Abstract
The purpose of this study was to evaluate the effects of a topical spray containing 0.0584% hydrocortisone aceponate (HCA) on canine atopic dermatitis (CAD) and to evaluate the skin barrier function during the treatment of CAD. Twenty-one dogs that fulfilled the diagnostic criteria for CAD were included in this study. The HCA spray was applied once a day to the lesions of all dogs for 7 or 14 days. Clinical assessment was performed before (day 0) and after treatment (day 14), and clinical responses were correlated with changes in skin barrier function. CAD severity significantly decreased after 14 days of HCA treatment based on the lesion scores (p < 0.0001), which were determined using the CAD extent and severity index (CADESI-03) and pruritus scores (p < 0.0001) calculated using a pruritus visual analog scale. Transepidermal water loss, a biomarker of skin barrier function, was significantly reduced compared to baseline (day 0) measurements (p = 0.0011). HCA spray was shown to be effective for significantly improving the condition of dogs suffering from CAD. This treatment also significantly improved cutaneous hydration and skin barrier function in the animals.
Keywords: atopic dermatitis, canine, hydrocortisone aceponate, transepidermal water loss
Introduction
Canine atopic dermatitis (CAD) is a chronic pruritic skin disorder with characteristic clinical features associated with the production of IgE antibodies against environmental allergens [10,16]. The pathogenesis of CAD is not fully understood but this is a complex, multifactorial disease involving interactions between environmental factors, immunologic abnormalities, and skin barrier defects [7,15,19]. Treatment options include allergen avoidance, allergen-specific immunotherapy, topical and systemic treatments such as administration of glucocorticoids, cyclosporine, anti-histamines, or essential fatty acids, and management of secondary bacterial or yeast infections [23,25,27].
Glucocorticoids are the most commonly used drugs for treating a variety of inflammatory skin diseases including CAD [28]. However, the adverse effects of glucocorticoids such as skin atrophy and iatrogenic Cushing's syndrome has limited the use of these compounds [2]. Topical glucocorticoids are an attractive treatment option since the drug can be directly absorbed at the site of inflammation, thereby avoiding systemic exposure. However, this mode of application is not recommended in veterinary dermatology due to a lack of penetration into the skin through the hair coat and concerns regarding the ingestion of the product [28,35].
Although ideal topical glucocorticoids with high potency and a low risk of side effects have not yet been synthesized, esterification to a hydrocortisone template molecule was performed to produce new glucocorticoid products with significant anti-inflammatory effects and minimal adverse effects [5,32]. Diester molecules can rapidly penetrate the stratum corneum and undergo metabolism within the dermis, thus mitigating local and systemic side effects [12]. These compounds are widely used for treating atopic dermatitis and other forms of eczema in humans [3,30]. Recently, a new generation of topical glucocorticoids with excellent pharmacological activities and low benefit-risk ratios was developed for use in veterinary dermatology [4,20]. The purpose of this study was to evaluate the effects of a topical diester formula spray containing 0.0584% hydrocortisone aceponate (HCA) on canines with CAD, and to evaluate skin barrier function during the treatment of CAD by measuring transepidermal water loss (TEWL).
Materials and Methods
Study population
The CAD dogs used in the study were recruited from the Seoul National University Hospital for Animals (Korea) between June 2009 and April 2010. CAD was diagnosed based on the animal's history, clinical findings, positive results of a serum allergen-specific IgE test and/or intradermal skin test, and exclusion of ectoparasites and skin infections. A food elimination trial using a commercial single protein diet (Natural Balance Potato and Duck formula; Natural Balance Pet Foods, USA) or a commercial hydrolyzed hypoallergenic diet (Royal Canin Hypoallergenic; Royal Canin, France) for a minimum of 6 weeks was performed to rule out adverse food reactions, and dogs with a partial or complete resolution of clinical signs while being fed the diet were excluded.
