Abstract
The aim of this study was to demonstrate and assess C-reactive protein (CRP) changes in dogs with induced bacterial cystitis with or without antibiotics. We also evaluated availability of CRP levels to serve as an indicator for monitoring or diagnosing bacterial cystitis. Serial CRP concentrations in dogs with induced bacterial cystitis were higher than those of controls (p < 0.001). CRP concentrations peaked on day 7 and gradually decreased thereafter. In the treatment group, CRP concentrations decreased after medication compared to the untreated group (p = 0.032). CRP levels had a linear correlation with urine white blood cell counts among all groups (r = 0.837, p < 0.001, n = 140). Compared to the negative urine culture group, dogs with positive urine culture results had higher CRP concentrations (median 43.8 mg/L vs. 5.9 mg/L; p < 0.001). Area under the receiver operating characteristic curve was 0.955; when cut-off value was 12.2 mg/L, CRP measurements were found to have a sensitivity of 92.3% and specificity of 86.4%. This result indicates that rapid increases of CRP occurred after inducing bacterial cystitis and CRP may be a useful indicator for monitoring or diagnosing canine bacterial cystitis together with sediment urinalysis and urine bacterial culture.
Keywords: bacterial cystitis, C-reactive protein, dog, Proteus mirabilis, urine WBC
Introduction
A urinary tract infection (UTI) is the most common urinary tract disease in dogs and typically involves bacteria. Bacterial cystitis is the most frequently observed inflammatory disease affecting the urinary bladder (UB) of dogs and humans [1]. A diagnosis of UTI in dogs is based on urine bacterial culturing, urinalysis including sedimentation assessment [urine white blood cell (WBC) and urine red blood cell (RBC) counts], and clinical symptoms such as dysuria, hematuria, and pollakiuria. Although the presence of WBCs greater than five cells per high power field (HPF) (×400) and detection of bacteria in urine are indicative of infection, urine culturing is the gold standard for confirming cases of bacterial cystitis. However, failure to detect bacteria in urine sediment does not rule out urinary tract infection [2,16]. The results of urine culturing can be negative when antibiotic therapy has been initiated prior to urine collection or if handling of the urine sample during collection, preservation, or transportation is inadequate [25]. For treating bacterial cystitis in veterinary medicine, assessment of the medical history, clinical signs, serial urinalysis, and urine culturing are the best methods for monitoring the infection and determining whether to continue treatment [23]. Acute phase proteins (APPs) are a group of factors in blood whose concentrations change rapidly in response to infection and tissue damage [5]. C-reactive protein (CRP) is a major APP synthesized in the liver in response to tissue damage caused by infection, inflammation, or trauma. Thus, increases in CRP concentrations are indicative of the presence, extent, and severity of tissue injury [6].
In human medicine, CRP measurement is considered a sensitive method of screening febrile children for possible occult bacterial infections including UTI, bacteremia, meningitis, pneumonia, and septic arthritis [13,23,26]. Since UTI is the most common cause of serious occult bacterial infections in young children seen in emergency departments [29], researchers have attempted to investigate the use of CRP for diagnosing UTIs in children compared to radiologic, laboratory, and clinical findings such as dimercaptosuccinic acid, renal scintigraphy, WBC counts, erythrocyte sedimentation rates, and duration of fever [10,12]. In veterinary medicine, numerous authors have investigated the usefulness of canine serum CRP measurement as a tool for diagnosing and monitoring various diseases including immune-mediated hemolytic anemia [17], pyometra [8], chronic valvular disease [27], acute gastric mucosal injury [24], idiopathic polyarthritis [22], and surgical trauma [32]. However, alterations in CRP concentration have not been evaluated in cases of canine bacterial cystitis.
The objectives of this study were to assess differences of serum CRP concentration in dogs with experimentally induced bacterial cystitis compared to control animals, and measure changes in CRP concentration following antibiotic treatment. The use of serum CRP concentrations as an indicator for monitoring or diagnosing bacterial cystitis was evaluated based on the correlation between CRP concentration and urine WBC counts, and variation of CRP levels according to urine bacterial culture results. The sensitivity and specificity with optimal cut-off values from the receiver operating characteristic (ROC) curve were evaluated as well.
Materials and Methods
Dogs
Twenty 2- to 6-year old male beagles weighing 6.5~8.5 kg were used for our study. All animals were determined to be clinically healthy with no evidence of urinary tract infection or inflammation based on physical examination, complete blood count (CBC), serum biochemistry analysis, urinalysis, and urine culturing. The dogs were housed in individual cages, and were given commercial dry food twice a day and water ad libitum except for 24 h prior to general anesthesia. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Seoul National University (approval No. SNU-080821-1).
