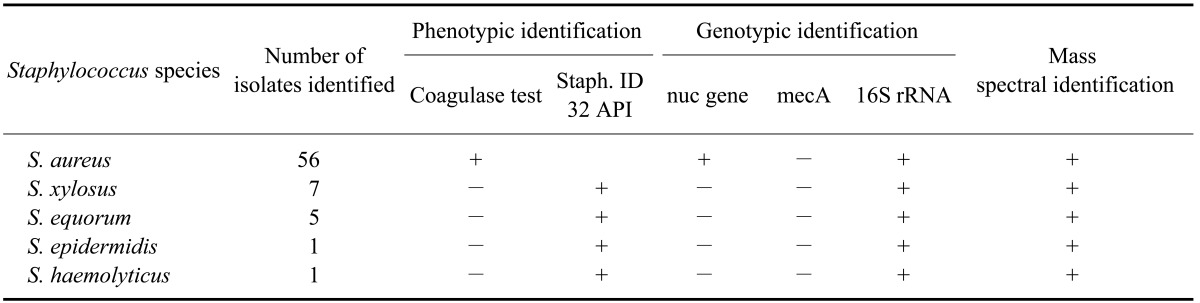
A total of 70 S. aureus and CNS isolates had been recovered from subclinical cases of bovine mastitis during a survey carried out for 6 months. Isolation of all isolates was performed according to the NMC recommendations [20] for examining quarter-milk samples. S. aureus strains were preliminarily identified by colony morphology, ability to induce hemolysis, and Gram staining. Creamy, grayish-white, or golden-yellow colonies that were catalase-positive, coagulase-positive, and Gram-positive cocci and induced complete and/or incomplete hemolysis were identified as S. aureus. Specific identification of the S. aureus strains and CNS was made phenotypically with a tube coagulase test and Staph ID 32 API systems (BioMérieux, France), and genotypically by PCR and MALDI-TOF-MS. The reference S. aureus strains used for each trial were American Type Culture Collection 25923 (ATCC 25923; ATCC, USA) and Deutsche Sammlung von Mikroorganismen 799 (DSM 799; DSM, Germany).
Teat dips
Thirty isolates of different Staphylococcus species (17 coagulase-positive S. aureus and 13 CNS) were derived from the first group of cows with teats that were regularly treated with a solution of nonoxinol-9 iodine complex (2,700 ppm as nonoxinol-9 iodine complex and less that 8% glecrol; Kesla, Germany). Another 30 isolates of various Staphylococcus species (29 coagulase-positive S. aureus and one CNS) were collected from a second group of cows with teats that were regularly dipped in a preparation of chlorhexidine digluconate [3,500 ppm as chlorhexidine digluconate along with glycerol, polysorbate 20, sodiumsalt (E141), chlorophyllin copper complex, and purified water; Eimermacher, Germany]. Finally, ten isolates of S. aureus were isolated from a third group of cows that had not been subjected to teat dipping (control group).
Broth macrodilution method for measuring the in vitro susceptibility of S. aureus to commercial teat dips
Serial dilutions (100, 50, 25, 12.5, 6.25, 3.12, 1.56 and 0.78%) of disinfectant were added to tryptose soya broth (TSB) (Oxoid, England) inoculated with a standardized number (ca 1 × 108 KbE/mL) of S. aureus and incubated at 37℃ for a prescribed time (18~24 h). Turbidity of the actively growing broth culture was adjusted by a Nephelometer (TREK Diagnostic Systems, UK) with sterile saline (NaCl) to obtain a level of turbidity optically comparable to that of 0.5 McFarland standards (ca 1 × 108 KbE/mL). The lowest concentration (highest dilution) of disinfectant preventing the appearance of turbidity was considered to be the minimum inhibitory concentration (MIC). This method was repeated twice in the same manner for the nonoxinol-9 iodine complex, chlorhexidine digluconate, and the negative control.
Induction of S. aureus resistance to disinfectants with sub-lethal concentrations of the chemicals using a broth macrodilution method
Ten different isolates of S. aureus previously tested in the preceding subsection with nonoxinol-9 iodine complex and chlorhexidine digluconate were included in this study. The purpose of this investigation was to compare the MIC of each active agent before and after ten passages. All strains were passed ten times in a liquid medium (TSB) with a sub-lethal concentration (concentration of each disinfectant below the MIC, where the isolates still show growth (12.5 and 25.0% for nonoxinol-9 iodine complex and chlorhexidine digluconate, respectively)) of the disinfectants within a 72-h interval under complete hygienic conditions to avoid contamination. The MIC values for the isolates were again determined after the tenth passage and compared to the original MIC values measured before passaging. Purity of the S. aureus cultures was evaluated by streaking on Mueller-Hinton agar (Oxoid, England). The stability of disinfectant resistance was determined by continuously subculturing the resistant strains in disinfectant-free nutrient broth (Oxoid, England). Subculturing was performed every 24 h for ten passages and MIC was measured after the tenth passage. Culture purity was evaluated at each passage.