Study protocol
The 0.0584% HCA spray (Cortavance; Virbac, France) was applied to all atopic dogs once a day for 7 or 14 days. The spray was administered according to the manufacturer's instruction. The owners applied two pumps of the spray over the severe skin lesions (a surface area of 100 cm2) from a distance of 10 cm. A clinician (C.Y. Hwang, Seoul National University, Korea) conducted an assessment of the animals on days 0 and 14 as described below.
Lesion scores
To estimate the clinical severity of the lesions at the spray application sites, the clinician used a modified lesion scoring system based on the CAD extent and severity index (CADESI-03) [26]. This approach evaluated four clinical factors (erythema, excoriation, lichenification, and self-induced alopecia) that were graded from 0 (normal) to 5 (most severe), thereby yielding a score of 0~20 for each body site examined.
Pruritus scores
The owners were asked to evaluate the degree of their dog's pruritus using a pruritus visual analog scale [31]. This scale consisted of an 11-point linear scale with which the condition of the animals was graded from 0 (not itchy: no scratching, chewing, rubbing, or licking observed) to 10 (extremely itchy: scratching, chewing, rubbing, or licking constantly).
TEWL assessment
TEWL, a marker of epidermal barrier function, was measured in lesions of the CAD dogs using a VapoMeter (Delfin Technologies, Finland), a hand-held, closed chamber evaporimeter, according to the manufacturer's instructions. All dogs were allowed to acclimate to an environmentally controlled room maintained at 20~22℃ with 40~60% relative humidity for at least 10 min prior to testing. TEWL was measured three times at the spray application sites [21,22].
Assessment of the HCA spray safety
Clinicians (C.Y. Hwang and E.H. Nam, Seoul National University, Korea) evaluated any adverse events by performing thorough clinical examinations at each visit. On days 0, 14, and 28 of the study, blood samples for complete blood cell count and biochemical analysis of hepatic enzyme activities were taken, and adrenocorticotrophic hormone (ACTH) stimulation tests were performed. Briefly, serum was collected for cortisol assay before and 1 h after administration of synthetic ACTH 0.25 mg/dog, IV (Synacthen; Alliance Pharmaceuticals, UK).
Statistical analysis
Results of the clinical evaluation (lesion score, pruritus score, and TEWL findings) were compared to the data obtained before treatment. Statistical comparisons were performed using a paired Student's t-test and Wilcoxon signed rank test depending on the validity of the assumptions. All data were analyzed using the SPSS statistical software package (ver. 12.0; SPSS, USA). Data are presented as the mean ± SD. p-values < 0.05 were considered statistically significant.
Results
Twenty-one dogs with CAD were enrolled in this study (Table 1). The study group included three intact males, six castrated males, six intact females, and six spayed females. The mean age of the animals was 8.5 years (range: 5~13 years). The breeds of the dogs were Shih Tzu (n = 10), mixed (n = 3), Maltese (n = 2), Yorkshire Terrier (n = 1), Cocker Spaniel (n = 1), Beagle (n = 1), Pomeranian (n = 1), Schnauzer (n = 1), and Pekingese (n = 1).
Table 1.
Data for the 21 dogs with canine atopic dermatitis enrolled in this study
F: female, FS: spayed female, M: male, MC: castrated male.