Experimental groups
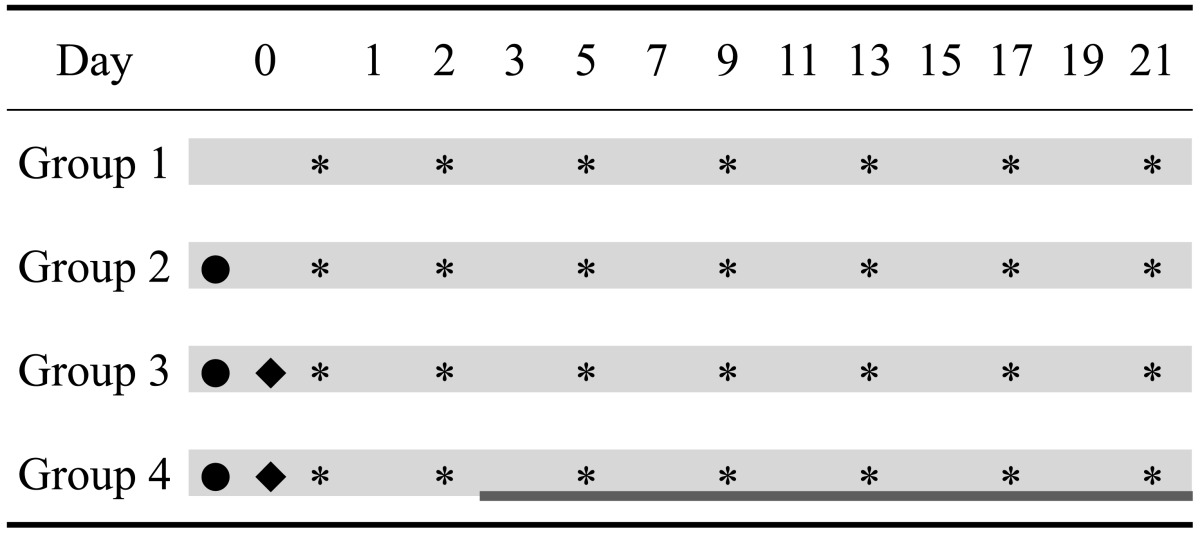
The twenty dogs were divided four groups of five animals each: Group 1, healthy controls; Group 2, UB irrigation only; Group 3, untreated bacterial cystitis (bacteria inoculation following UB irrigation); and Group 4, bacterial cystitis treated with antibiotics. Details of the study design and treatment schedules are presented in Table 1.
Table 1.
Study design and procedure schedule for the experiment
•: urinary bladder irrigation, ♦: bacterial infusion, ▓: antibiotic treatment, ░: blood sampling, *: urine sampling.
Proteus mirabilis and antimicrobial susceptibility test
Field isolates of Proteus (P.) mirabilis from the urine of dogs diagnosed with bacterial cystitis were identified based on characteristic swarming colony morphology, foul odor of blood agar cultures, and standard chemical tests (oxidase-negative, indole-negative, and ornithine decarboxylase-positive). Identity of the bacteria was confirmed with a VITEK II system (Bio Merieux Vitek, USA). Prior to inoculation of the experimental dogs with P. mirabilis, an antimicrobial susceptibility test was performed using an agar diffusion method (Sensi-Disc; Becton Dickinson, USA) with Mueller-Hinton agar (Hanil Komed, Korea).
Cystitis induction and subsequent treatment
Cystitis was induced using a previously established protocol [3,28]. Briefly, general anesthesia was induced with Zoletil 50 (0.075 mL/kg intravenous; Virbac Laboratories, France). The external genitalia were then prepared by clipping the hair and cleaning with a 0.1% organic iodine solution. Using a sterile urethral catheter, the UB was emptied and infused with 10 mL of a 0.1% solution of salicylic acid in ethanol. After 10 min, the infused solution was removed and the bladder was rinsed three times with 50 mL of a sterile saline (0.9% NaCl) solution. Subsequently, 2.5 mL PBS containing approximately 108 colony-forming units of P. mirabilis per mL that was isolated from the urine sample of cystitic patient and identified on the basis of characteristic swarming colony morphology, foul odor in blood agar, standard chemical tests (oxidase negative, indole negative, ornithine decarboxylase positive) and the VITEK II system (Bio Merieux Vitek, USA) were infused into the bladder. The catheter was flushed with 1 mL of a sterile saline solution and removed. The treatment group received amoxicillin-clavulanic acid (12.5 mg/kg, bid per oral) from day 3 (after blood collection) until day 21 according to the results of antimicrobial susceptibility test for P. mirabilis.
Serum collection and complete blood count
Food was withheld from the dogs for at least 6 h before samples of blood were collected to prevent postprandial lipemia. Blood samples were collected slowly from the jugular vein to prevent hemolysis into serum separating tubes (Vacutainer SST; Becton Dickinson, USA) for CRP measurement and EDTA tubes (Vacutainer EDTA; Becton Dickinson, USA) for complete blood counts on the sampling day. Serum samples were prepared by centrifugation (1,500 × g for 10 min) and stored in plain micro tubes (Eppendorf, Germany) at -20℃ until CRP analysis. Complete blood counts were performed with an automated blood count analyzer (Celltax Alpha MEK-6318K; Nihon Kohden, Japan) while differential counts and blood cell morphology evaluations were performed manually.
CRP measurement
Serum CRP concentrations in the dogs were measured using a commercial canine CRP enzyme-linked immunosorbent assay kit (BD Biosciences, USA). Serum samples were diluted (500-fold), 100 µL of sample were added to each well, and incubated for 30 min at room temperature. After washing four times with wash buffer, 100 µL of detection antibody/enzyme conjugate was then added and the plate was incubated for 30 min at room temperature. After washing the plate four times with provided wash buffer, 100 µL of a 3,3',5,5'-tetramethylbenzidine substrate solution was added to each well for the color reaction and incubated for 10 min. Phosphoric acid (100 µL) was added to stop the reaction and absorbance was read at 450 nm within 10 min using a microplate reader (Bio-Rad, USA). Canine CRP concentrations were determined according to the standard curve made with standard solutions (1.25~200 mg/L).