Assessment of antimicrobial drug resistance of S. aureus strains and CNS using the agar disk diffusion method
A total of 70 strains of S. aureus and CNS were evaluated in this experiment. Six antibiotics were chosen according to the frequency of use for mastitis therapy. These included penicillin (penicillin G and oxacillin), aminoglycosides (gentamycin), macrolides (erythromycin), tetracyclines (tetracycline), and chloramphinicol. An agar disc diffusion test was carried out to determine the drug susceptibility for all strains. Cells from four to five colonies of S. aureus from pure cultures were picked with a sterile loop and suspended in 5 mL of sterile physiologic saline (NaCl) solution. Turbidity of the actively growing broth culture (TSB) was adjusted with 5 mL of sterile saline (NaCl) to obtain turbidity optically comparable to that of 0.5 McFarland standards (ca 1 × 108 KbE/mL). The entire surface of a Mueller-Hinton agar plate was inoculated with bacterial culture using a sterile swab. Disks (Mast Diagnostika, Germany) containing 10 I.U. penicillin G, 10 µg gentamycin, 5 µg oxacillin, 15 µg erythromycin, 30 µg tetracycline, or 30 µg chloramphinicol were placed onto the agar surface using sterile forceps and gently pressed to ensure contact. The plates were incubated at 37℃ for 24 h and the diameter of the zone of inhibition around each disk was then measured. The zones of growth inhibition were compared to the zone-size interpretative table, and all strains were designated as susceptible, intermediate, or resistant to each drug tested. The presence of colonies within a zone of inhibition was considered to indicate eventual resistance to that agent.
Evaluation of cross-resistance to teat dips and antibiotics using the broth microdilution method
Cross-resistance to a panel of antibiotics was assessed with Sensititre plates (TREK Diagnostic Systems, UK) using the broth microdilution method of the Federal Institute for Risk Assessment (Germany) according to instructions M7-A8 of the Clinical Laboratory Standards Institute [4]. Briefly, Mueller-Hinton agar plates were streaked with bacterial cryobank to obtain isolated colonies of S. aureus. After an overnight incubation at 37℃, four or five isolated colonies were selected and transferred with an inoculating needle or loop to a tube containing 5 mL of sterile saline solution and vortexed thoroughly. Turbidity of the actively growing broth culture (TSB) was adjusted with sterile saline using a nephelometer (TREK Diagnostic Systems, UK) to obtain turbidity optically comparable to that of 0.5 McFarland standards (ca 1 × 105 KbE/mL) and then inoculate 11 mL cation adjusted Mueller-Hinton broth tube with 15~50 µL of the adjusted broth culture. Screw the dosing head on the tube and inoculate 50 µL in each well of the microtitre plate (European susceptibility testing). The microtitre plates were sealed with foil, incubated at 37℃ for 18~24 h, and read with a Sensititre automatic reader (TREK Diagnostic Systems, UK).
Results
All S. aureus and CNS isolates were identified phenotypically by a tube coagulase test and Staph ID 32 API system, and genotypically using PCR and MALDI-TOF-MS (Table 1). Susceptibility of S. aureus to the nonoxinol-9 iodine complexes and chlorhexidine digluconate was measured using the broth macrodilution method. The MIC values of nonoxinol-9 iodine for the control group ranged from 41 to 46%. These values ranged from 41 to 49% for the dipped group. The mean MIC values of nonoxinol-9 iodine for the dipped and control groups were 45.7 ± 2.54% and 42.6 ± 1.64%, respectively. The MIC values of chlorhexidine digluconate for the control group ranged from 96 to 98% and from 95 to 99% for the dipped group. The mean MIC values of chlorhexidine digluconate for the dipped and control groups were 97.51 ± 0.98% and 96.8 ± 0.78%, respectively. No significant differences (p < 0.05) were observed between the dipped and control groups for either teat dip.
Table 1.