Lesion scores
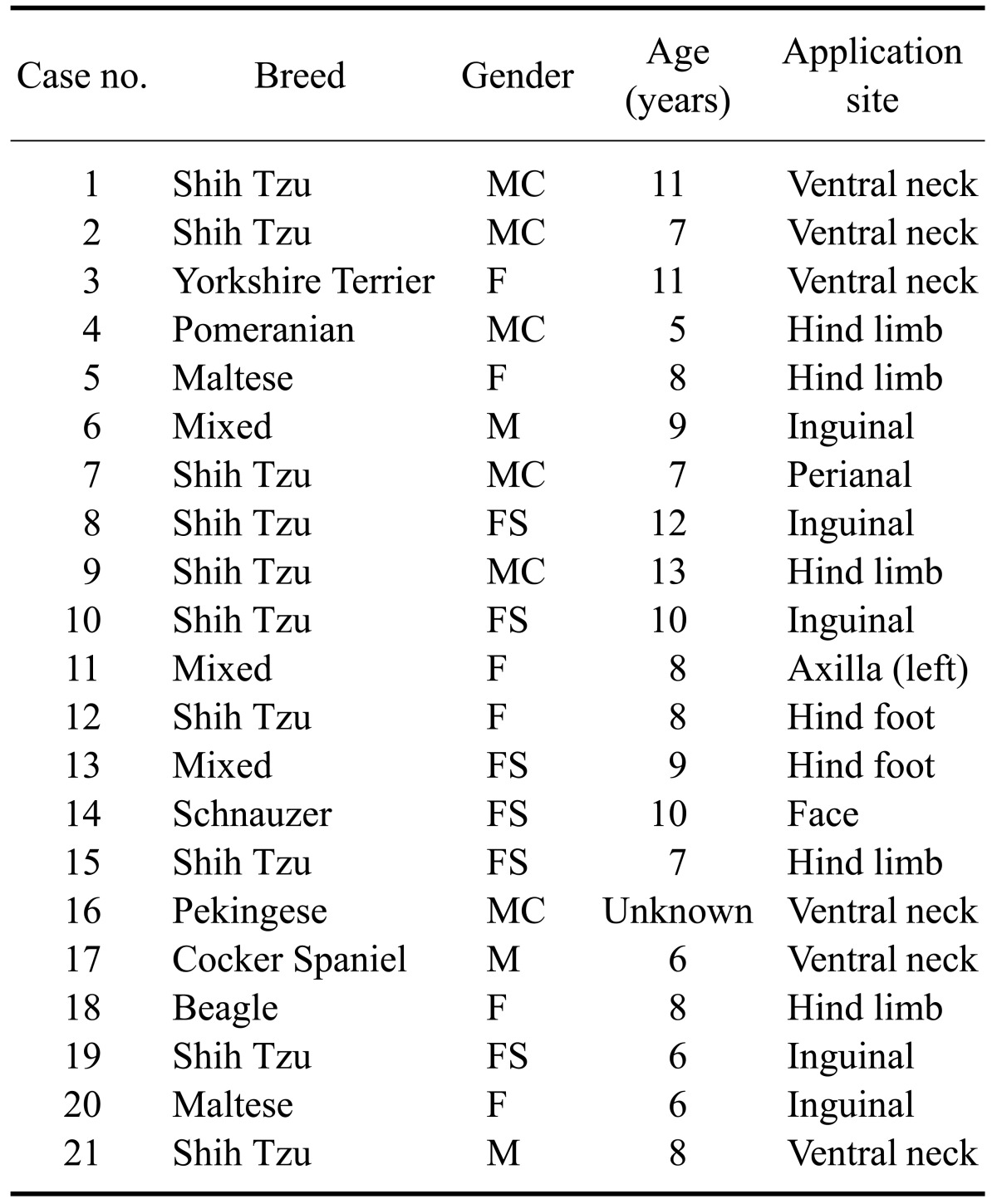
The lesion scores at the spray application sites significantly decreased by day 14 (mean: 5.5 ± 3.7, range: 3~17) after HCA treatment compared to day 0 (mean: 11.9 ± 4.1, range: 0 ~ 12; p < 0.0001, Fig. 1). The lesion scores for 15 of the 21 atopic dogs were reduced by 50% or more following HCA treatment compared to the day 0 scores.
Fig. 1.
Changes in lesion scores during treatment with 0.0584% hydrocortisone aceponate (HCA) spray (n = 21). A statistically significant difference in lesion scores was observed between day 0 and day 14 (p < 0.0001). Box and whisker plots show the median, 25th and 75th percentiles, and range of the scores.