Urinalysis and urine bacterial culturing
The UB was examined with ultrasonography and urine samples were collected through a urethral catheter on days 0, 2, 5, 9, 13, 17, and 21. Proteins in the urine samples were measured with dipsticks (Combur10-Test M; Roche, Germany) and urine sediment was collected by centrifugation (47 × g for 5 min) after discarding supernatant. Urine WBC and RBC counts were determined based on the mean of the WBC counts in ten HPF (×400). Urine specimens were spread onto blood agar and MacConkey's agar plates (Becton Dickinson, USA) and incubated at 37° for 24 h. Dogs were considered to have an infection if the bacterial culture yielded >104 CFU/mL of urine [23].
Statistical analysis
Differences in CRP concentrations between Group 1 (control animals), Group 2 (UB irritation), and Group 3 (induced bacterial cystitis) were analyzed with a repeated measures analysis of variance (ANOVA) with a post hoc test (Scheffe's multiple comparison test) using SPSS 12.0K for Windows (SPSS, USA). Differences in CRP concentrations between Group 3 and Group 4 (induced bacterial cystitis plus antibiotic medication) were evaluated with a repeated measures ANOVA. CRP concentrations before and after medication in Group 4 were compared with a paired t-test. The correlations among CRP concentration, urine WBC counts, urine RBC counts, and urine protein levels were tested with Pearson's and Spearman's correlation coefficients. A Mann-Whitney test was used to compare the CRP distribution in dogs with the bacteria-positive urine cultures to those with negative urine culture results (absence of bacteria). Data were expressed as the mean ± SD or median (range), and p-values <0.05 were considered to be statistically significant.
Results
All experimental dogs were alive during the study period. Aside from pollakiuria, stranguria, and hematuria, no other clinical symptoms were noticed. Changes in blood WBC counts, band neutrophils, and body temperature did not significantly differ among the four groups during the experimental period (data not shown).
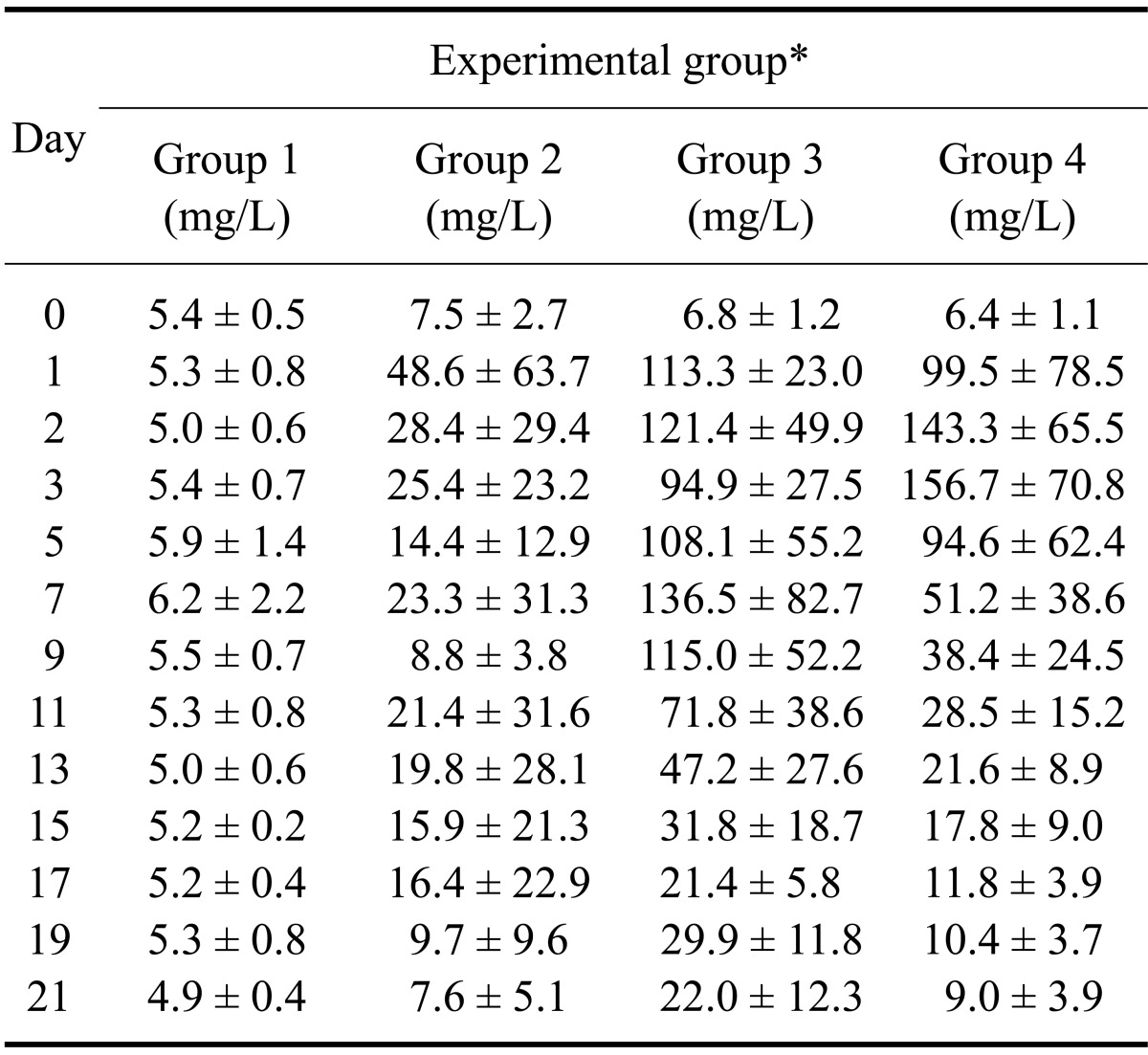
No remarkable change in CRP levels was observed in Group 1 (Table 2). Although Group 2 had a slightly higher CRP concentration than Group 1 during the experiment period, no significance difference was found (p = 0.376). On the other hand, CRP concentrations of Group 3 increased immediately from day 1, peaked on day 7 (136.5 ± 82.7 mg/L), and then decreased slowly from day 13 to day 21 (22.0 ± 12.3 mg/L). Serially measured CRP concentrations of Group 3 significantly differed from Group 1 (p < 0.001) and Group 2 (p = 0.001) throughout the entire experiment.