Phenotypic, genotypic, and mass spectral identification of Staphylococcus (S.) aureus isolates and coagulase negative species (CNS) from cows with subclinical cases of mastitis
Induction of S. aureus resistance
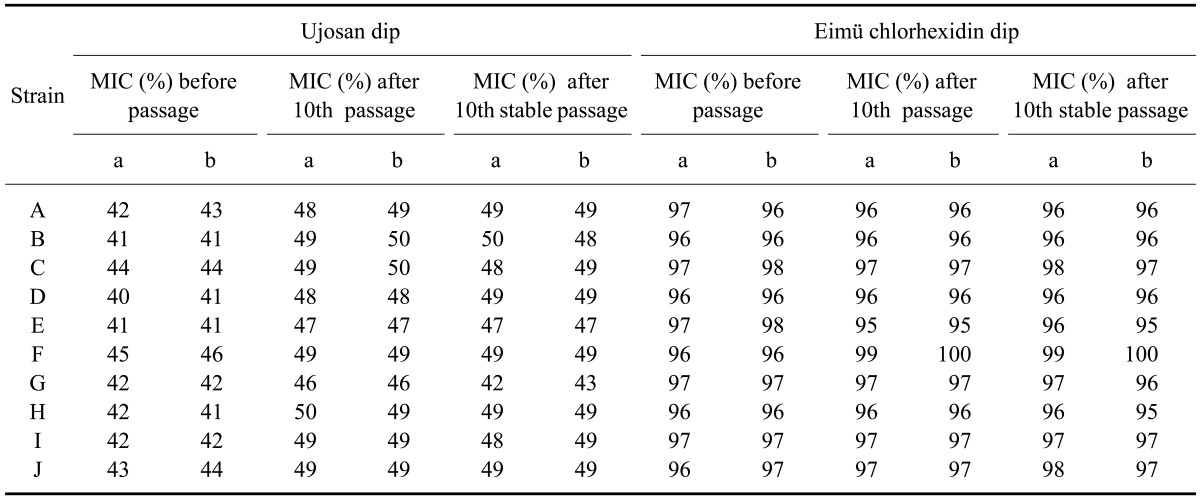
Induction of resistance for S. aureus was readily achieved by repeated passage with sub-lethal concentrations of the nonoxinol-9 iodine complex and chlorhexidine digluconate (concentration of each disinfectant below the MIC, where the isolates still show growth (12.5 and 25% for nonoxinol-9 iodine complex and chlorhexidine digluconate, respectively)). Nine strains of S. aureus (Table 1) showed a significant reduction in susceptibility to the nonoxinol-9 iodine complex while one strain showed a small reduction in susceptibility to this reagent. In contrast, susceptibility to chlorhexidine digluconate did not changed in any strain exposed to sub-lethal concentration of this compound except for strain F which showed a significant reduction in susceptibility (Table 2). The stability of acquired resistance was noticed in all S. aureus strains that had adapted to the nonoxinol-9 iodine complex and chlorhexidine digluconate. The percentages of S. aureus strains that developed stable resistance against the nonoxinol-9 iodine complex and chlorhexidine digluconate were 90 and 10%, respectively. It was impossible to increase resistance to chlorhexidine of most S. aureus strains via serial passage. Taken together, the results of this experiment support the hypothesis that prolonged exposure to commercial teat dips alters the germicidal susceptibility of S. aureus. The exception to this conclusion was that S. aureus did not exhibit enhanced tolerance of chlorhexidine digluconate similar to that observed with the nonoxinol-9 iodine complex.
Table 2.
The minimum inhibitory concentration (MIC) values of the Ujosan and Eimü chlorhexidine dips administered at sub-lethal concentrations before ten passages, after the tenth passage, and after the tenth stable passage of ten S. aureus strains
a: first test, b: second test, passage: passage of S. aureus with sub-lethal concentrations of the nonoxinol-9 iodine complex and chlorhexidine digluconate, stable passage: passage of S. aureus without active substances (nonoxinol-9 iodine complex and chlorhexidine digluconate).
Antimicrobial drug resistance of S. aureus strains and CNS
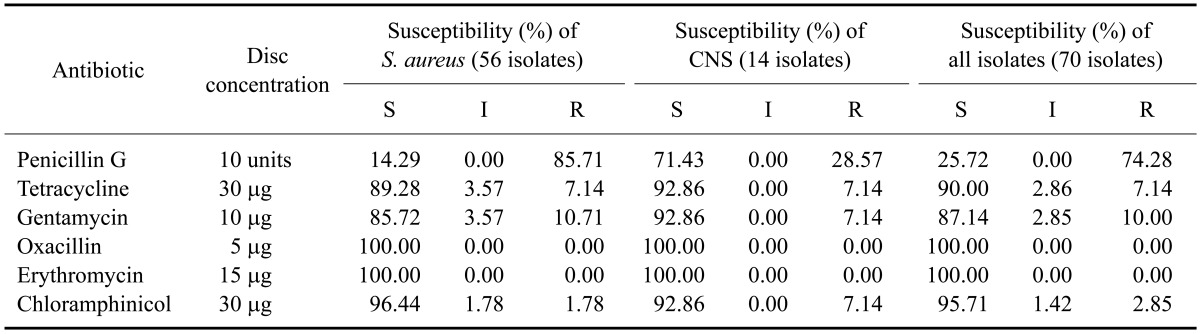
As shown in Table 3, the S. aureus isolates showed the highest level of in vitro resistance to penicillin G (85.72%) while that of CNS was lower (28.57%), In addition 7.14% of the S. aureus and CNS specimens were resistant to tetracycline while only 10.71% of S. aureus and 7.14% of CNS were resistant to gentamycin. The percentages of S. aureus and CNS isolates resistant to chloramphinicol were 1.78 and 7.14%, respectively. On the other hand, all staphylococci strains were susceptible to oxacillin and erythromycin.
Table 3.
Percentages of in vitro susceptibility to six selected antimicrobial agents for 70 Staphylococcus species obtained from subclinical cases of bovine mastitis
CNS: coagulae-negative Staphylococcus, S: susceptible, I: intermediate, R: resistant.