Pruritus scores
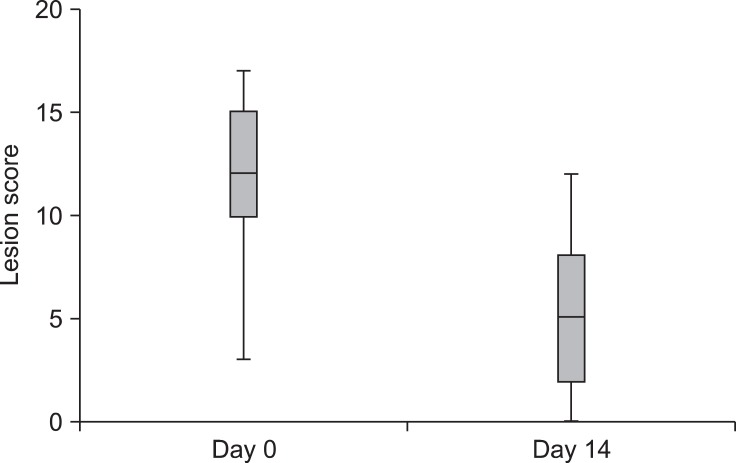
The pruritus scores were significantly lower on day 14 (mean: 2.4 ± 1.4, range: 0~6) after HCA treatment compared to day 0 (mean: 6.8 ± 1.5, range: 3~8; p < 0.0001, Fig. 2). The scores for 16 of the 21 atopic dogs improved by 50% or more following HCA treatment.
Fig. 2.
Changes in pruritus scores during treatment with 0.0584% HCA spray (n = 21). A statistically significant difference in pruritus scores was observed between day 0 and day 14 (p < 0.0001). The box represents the 25th and 75th percentiles with the bold lines indicating the median. The whiskers indicate the range. Outliers (asterisks) are also indicated.
TEWL findings
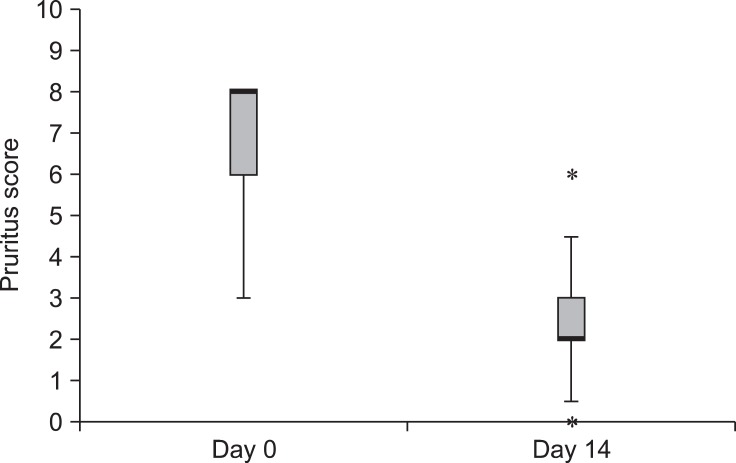
To evaluate the effect of the HCA spray treatment on skin barrier function, TEWL was measured in the lesions of the CAD dogs. Post-treatment TEWL values (mean: 17.5 ± 6.9 g/m2/h, range: 9.5~29.8 g/m2/h) were significantly lower than the pre-treatment values (mean: 48.7 ± 42.4 g/m2/h, range: 12.9~211.0 g/m2/h; p = 0.0011, Fig. 3). Reductions in TEWL values of 50% or more were observed in 13 of the 21 atopic dogs following HCA treatment.
Fig. 3.
Changes in transepidermal water loss (TEWL) during treatment with 0.0584% HCA spray (n = 21). TEWL values differed significantly between day 0 and day 14 (p = 0.0011). Box and whisker plots show the median, 25th and 75th percentiles, and range of the TEWL values. Outlier (asterisk) are also indicated.
Adverse events
No adverse events were observed in any of the 21 dogs during the study period by the owners or clinicians.