Table 2.
Mean serum C-reactive protein concentrations of the four experimental groups
*Group 1: control, Group 2: urinary bladder irrigation, Group 3: bacterial cystitis without treatment, Group 4: bacterial cystitis with treatment. Data are expressed as the mean ± SD.
In Group 4, serum CRP concentrations increased in a manner similar those of Group 3. After administrating the antibiotic medication (day 3), CRP concentrations decreased rapidly from day 5 until the end of the experiment. CRP concentrations significantly differed (p = 0.032) between Group 3 and Group 4 during the treatment period (from day 5 to day 21). After treatment with antibiotics, CRP values significantly decreased from day 5 compared to pretreatment (day 3) levels (p = 0.017) in Group 4. On the other hand, CRP values for Group 3 (untreated group) did not decreased significantly until day 11 (p = 0.245) and gradually decreased thereafter.
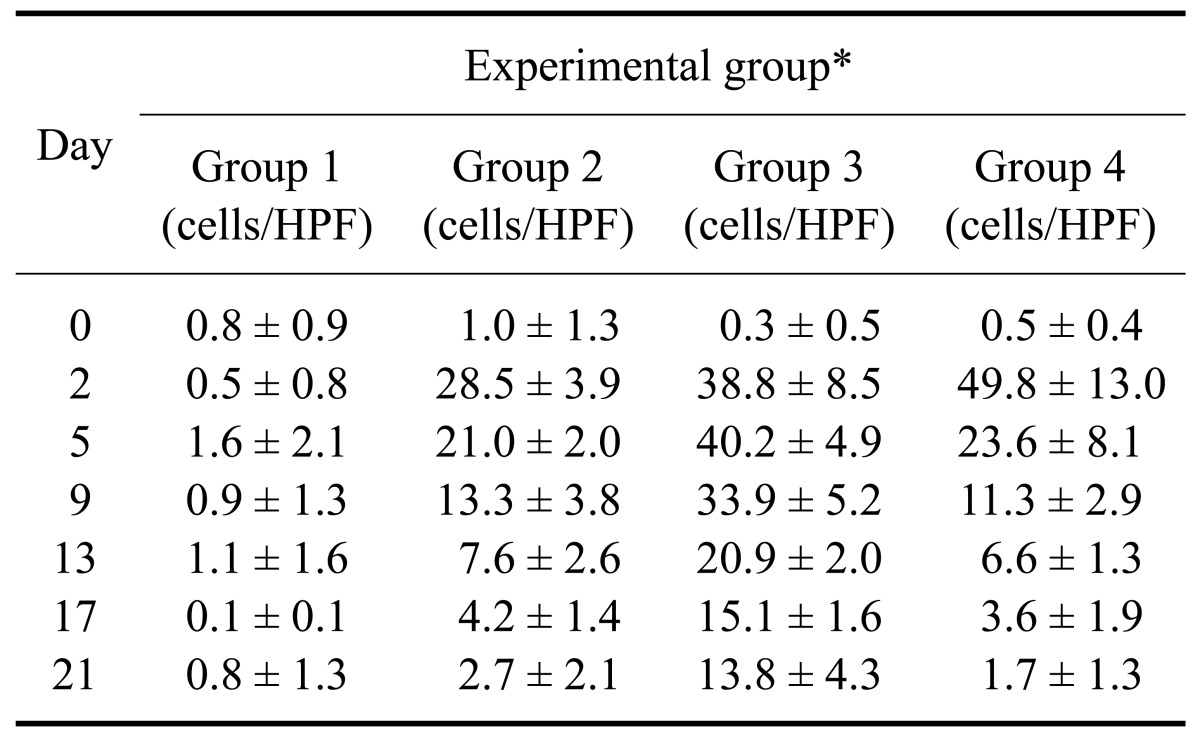
Urine WBC counts, urine RBC counts, and urine culture results are shown in Table 3 and Fig. 1. A correlation was found between CRP concentration and urine WBC counts (r = 0.837, p < 0.001, n = 140), urine RBC counts (r = 0.692, p < 0.001, n = 140), and urine protein levels (r = 0.565, p < 0.001, n = 140). The urine WBC counts for Group 4 decreased from 49.8 ± 13.0 cells/HPF on day 2 (prior to administration of the antibiotics) to 11.3 ± 2.9 cells/HPF on day 9, and then decreased gradually to 1.7 ± 1.3 cells/HPF. In contrast, urine WBC counts for Group 3 did not change significantly until day 9 (p = 0.204) before gradually decreasing.
Table 3.
Mean urine white blood cell and red blood cell counts for the four experimental groups
*Group 1: control, Group 2: urinary bladder irrigation, Group 3: bacterial cystitis without treatment, Group 4: bacterial cystitis with treatment. HPF: high power field (×400). Data are expressed as the mean ± SD.