Cross-resistance to teat dips and antibiotics
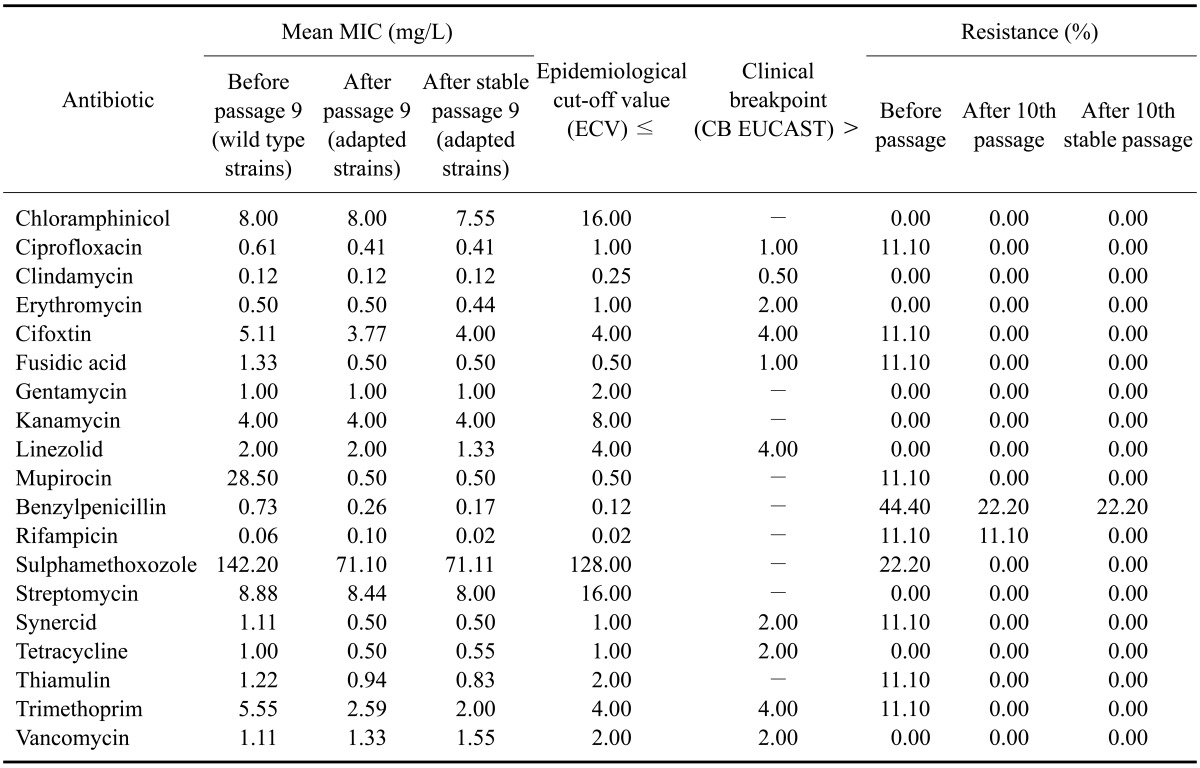
The antibiotic susceptibility rates of S. aureus isolated from cases of bovine mastitis according to the National Reference Laboratory for Antibiotic Resistance (Federal Institute for Risk Assessment, Germany) are shown in Table 4. All isolates showed the highest in vitro susceptibility to all types of antibiotics commercially available for treating bovine S. aureus mastitis. Resistance to antibiotics was observed only in a small number of strains. S. aureus F and H that were resistant to the nonoxinol-9 iodine complex also showed decreased susceptibility to penicillin G in a panel of different antimicrobial agents for which MIC values ranged from 0.25 to 2 mg/L. Additionally, no resistance to most antimicrobial agents was observed in the S. aureus isolates. Differences in MIC values did not affect the classifications of all strains, which were established according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint guidelines.
Table 4.
Mean MIC values (mg/L) of 19 commercial antimicrobial agents used to treat bovine mastitis for the wild type and Ujosan dip-resistant S. aureus strains
EUCAST: European Committee on Antimicrobial Susceptibility.
Discussion
The current strategy for controlling mastitis includes a combination of post-milking dipping and dry cow therapy associated with good veterinary practice of administrating antimicrobials to prevent or treat new infections on farms. However, this strategy is not always been fully successful. Some studies have stated that failure of these programs to control bovine mastitis can be partly attributed to teat disinfectants and/or antibiotics which do not afford sufficient protection against multiple pathogens that cause mastitis, particularly S. aureus and CNS [12,26]. Given that post-milking application of teat disinfectants and antibiotic therapy are very important tools for controlling bovine S. aureus mastitis, the current study concentrated on studying the induction of S. aureus resistance to different types of disinfectants as well as potential S. aureus cross-resistance to these teat dips and different types of antibiotics commonly used for treating bovine mastitis.
After measuring the susceptibility of S. aureus strains to commercial teat dips, it was observed that there were no significant differences between the quarters treated with nonoxinol-9 iodine complexes or chlorhexidine digluconate and the control group. This finding could be due to the fact that many of this pathogen has already been established in the udder before teat dipping [5,22]. It is not expected that post-milking teat dips would have any effect on previously established infections as reported by Whist et al. [39] who observed higher somatic cell counts in older cows with a greater prevalence of S. aureus dipped with iodine. The outcomes of the present study along with the effect of the nonoxinol-9 iodine complex and chlorhexidine digluconate on infected quarters need to be investigated further.