Discussion
CAD is a multigenetic and multifactorial disease caused by various factors including pharmacologic and immunologic abnormalities, and skin barrier defects [6,7]. Currently, there is evidence indicating that abnormal skin barrier function contributes to the pathogenesis of CAD, which is similar to that seen in human with atopic dermatitis [8,9,19,24,34]. The skin barrier is located in the stratum corneum of the epidermis. This barrier controls percutaneous absorption of external irritants and allergens, and regulates TEWL [8,9,13]. Impairment of the skin barrier can be caused by a primary defect or is a consequence of inflammation. Once the barrier function has been impaired, progressive worsening of this function occurs. Thus, impaired barrier function might play a key role in the development and aggravation of the clinical signs of CAD [17,29]. Recent studies have revealed that there is a correlation between improved skin barrier function and alleviation of CAD symptoms [13,18]. Therefore, it was suggested that skin barrier function is crucial for the successful treatment of CAD [1,11,19,36].
The present study was carried out to examine the potential beneficial role of skin barrier function as well as the efficacy of a 0.0584% HCA spray for treating CAD. We found that application of the HCA spray significantly improved both pruritus and other clinical signs associated with CAD in atopic dogs. After 14 days, scores for lesion severity (calculated according to the modified CADESI-03) and pruritus at the spray application sites were reduced from the baseline values by an average of 54.63% and 60.57%, respectively, with the majority of atopic dogs achieving more than a 50% reduction. To evaluate skin barrier function, TEWL, which is defined as the total amount of water loss that occurs by passive diffusion through the epidermal layer, was measured [14,33,37]. Measurement of TEWL values, which increase with progressive damage to the skin, is one of the most commonly used noninvasive methods to assess skin barrier function [19]. In our study, significant improvement in TEWL values was observed for the skin of atopic dogs following HCA spray treatment. The mean reduction in TEWL scores was 47.43%, and the TEWL values for 13 of the 21 dogs decreased by more than 50%.
HCA has been show to exert potent anti-inflammatory effects in the skin with few adverse side effects [5,20,32]. HCA, which is derived from the esterification of C21-acetate and C17-propionate, has increased hydrophilicity and lipophilicity compared to the parent drugs. This enhances its accumulation in the epidermis-dermis without entering the blood circulation [4,20]. In recent studies, HCA was show to be very well tolerated and the repeated use of the HCA had no observable adverse reactions over 70 days in dogs [4,20]. As shown in the present study, 7-day treatment with the HCA spray may be sufficient for reducing the severity of pruritus and skin lesions although long-term use of this product will be required to control the clinical signs of CAD. It may also be possible that the dose and frequency of administration can be reduced when using the HCA spray relative to systemic glucocorticoids and calcineurin inhibitors. Therefore, HCA may be more effective and safer than traditional topical glucocorticoids for treating CAD and other forms of pruritic dermatitis.
In conclusion, our findings suggest that daily application of a 0.0584% HCA spray not only provided short-term alleviation of clinical signs associated with CAD, but also improved skin barrier function, which is associated with the pathogenesis of CAD. Further investigation is required to monitor adverse effects resulting from the prolonged use of this product although no adverse effects following treatment for up to 70 days were observed in a previous study [20].
Acknowledgments
This study was partially supported by Virbac Korea.