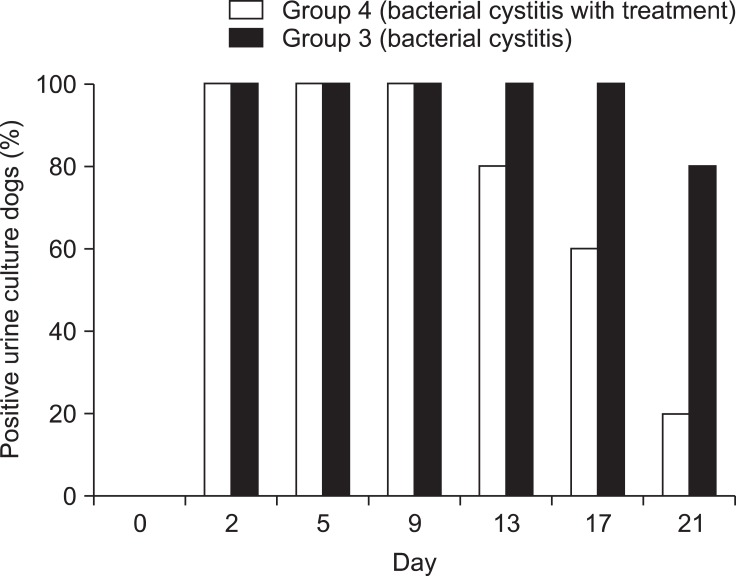
Fig. 1.
Positive urine culture results of group 3 and group 4. Note that negative results appeared on day 13 after 10 days of antibiotic treatment in group 4.
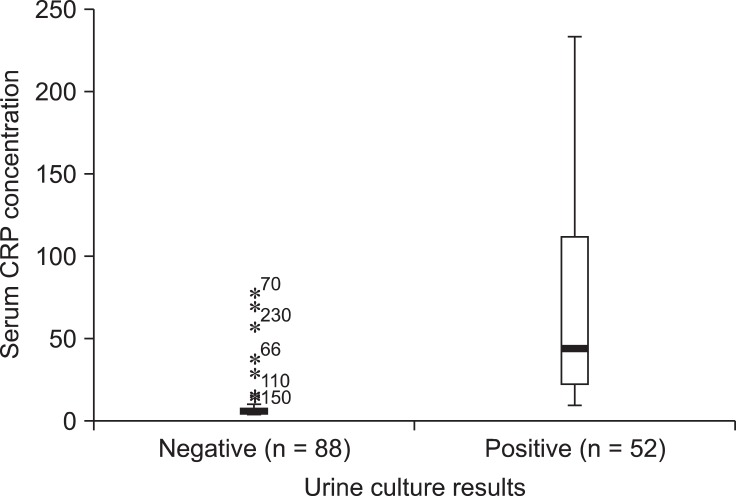
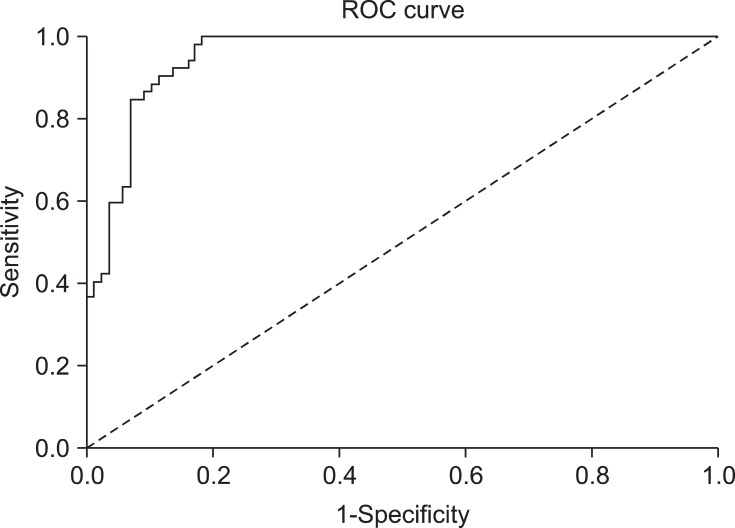
Compared to animals with negative urine culture results (Fig. 2), dogs with positive urine cultures had higher serum CRP concentrations [median 43.8 mg/L (range: 9.4~33.4 mg/L) vs. 5.9 mg/L (4.2~76.5 mg/L); p < 0.001]. The area under the ROC curve was 0.955 for CRP measurement (Fig. 3). If the cut-off value was 12.2 mg/L, CRP measurement had a sensitivity of 92.3%, specificity of 86.4%, positive predictive value of 80%, and negative predictive value of 94%. The 95% confidence intervals for CRP concentrations were 54.1~87.5 mg/L for the dogs with positive urine cultures and 7.1~12.4 mg/L for dogs with negative urine cultures.
Fig. 2.
Serum C-reactive protein (CRP) distribution in the negative and positive urine culture group. There was little overlap between two groups. The median values of each group were 43.8 mg/L (range: 9.4~233.4 mg/L) vs. 5.9 mg/L (range: 4.2~76.5 mg/L) respectively.
Fig. 3.
Receiver operating characteristic (ROC) curve for the CRP measurement. The area under the curve was 0.955. Considering 12.2 mg/L of cut-off value, CRP measurement had sensitivity of 92.3%, specificity of 86.4%, positive predictive value of 80%, and negative predictive value of 94%.