Induction of S. aureus resistance was readily achieved by repeated passage with sub-lethal concentrations of the nonoxinol-9 iodine complex and chlorhexidine digluconate. Exposure to relatively low concentrations of nonoxinol-9 iodine led to a high level of resistance within ten passages for most strains (90%). All strains were initially extremely sensitive to low concentration of the nonoxinol-9 iodine complex. Most subsequently acquired high levels of resistance after ten rounds of exposure to sub-lethal concentrations of the germicide. Thus, resistance to disinfecting agents induced by exposure to sub-lethal concentrations of the active compound led to significantly increased (p > 0.001) MIC values for the nonoxinol-9 iodine complex in most strains. However, such resistance to chlorhexidine digluconate was not observed except for one strain in which the MIC value significantly increased after the tenth stable passage.
Similar findings were reported in one study for a variety of biocides [25]. In addition, Gilbert et al. [11] reported a phenotypic change resulting in resistance to several unrelated compounds in vitro following exposure to a low concentration of a biocide. Moreover, Escalada et al. [8] observed decreased in growth rates in Escherichia coli and Pseudomonas aeruginosa following exposure to sub-lethal concentrations of the biocide triclosan, indicating the generation of stress in the organism. However, the results of our study were different from ones obtained by Hogan and Smith [13] who tested eight strains of S. aureus to determine if repeated in vitro exposure (15 times) to sub-lethal concentrations of four commercial teat dips could enhance bacterial tolerance. They found that the responses of S. aureus to chlorhexidine, sodium hypochlorite, and iodophor were not affected by prolonged exposure to these reagents. Inappropriate use of antimicrobial drugs as well as biocides in human and veterinary medicine (needless use, incorrect choice, low dosage, short contact, or irregular application) is primarily responsible for the emergence of many resistant bacteria species, including staphylococci [40].
The Scientific Committee on Emerging and Newly Identified Health Risks [32] reported that bacterial resistance arises from a mechanism causing decreases in intracellular concentrations of biocides below a threshold level that is harmful to the bacterium. McDonnell and Russell [19] also found that resistance is either a hereditary natural property of an organism, or acquired by mutation and acquisition of plasmids or transposons. However, although the mechanisms by which bacteria resist different types of antibiotics and biocides are still poorly defined, prolonged in vitro exposure to sub-lethal concentrations of antimicrobial agents undoubtedly contributes to resistance development. In the present study, acquired tolerance of S. aureus to the teat dips we tested was measured after the bacteria were subcultured 24 h in a media void of germicides and acquired stable tolerance of S. aureus to iodine and chlorhexidine was shown. The development of strains that retain resistance to a germicide in the absence of the compound is thought to be due to selection for or emergence of stable mutants.
At present, it is unknown which mechanisms contribute to the adaptive resistance observed in the strains examined in the present study. However, this resistance is likely due to the presence of active efflux. This process has gained increased recognition as a resistance mechanism over the past decade and received considerable attention in recent years [14]. Efflux pumps decrease the intracellular concentration of toxic compounds [23]. These pumps are an important mechanism by which bacteria can evade the effect(s) of antimicrobial agents. The role of efflux pumps in the development of bacterial resistance to biocides might be considered modest. This is because increased bacterial susceptibility to selected biocides as the results of efflux pump expression is usually measured as an increase in MIC values rather than resistance to high concentrations of an active ingredient. Efflux pumps have been shown to decrease the efficacy of numerous biocides including quaternary ammonium compounds (Qacs), phenolic parabens, and intercalating agents, most notably in S. aureus that express pumps such as QacA-D, QacG, and QacH [14].
Antimicrobial susceptibility of S. aureus isolated from bovine mastitis varies widely by region. In the present study, our results indicated that S. aureus isolates exhibited the highest degree of resistance to penicillin G (85.72%) whereas resistance to other antimicrobial agents was limited. This result was similar to those obtained by Shi et al. [34] who isolated 206 S. aureus strains from Inner Mongolia and found that most (87.30%) were resistant to penicillin. In contrast, Aarestrup and Jensen [1] recorded a low penicillin resistance rate (10%) in Denmark, Norway, and Sweden. In the rest of the Europe, the proportion of penicillin-resistant isolates was found to range from 23% up to 69% [21]. Large scale studies on antimicrobial resistance of up to 5,240 bovine S. aureus isolates per year were conducted as part of a national monitoring program in Germany during 1992~1997 and found that 38~57% of the isolates were resistant to penicillin [21,38].