References
- 1.Aalto-Korte K. Improvement of skin barrier function during treatment of atopic dermatitis. J Am Acad Dermatol. 1995;33:969–972. doi: 10.1016/0190-9622(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 2.Behrend EN, Kemppainen RJ. Glucocorticoid therapy. Pharmacology, indications, and complications. Vet Clin North Am Small Anim Pract. 1997;27:187–213. doi: 10.1016/s0195-5616(97)50027-1. [DOI] [PubMed] [Google Scholar]
- 3.Bieber T, Vick K, Folster-Holst R, Belloni-Fortina A, Stadtler G, Worm M, Arcangeli F. Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy. 2007;62:184–189. doi: 10.1111/j.1398-9995.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonneau S, Skowronski V, Sanquer A, Maynard L, Eun HM. Therapeutic efficacy of topical hydrocortisone aceponate in experimental flea-allergy dermatitis in dogs. Aust Vet J. 2009;87:287–291. doi: 10.1111/j.1751-0813.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 5.Brazzini B, Pimpinelli N. New and established topical corticosteroids in dermatology: clinical pharmacology and therapeutic use. Am J Clin Dermatol. 2002;3:47–58. doi: 10.2165/00128071-200203010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ, Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 7.DeBoer DJ. Canine atopic dermatitis: new targets, new therapies. J Nutr. 2004;134:2056S–2061S. doi: 10.1093/jn/134.8.2056S. [DOI] [PubMed] [Google Scholar]
- 8.Elias PM. Barrier repair trumps immunology in the pathogenesis and therapy of atopic dermatitis. Drug Discov Today Dis Mech. 2008;5:e33–e38. doi: 10.1016/j.ddmec.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:282–295. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin CE, DeBoer DJ. The ACVD task force on canine atopic dermatitis (XIV): clinical manifestations of canine atopic dermatitis. Vet Immunol Immunopathol. 2001;81:255–269. doi: 10.1016/s0165-2427(01)00346-4. [DOI] [PubMed] [Google Scholar]
- 11.Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989;30:323–333. [PubMed] [Google Scholar]
- 12.Gupta AK, Chow M. Prednicarbate (Dermatop®): profile of a corticosteroid. J Cutan Med Surg. 2004;8:244–247. doi: 10.1007/s10227-004-0120-x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, Assa'ad AH, Khurana Hershey GK. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725–730. doi: 10.1016/j.jaci.2007.12.1161. [DOI] [PubMed] [Google Scholar]
- 14.Hightower K, Marsella R, Flynn-Lurie A. Effects of age and allergen exposure on transepidermal water loss in a house dust mite-sensitized beagle model of atopic dermatitis. Vet Dermatol. 2010;21:88–95. doi: 10.1111/j.1365-3164.2009.00839.x. [DOI] [PubMed] [Google Scholar]
- 15.Hill PB, DeBoer DJ. The ACVD task force on canine atopic dermatitis (IV): environmental allergens. Vet Immunol Immunopathol. 2001;81:169–186. doi: 10.1016/s0165-2427(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 16.Hillier A, Griffin CE. The ACVD task force on canine atopic dermatitis (I): incidence and prevalence. Vet Immunol Immunopathol. 2001;81:147–151. doi: 10.1016/s0165-2427(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 17.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 18.Kim DW, Park JY, Na GY, Lee SJ, Lee WJ. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. Int J Dermatol. 2006;45:698–701. doi: 10.1111/j.1365-4632.2005.02644.x. [DOI] [PubMed] [Google Scholar]
- 19.Marsella R, Samuelson D. Unravelling the skin barrier: a new paradigm for atopic dermatitis and house dust mites. Vet Dermatol. 2009;20:533–540. doi: 10.1111/j.1365-3164.2009.00809.x. [DOI] [PubMed] [Google Scholar]
- 20.Nuttall T, Mueller R, Bensignor E, Verde M, Noli C, Schmidt V, Reme C. Efficacy of a 0.0584% hydrocortisone aceponate spray in the management of canine atopic dermatitis: a randomised, double blind, placebo-controlled trial. Vet Dermatol. 2009;20:191–198. doi: 10.1111/j.1365-3164.2009.00756.x. [DOI] [PubMed] [Google Scholar]