Discussion
The levels of CRP, a major acute phase protein, have no specificity for disease but levels of this protein increase in the presence of inflammation or tissue damage [11]. CRP has been reported to be a suitable marker of inflammation in dogs [6], and its usefulness for the early diagnosis and therapeutic monitoring of various diseases has been evaluated in veterinary medicine [7,9,21,22,24]. In humans, CRP concentrations have been shown to be elevated in patients with urinary tract infections as an acute phase response [15,20]. There has been no report about changes in CRP concentration associated with UTIs including cystitis in veterinary medicine. The present study is the first to evaluate changes of serum CRP concentration in dogs with experimentally induced bacterial cystitis. We measured serial CRP concentrations in canines suffering from cystitis along with healthy controls. Results from the infected dogs that did not receive treatment were compared to those of the antibiotic treatment group to monitor changes in CRP concentration according to antibiotic administration. The use of serum CRP concentration as an indicator for monitoring and diagnosing bacterial cystitis was also evaluated.
Acute increases of CRP levels in dogs with induced bacterial cystitis (Group 3) were higher than those of the tissue damage control group (Group 2), suggesting that CRP is produced rapidly in response to not only tissue damage but also the presence of bacteria in the UB. This could be explained by the fact that bacterial infection activates a diverse number of cytokines, including interleukin (IL)-6 which primarily stimulates the synthesis of CRP in the liver, and has a role in CRP activation of the classical complement system needed for initiating the opsonization, phagocytosis, and lysis of invading microorganisms [18,33]. In cases of infection with Gram-negative bacteria expressing lipopolysaccharides, CRP may play an important role in protecting tissue and limiting inflammatory responses by interacting with FcγR, leading to production of the anti-inflammatory cytokine IL-10 and down-regulation of IL-12 [19].
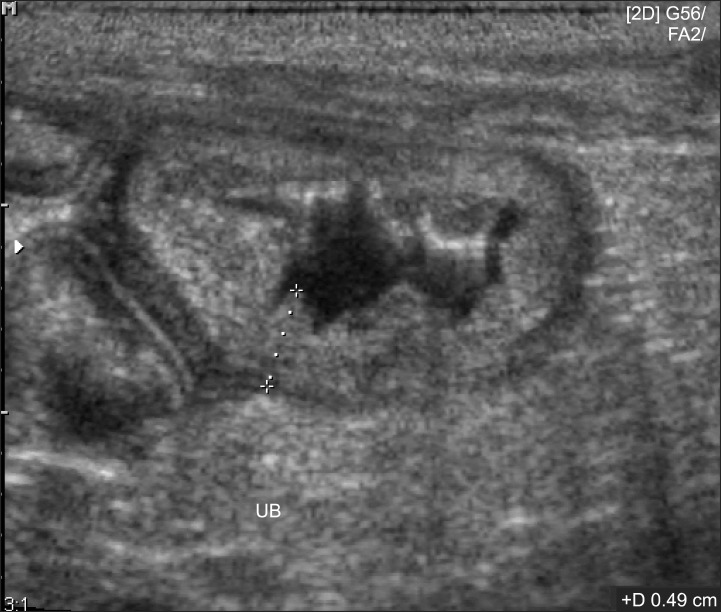
Mildly increased CRP levels in Group 3 observed at the end of the experiment may have been caused by chronic bacterial cystitis. Moderate to severe changes in the UB walls were observed by ultrasonography after cystitis induction. These morphology of the UB walls was almost normal at the end of experiment although a biopsy was not performed (Fig. 4). In one study of experimentally induced canine cystitis, some dogs spontaneously recovered in 3.5 weeks without treatment [31]. Therefore, mildly increased CRP concentrations may be observed in cases of chronic bacterial cystitis that are commonly encountered in veterinary clinics. Mildly increased CRP levels are associated with other chronic diseases in dogs such as chronic valvular disease [median 2.17 mg/L (range: 0.86~3.8 mg/L)] and inflammatory bowel disease (15.33 ± 4.85 mg/L) as well [14,27]. Compared to the levels observed before treatment, CRP concentrations in Group 4 decreased rapidly and significantly starting on day 3 (the time at which antibiotic treatment was initiated) until the end of the experiment. In contrast, CRP concentrations in Group 3 decreased significantly starting on day 13 relative to the initial level.
Fig. 4.
Ultrasonographic appearance of urinary bladder (UB) wall of group 3 (no treatment) on day 21. Even though the dog had bacterial cystitis, the morphology of UB wall was mildly thickened compared to dogs without cystitis.
Changes in urine WBC counts and urine bacterial culturing results showed similar patterns. These findings indicate that CRP values could be used to monitor early responses to antibiotic administration in our model of induced bacterial cystitis. Inflammation in the UB of dogs is generally not accompanied by changes in CBC or other serum parameters [23]. According to another previous report, blood WBC counts may also vary depending upon the disorder and severity of inflammation [32]. In the current study, the relationship between blood WBC count and CRP concentration was found to be significant (r = 0.382); however, the correlation coefficient was low and differences in blood WBC counts among the four groups were not significant (p = 0.499). Burton et al. [4] reported a low correlation coefficient (r = 0.34) between CRP concentration and band neutrophils in dogs following surgery. In a study of CRP levels and WBC counts in cases of canine polyarthritis, the levels of CRP were found to rapidly decrease in response to treatment whereas the WBC counts did not [22]. This finding suggests that blood WBC counts cannot serve as a reliable indicator of bacterial cystitis severity or predict responses to antibiotic therapy. However, serial CRP levels could be used to monitor early responses to antibiotic treatment and might alert veterinarians to the need for further evaluation or additional treatment.