The high rate penicillin resistance amongst S. aureus isolated from milk of cow mastitis is likely due to the wide use of intramammary preparations containing combinations of different antibiotics and broad-spectrum antimicrobials [24]. Numerous factors can influence the overall susceptibility patterns of mastitis pathogens. Scar tissue in the udders of cattle chronically infected by S. aureus is an important factor which prevents the penetration of antimicrobial agents [7]. Moreover, penicillin resistance can be due to the expression of inducible β-lactamase encoded by the blaZ gene, which causes hydrolysis of the β-lactam ring of penicillin [34]. The first report on the ability of S. aureus to metabolize penicillin were published in 1940, a year before the antimicrobial was introduced for therapeutic use [23]. Impaired treatment response is associated with penicillin resistance of infectious S. aureus strains. Jones et al. [15] noted over 30 years ago that S. aureus isolates have relatively high MIC values for penicillin and ampicillin, and attributed this to β-lactamase inhibition of the antimicrobial drugs. β-lactamase production is induced in some bacteria upon exposure to β-lactam drugs. The importance of prolonged β-lactamase-associated resistance in S. aureus was underscored by the Watts and Salmon [37] report of higher MIC values in isolates that produced this enzyme compared to microorganisms that did not. No evidence exists which suggests that this adaptation of S. aureus, or resistance to other classes of antibacterial drugs, is different from those noted 35 years ago. The MIC values and disk diffusion test results have demonstrated that ampicillin and penicillin are consistently the antimicrobial drugs to which S. aureus are most commonly resistant [37]. However, comparing values from one time period to another should be avoided. Any comparison of this kind should be done with caution because of differences in geography, the numbers of isolates evaluated, and inconsistencies in laboratory methods. For example, two different studies performed in the same year by Gentilini et al. [9] reported the proportion of oxacillin-resistant strains among S. aureus as 42 and 0%, respectively.
Cross-resistance of Ujosan dip (Kesla, Germany) and chlorhexidine digluconate-resistant S. aureus to a panel of antibiotics was investigated among 29 strains. When MIC values for the susceptible and adapted strains were determined by the microdilution method, S. aureus strains that had adapted to the Ujosan dip and chlorhexidine digluconate were found to be susceptible to all tested antibiotics. Differences in MIC values did not affect classifications of all strains, which were all susceptible to antimicrobial agents according to EUCAST breakpoint guidelines. Similar findings were reported by Jurgens et al. [16], Birošová and Mikulášová [2], and Cottell et al. [6]. However, our result was not in agreement with those obtained by Karatzas et al. [17] or Randall et al. [27]. They observed cross-resistance among bacterial species with reduced susceptibility to biocides and antibiotic resistance. There is no indication from these results suggesting that strains resistant to nonoxinol-9 iodine complexes and chlorhexidine digluconate are also resistant to antibiotics according to EUCAST. The association between biocide non-susceptibility and antibiotic resistance is still unclear. Several investigators were able to demonstrate cross-resistance between antibiotics and biocides. However, when cross-resistance was identified, it was often reported for second-line drugs or compounds not usually used for therapeutic purposes [17]. Moreover, nearly all of this research has been performed in the laboratory, and thus may not accurately reflect real world situations. These studies only examined antibiotic and biocide sensitivities in vitro. A lack of cross-resistance of the nonoxinol-9 iodine complex and chlorhexidine digluconate-resistant mutants to the antibiotics tested in this study suggested that the mechanisms underlying resistance to biocides are distinct from those affecting antibiotic resistance.
Although bacterial susceptibilities to antimicrobial drugs are well characterized, the relevance of changes in the MIC of an antiseptic is currently unknown. Even so, Rogers [28] noted that growing bacterial isolates in sub-lethal concentrations of biocide can induce changes in the profile of antibiotic susceptibility, especially if changes in biocide susceptibilities can be related to therapeutic levels of antibiotics. Multiple studies have suggested that an efflux mechanism is involved in biocide resistance [19,29]. Current knowledge of efflux mechanisms suggests that these pumps can utilize a variety of substrates, including antibiotics and biocides, and therefore may represent a problem in induction of resistance to different antimicrobial agents. There is incomplete understanding as to whether the use of biocides might give rise to resistance to currently used antibiotics, or prevent the development of new antibiotics. Clearly, more research is needed to characterize the relationship between biocide non-susceptibility and antibiotic resistance. Based on the results from the present study, resistance of S. aureus to chemical disinfectants may be more likely to develop if these compounds are used at concentrations lower than ones required for optimal biocidal effects. This reinforces the importance of always using disinfectants at the recommended concentrations and according to the manufacturer's directions.