- 21.Nuutinen J, Alanen E, Autio P, Lahtinen MR, Harvima I, Lahtinen T. A closed unventilated chamber for the measurement of transepidermal water loss. Skin Res Technol. 2003;9:85–89. doi: 10.1034/j.1600-0846.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 22.Oh WS, Oh TH. Measurement of transepidermal water loss from clipped and unclipped anatomical sites on the dog. Aust Vet J. 2009;87:409–412. doi: 10.1111/j.1751-0813.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 23.Olivry T, DeBoer DJ, Favrot C, Jackson HA, Mueller RS, Nuttall T, Prelaud P. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol. 2010;21:233–248. doi: 10.1111/j.1365-3164.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 24.Olivry T, Hill PB. The ACVD task force on canine atopic dermatitis (VIII): is the epidermal lipid barrier defective? Vet Immunol Immunopathol. 2001;81:215–218. doi: 10.1016/s0165-2427(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 25.Olivry T, Mueller RS. Evidence-based veterinary dermatology: a systematic review of the pharmacotherapy of canine atopic dermatitis. Vet Dermatol. 2003;14:121–146. doi: 10.1046/j.1365-3164.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 26.Olivry T, Mueller R, Nuttall T, Favrot C, Prelaud P. Determination of CADESI 03 thresholds for increasing severity levels of canine atopic dermatitis. Vet Dermatol. 2008;19:115–119. doi: 10.1111/j.1365-3164.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 27.Olivry T, Sousa CA. The ACVD task force on canine atopic dermatitis (XIX): general principles of therapy. Vet Immunol Immunopathol. 2001;81:311–316. doi: 10.1016/s0165-2427(01)00347-6. [DOI] [PubMed] [Google Scholar]
- 28.Olivry T, Sousa CA. The ACVD task force on canine atopic dermatitis (XX): glucocorticoid pharmacotherapy. Vet Immunol Immunopathol. 2001;81:317–322. doi: 10.1016/s0165-2427(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 29.Reiter LV, Torres SMF, Wertz PW. Characterization and quantification of ceramides in the nonlesional skin of canine patients with atopic dermatitis compared with controls. Vet Dermatol. 2009;20:260–266. doi: 10.1111/j.1365-3164.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 30.Ruzicka T. Methylprednisolone aceponate in eczema and other inflammatory skin disorders -- a clinical update. Int J Clin Pract. 2006;60:85–92. doi: 10.1111/j.1368-5031.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 31.Rybnicek J, Lau-Gillard PJ, Harvey R, Hill PB. Further validation of a pruritus severity scale for use in dogs. Vet Dermatol. 2009;20:115–122. doi: 10.1111/j.1365-3164.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- 32.Schackert C, Korting HC, Schafer-Korting M. Qualitative and quantitative assessment of the benefit-risk ratio of medium potency topical corticosteroids in vitro and in vivo: characterisation of drugs with an increased benefit-risk ratio. BioDrugs. 2000;13:267–277. doi: 10.2165/00063030-200013040-00005. [DOI] [PubMed] [Google Scholar]
- 33.Shimada K, Yoshihara T, Yamamoto M, Konno K, Momoi Y, Nishifuji K, Iwasaki T. Transepidermal water loss (TEWL) reflects skin barrier function of dog. J Vet Med Sci. 2008;70:841–843. doi: 10.1292/jvms.70.841. [DOI] [PubMed] [Google Scholar]
- 34.Sugarman JL. The epidermal barrier in atopic dermatitis. Semin Cutan Med Surg. 2008;27:108–114. doi: 10.1016/j.sder.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Thomas RC, Logas D, Radosta L, Harrison J. Effects of a 1% hydrocortisone conditioner on hematologic and biochemical parameters, adrenal function testing, and cutaneous reactivity to histamine in normal and pruritic dogs. Vet Ther. 2000;1:25–34. [PubMed] [Google Scholar]
- 36.Watson AL, Fray TR, Bailey J, Baker CB, Beyer SA, Markwell PJ. Dietary constituents are able to play a beneficial role in canine epidermal barrier function. Exp Dermatol. 2006;15:74–81. doi: 10.1111/j.0906-6705.2005.00385.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoshihara T, Shimada K, Momoi Y, Konno K, Iwasaki T. A new method of measuring the transepidermal water loss (TEWL) of dog skin. J Vet Med Sci. 2007;69:289–292. doi: 10.1292/jvms.69.289. [DOI] [PubMed] [Google Scholar]