Considering the linear relationships between serum CRP levels and urine WBC counts (r = 0.837), urine RBC counts (r = 0.692), and urine protein levels (r = 0.565), serum CRP concentrations provide useful information about the severity of inflammation inside the UB. These correlations suggest that CRP concentrations can represent a safe, convenient, and alternative method for evaluating the status of bacterial cystitis. This would eliminate the need for repeated cystocentesis and catheterization that can directly damage the bladder wall and urethra or result in infection via the leaking of urine containing bacteria [30].
Urine bacterial culturing is regarded as the gold standard for diagnosing urinary tract bacterial infections. There are currently no specific or effective methods that can replace bacterial culturing [23]. However, bacterial culture results can be affected by various factors such as previous antibiotic administration, low urine collection volume after voiding, contamination caused by inappropriate collection procedures, and false negative results due to poor sample handling. Given the high sensitivity, specificity, and positive/negative predictive values observed in our experimental model, serum CRP concentrations may be a useful indirect parameter for predicting the progression of bacterial cystitis alone or in addition to urine bacterial culturing, especially when repeated urine collection and/or bacterial culturing is not practical.
Despite our encouraging results, the present study was limited by the fact that the pattern and severity of inflammation in cases of spontaneous bacterial cystitis can differ from those observed in our model of experimental inflammation. As previously mentioned, incidents of bacterial cystitis encountered in the clinic may be chronic and relatively difficult to detect. Therefore, further investigations of these types of cases are needed.
In conclusion, the results of our study demonstrated that changes of serum CRP levels in dogs with experimentally induced bacterial cystitis could be useful for detecting inflammation. These changes could also help monitor the responses to treatment with antibiotics during the early stages of bacterial cystitis although more studies are needed prior to application of this technique in veterinary practice. Considering the limitations of CRP as a sensitive and nonspecific protein, clinicians should serially measure the levels of this protein in conjunction with assessing clinical signs and the results of other biological tests when interpreting the significance of CRP levels.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea funded by the Korean Government (No. 2011-0000226). The authors also thank the Research Institute for Veterinary Science, College of Veterinary Science and BK21 Program for Veterinary Science, Seoul National University, Korea.
References
- 1.Barsanti JA. Genitourinary infections. In: Green CE, editor. Infectious Disease of the Dogs and Cat. 2nd ed. Philadelphia: Saunders; 1998. pp. 626–645. [Google Scholar]
- 2.Bartges JW. Urinary tract infections. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 6th ed. St. Louis: Elsvier Saunders; 2005. pp. 1800–1808. [Google Scholar]
- 3.Bowles MH, Welsh RD, Hoffman J, Turnwald GH. Evaluation of a method using Proteus mirabilis and Pseudomonas aeruginosa to experimentally induce dual infection of the urinary bladder in dogs. Am J Vet Res. 2000;61:1484–1486. doi: 10.2460/ajvr.2000.61.1484. [DOI] [PubMed] [Google Scholar]
- 4.Burton SA, Honor DJ, Mackenzie AL, Eckersall PD, Markham RJ, Horney BS. C-reactive protein concentration in dogs with inflammatory leukograms. Am J Vet Res. 1994;55:613–618. [PubMed] [Google Scholar]
- 5.Canova CR, Courtin C, Reinhart WH. C-reactive protein (CRP) in cerebro-vascular events. Atherosclerosis. 1999;147:49–53. doi: 10.1016/s0021-9150(99)00162-8. [DOI] [PubMed] [Google Scholar]
- 6.Cerón JJ, Eckersall PD, Martínez-Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol. 2005;34:85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 7.Conner JG, Eckersall PD, Ferguson J, Douglas TA. Acute phase response in the dog following surgical trauma. Res Vet Sci. 1988;45:107–110. [PubMed] [Google Scholar]
- 8.Dąbrowski R, Wawron W, Kostro K. Changes in CRP, SAA and haptoglobin produced in response to ovariohysterectomy in healthy bitches and those with pyometra. Theriogenology. 2007;67:321–327. doi: 10.1016/j.theriogenology.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Fransson BA, Karlstam E, Bergstrom A, Lagerstedt AS, Park JS, Evans MA, Ragle CA. C-reactive protein in the differentiation of pyometra from cystic endometrial hyperplasia/mucometra in dogs. J Am Anim Hosp Assoc. 2004;40:391–399. doi: 10.5326/0400391. [DOI] [PubMed] [Google Scholar]
- 10.Garin EH, Olavarria F, Araya C, Broussain M, Barrera C, Young L. Diagnostic significance of clinical and laboratory findings to localize site of urinary infection. Pediatr Nephrol. 2007;22:1002–1006. doi: 10.1007/s00467-007-0465-7. [DOI] [PubMed] [Google Scholar]