References
- 1.Aarestrup FM, Jensen NE. Development of penicillin resistance among Staphylococcus aureus isolated from bovine mastitis in Denmark and other countries. Microb Drug Resist. 1998;4:247–256. doi: 10.1089/mdr.1998.4.247. [DOI] [PubMed] [Google Scholar]
- 2.Birosová L, Mikulášová M. Development of triclosan and antibiotic resistance in Salmonella enterica serovar Typhimurium. J Med Microbiol. 2009;58:436–441. doi: 10.1099/jmm.0.003657-0. [DOI] [PubMed] [Google Scholar]
- 3.Capurro A. Diagnostic and epidemiological studies of staphylococci in bovine mastitis. Uppsala: Swedish University of Agricultural Sciences; 2009. Doctoral dissertation. [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard. 8th ed. Wayne: Clinical and Laboratory Standards Institute; 2009. pp. 1–65. [Google Scholar]
- 5.Compton CWR, Heuer C, Parker K, McDougall S. Risk factors for peripartum mastitis in pasture-grazed dairy heifers. J Dairy Sci. 2007;90:4171–4180. doi: 10.3168/jds.2006-882. [DOI] [PubMed] [Google Scholar]
- 6.Cottell A, Denyer SP, Hanlon GW, Ochs D, Maillard JY. Triclosan-tolerant bacteria: changes in susceptibility to antibiotics. J Hosp Infect. 2009;72:71–76. doi: 10.1016/j.jhin.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 7.De Oliveira AP, Watts JL, Salmon SA, Aarestrup FM. Antimicrobial susceptibility of Staphylococcus aureus isolated from bovine mastitis in Europe and the United States. J Dairy Sci. 2000;83:855–862. doi: 10.3168/jds.S0022-0302(00)74949-6. [DOI] [PubMed] [Google Scholar]
- 8.Escalada MG, Harwood JL, Maillard JY, Ochs D. Triclosan inhibition of fatty acid synthesis and its effect on growth of Escherichia coli and Pseudomonas aeruginosa. J Antimicrob Chemother. 2005;55:879–882. doi: 10.1093/jac/dki123. [DOI] [PubMed] [Google Scholar]
- 9.Gentilini E, Denamiel G, Llorente P, Godaly S, Rebuelto M, DeGregorio O. Antimicrobial susceptibility of Staphylococcus aureus isolated from bovine mastitis in Argentina. J Dairy Sci. 2000;83:1224–1227. doi: 10.3168/jds.S0022-0302(00)74988-5. [DOI] [PubMed] [Google Scholar]
- 10.Ghorbanpoor M, Seyfi Abad Shapouri MR, Moatamedi H, Jamshidian M, Gooraninejad S. Comparison of PCR and bacterial culture methods for diagnosis of dairy cattle's subclinical mastitis caused by Staphylococcus aureus. J Vet Res. 2007;62:87–91. [Google Scholar]
- 11.Gilbert P, Allison DG, McBain AJ. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J Appl Microbiol. 2002;92(Suppl):98S–110S. [PubMed] [Google Scholar]
- 12.Gruet P, Maincent P, Berthelot X, Kaltsatos V. Bovine mastitis and intramammary drug delivery: review and perspectives. Adv Drug Deliv Rev. 2001;50:245–259. doi: 10.1016/s0169-409x(01)00160-0. [DOI] [PubMed] [Google Scholar]
- 13.Hogan JS, Smith KL. Prolonged in vitro exposure of Staphylococcus aureus to germicidal teat dips. J Dairy Sci. 1989;72:1052–1056. doi: 10.3168/jds.S0022-0302(89)79201-8. [DOI] [PubMed] [Google Scholar]
- 14.Huet AA, Raygada JL, Mendiratta K, Seo SM, Kaatz GW. Multidrug efflux pump overexpression in Staphylococcus aureus after single and multiple in vitro exposures to biocides and dyes. Microbiology. 2008;154:3144–3153. doi: 10.1099/mic.0.2008/021188-0. [DOI] [PubMed] [Google Scholar]
- 15.Jones A, Higgs TM, Neave FK, Smith A. The sensitivity of bovine staphylococci, streptococci and corynebacteria to cloxacillin and various other antibiotics. J Dairy Res. 1967;34:249–255. [Google Scholar]
- 16.Jurgens DJ, Sattar SA, Mah TF. Chloraminated drinking water does not generate bacterial resistance to antibiotics in Pseudomonas aeruginosa biofilms. Lett Appl Microbiol. 2008;46:562–567. doi: 10.1111/j.1472-765X.2008.02354.x. [DOI] [PubMed] [Google Scholar]
- 17.Karatzas KA, Webber MA, Jorgensen F, Woodward MJ, Piddock LJ, Humphrey TJ. Prolonged treatment of Salmonella enterica serovar Typhimurium with commercial disinfectants selects for multiple antibiotic resistance, increased efflux and reduced invasiveness. J Antimicrob Chemother. 2007;60:947–955. doi: 10.1093/jac/dkm314. [DOI] [PubMed] [Google Scholar]
- 18.Martineau F, Picard FJ, Lansac N, Ménard C, Roy PH, Ouellette M, Bergeron MG. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44:231–238. doi: 10.1128/aac.44.2.231-238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Mastitis Council. Summary of Peer-Reviewed Publications on Efficacy of Premilking and Postmilking Teat Disinfectants Published since 1980. Verona: National Mastitis Council; 2009. pp. 213–227. [Google Scholar]
- 21.Nunes SF, Bexiga R, Cavaco LM, Vilela CL. Technical note: antimicrobial susceptibility of Portuguese isolates of Staphylococcus aureus and Staphylococcus epidermidis in subclinical bovine mastitis. J Dairy Sci. 2007;90:3242–3246. doi: 10.3168/jds.2006-739. [DOI] [PubMed] [Google Scholar]