- 11.Gewurz H, Mold C, Siegel J, Fiedel B. C-reactive protein and the acute phase response. Adv Intern Med. 1982;27:345–372. [PubMed] [Google Scholar]
- 12.Huang DT, Huang FY, Tsai TC, Tsai JD, Chiu NC, Lin CC. Clinical differentiation of acute pyelonephritis from lower urinary tract infection in children. J Microbiol Immunol Infect. 2007;40:513–517. [PubMed] [Google Scholar]
- 13.Isaacman DJ, Burke BL. Utility of the serum C-reactive protein for detection of occult bacterial infection in children. Arch Pediatr Adolesc Med. 2002;156:905–909. doi: 10.1001/archpedi.156.9.905. [DOI] [PubMed] [Google Scholar]
- 14.Jergens AE, Schreiner CA, Frank DE, Niyo Y, Ahrens FE, Eckersall PD, Benson TJ, Evans R. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291–297. doi: 10.1111/j.1939-1676.2003.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 15.Jodal U, Lindberg U, Lincoln K. Level diagnosis of symptomatic urinary tract infections in childhood. Acta Paediatr Scand. 1975;64:201–208. doi: 10.1111/j.1651-2227.1975.tb03822.x. [DOI] [PubMed] [Google Scholar]
- 16.Lees GE. Bacterial urinary tract infections. Vet Clin North Am Small Anim Pract. 1996;26:297–304. [PubMed] [Google Scholar]
- 17.Mitchell KD, Kruth SA, Wood RD, Jefferson B. Serum acute phase protein concentrations in dogs with autoimmune hemolytic anemia. J Vet Intern Med. 2009;23:585–591. doi: 10.1111/j.1939-1676.2009.0282.x. [DOI] [PubMed] [Google Scholar]
- 18.Mold C, Du Clos TW, Nakayama S, Edwards KM, Gewurz H. C-reactive protein reactivity with complement and effects on phagocytosis. Ann N Y Acad Sci. 1982;389:251–262. doi: 10.1111/j.1749-6632.1982.tb22141.x. [DOI] [PubMed] [Google Scholar]
- 19.Mold C, Rodriguez W, Rodic-Polic B, Du Clos TW. C-reactive protein mediates protection from lipopolysaccharide through interactions with FcγR. J Immunol. 2002;169:7019–7025. doi: 10.4049/jimmunol.169.12.7019. [DOI] [PubMed] [Google Scholar]
- 20.Naseri M. Alterations of peripheral leukocyte count, erythrocyte sedimentation rate, and C-reactive protein in febrile urinary tract infection. Iran J Kidney Dis. 2008;2:137–142. [PubMed] [Google Scholar]
- 21.Nielsen L, Toft N, Eckersall PD, Mellor DJ, Morris JS. Serum C-reactive protein concentration as an indicator of remission status in dogs with multicentric lymphoma. J Vet Intern Med. 2007;21:1231–1236. doi: 10.1892/07-058.1. [DOI] [PubMed] [Google Scholar]
- 22.Ohno K, Yokoyama Y, Nakashima K, Setoguchi A, Fujino Y, Tsujimoto H. C-reactive protein concentration in canine idiopathic polyarthritis. J Vet Med Sci. 2006;68:1275–1279. doi: 10.1292/jvms.68.1275. [DOI] [PubMed] [Google Scholar]
- 23.Osborne CA, Lees GE. Bacterial infections of the canine and feline urinary tract. In: Osborne CA, Finco DR, editors. Canine and Feline Nephrology and Urology. 1st ed. Baltimore: Williams & Wilkins; 1995. pp. 759–797. [Google Scholar]
- 24.Otabe K, Ito T, Sugimoto T, Yamamoto S. C-reactive protein (CRP) measurement in canine serum following experimentally-induced acute gastric mucosal injury. Lab Anim. 2000;34:434–438. doi: 10.1258/002367700780387679. [DOI] [PubMed] [Google Scholar]
- 25.Padilla J, Osborne CA, Ward GE. Effects of storage time and temperature on quantitative culture of canine urine. J Am Vet Med Assoc. 1981;178:1077–1081. [PubMed] [Google Scholar]
- 26.Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics. 2001;108:1275–1279. doi: 10.1542/peds.108.6.1275. [DOI] [PubMed] [Google Scholar]
- 27.Rush JE, Lee ND, Freeman LM, Brewer B. C-reactive protein concentration in dogs with chronic valvular disease. J Vet Intern Med. 2006;20:635–639. doi: 10.1892/0891-6640(2006)20[635:cpcidw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Senior DF, Gaskin JM, Buergelt CD, Harvey JW, Keefe TJ. Amoxicillin and clavulanic acid combination in the treatment of experimentally induced bacterial cystitis in dogs. J Am Anim Hosp Assoc. 1986;22:227–233. [Google Scholar]
- 29.Shaw KN, Gorelick M, McGowan KL, Yakscoe NM, Schwartz JS. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998;102:e16. doi: 10.1542/peds.102.2.e16. [DOI] [PubMed] [Google Scholar]
- 30.Specht A, Chan D, O'Toole T, Kent M, Benson J, Rozanski EA, Rush JE. Acute staphylococcal peritonitis following cystocentesis in a dog. J Vet Emerg Crit Care. 2002;12:183–187. [Google Scholar]
- 31.Turnwald GH, Gossett KA, Cox HU, Kearney MT, Roy AF, Thomas DE, Troy GC. Comparison of single-dose and conventional trimethoprim-sulfadiazine therapy in experimental Staphylococcus intermedius cystitis in the female dog. Am J Vet Res. 1986;47:2621–2623. [PubMed] [Google Scholar]
- 32.Yamamoto S, Shida T, Miyaji S, Santsuka H, Fujise H, Mukawa K, Furukawa E, Nagae T, Naiki M. Changes in serum C-reactive protein levels in dogs with various disorders and surgical traumas. Vet Res Commun. 1993;17:85–93. doi: 10.1007/BF01839236. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita K, Fujinaga T, Miyamoto T, Hagio M, Izumisawa Y, Kotani T. Canine acute phase response: relationship between serum cytokine activity and acute phase protein in dogs. J Vet Med Sci. 1994;56:487–492. doi: 10.1292/jvms.56.487. [DOI] [PubMed] [Google Scholar]