- 22.Oliver SP, King SH, Lewis MJ, Torre PM, Matthews KR, Dowlen HH. Efficacy of chlorhexidine as a postmilking teat disinfectant for the prevention of bovine mastitis during lactation. J Dairy Sci. 1990;73:2230–2235. doi: 10.3168/jds.S0022-0302(90)78903-5. [DOI] [PubMed] [Google Scholar]
- 23.Piddock LJV. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitkälä A, Haveri M, Pyörälä S, Myllys V, Honkanen-Buzalski T. Bovine mastitis in Finland 2001--prevalence, distribution of bacteria, and antimicrobial resistance. J Dairy Sci. 2004;87:2433–2441. doi: 10.3168/jds.S0022-0302(04)73366-4. [DOI] [PubMed] [Google Scholar]
- 25.Prince HN, Nonemaker WS, Norgard RC, Prince DL. Drug resistance studies with topical antiseptics. J Pharm Sci. 1978;67:1629–1631. doi: 10.1002/jps.2600671134. [DOI] [PubMed] [Google Scholar]
- 26.Pyörälä S, Taponen S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet Microbiol. 2009;134:3–8. doi: 10.1016/j.vetmic.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Randall LP, Cooles SW, Coldham NG, Penuela EG, Mott AC, Woodward MJ, Piddock LJ, Webber MA. Commonly used farm disinfectants can select for mutant Salmonella enterica serovar Typhimurium with decreased susceptibility to biocides and antibiotics without compromising virulence. J Antimicrob Chemother. 2007;60:1273–1280. doi: 10.1093/jac/dkm359. [DOI] [PubMed] [Google Scholar]
- 28.Rogers CK Non-prescription Drugs Advisory Committee, editors. Consumer Antiseptic Drug Products Review. Silver Spring: Food and Drug Administration; 2005. FDA reviewer's literature summary regarding potential environmental hazards of biocides; pp. 1–11. [Google Scholar]
- 29.Russell AD, Tattawasart U, Maillard JY, Furr JR. Possible link between bacterial resistance and use of antibiotics and biocides. Antimicrob Agents Chemother. 1998;42:2151. doi: 10.1128/aac.42.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saei HD, Ahmadi M, Mardani K, Batavani RA. Genotyping of Staphylococcus aureus isolated from bovine mastitis based on PCR-RFLP analysis of the aroA gene. Comp Clin Pathol. 2010;19:163–168. [Google Scholar]
- 31.Sauer S, Freiwald A, Maier T, Kube M, Reinhardt R, Kostrzewa M, Geider K. Classification and identification of bacteria by mass spectrometry and computational analysis. PLoS One. 2008;3:e2843. doi: 10.1371/journal.pone.0002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scientific Committee on Emerging and Newly Identified Health Risk. Assessment of the Antibiotic Resistance Effects of Biocides. Brussels: Scientific Committee on Emerging and Newly Identified Health Risk; 2009. pp. 1–87. [Google Scholar]
- 33.Sears PM, McCarthy KK. Management and treatment of staphylococcal mastitis. Vet Clin North Am Food Anim Pract. 2003;19:171–185. doi: 10.1016/s0749-0720(02)00079-8. [DOI] [PubMed] [Google Scholar]
- 34.Shi D, Hao Y, Zhang A, Wulan B, Fan X. Antimicrobial resistance of Staphylococcus aureus isolated from bovine mastitis in China. Transbound Emerg Dis. 2010;57:221–224. doi: 10.1111/j.1865-1682.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 35.Sutra L, Poutrel B. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J Med Microbiol. 1994;40:79–89. doi: 10.1099/00222615-40-2-79. [DOI] [PubMed] [Google Scholar]
- 36.Szumała A, Pernak J. The natural and laboratory resistance of gram-negative rods to quaternary ammonium chlorides with alkylthiomethyl radical. Pharmazie. 1986;41:521. [PubMed] [Google Scholar]
- 37.Watts JL, Salmon SA. Activity of selected antimicrobial agents against strains of Staphylococcus aureus isolated from bovine intramammary infections that produce β-lactamase. J Dairy Sci. 1997;80:788–791. doi: 10.3168/jds.S0022-0302(97)75999-X. [DOI] [PubMed] [Google Scholar]
- 38.Werckenthin C, Cardoso M, Martel JL, Schwarz S. Antimicrobial resistance in staphylococci from animals with particular reference to bovine Staphylococcus aureus, porcine Staphylococcus hyicus, and canine Staphylococcus intermedius. Vet Res. 2001;32:341–362. doi: 10.1051/vetres:2001129. [DOI] [PubMed] [Google Scholar]
- 39.Whist AC, Østerås O, Sølverød L. Staphylococcus aureus and Streptococcus dysgalactiae in Norwegian herds after introduction of selective dry cow therapy and teat dipping. J Dairy Res. 2007;74:1–8. doi: 10.1017/S0022029906002135. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz A, Kaleta EF. Suitability of two commercial preparations for disinfections against methicillin-resistant Staphylococcus aureus in veterinary medicine. Dtsch Tierarztl Wochenschr. 2009;116:180–185. [PubMed] [Google Scholar]