Abstract
BACKGROUND
While agonist replacement therapies are effective for managing opioid dependence, community treatment programs are increasingly choosing detoxification. Unfortunately, success rates for opioid detoxification are very low, in part, due to physical and psychological symptoms associated with opioid withdrawal. Few behavior therapies specifically address the distressing experiences specific to opioid withdrawal. A novel behavioral treatment, Acceptance and Commitment Therapy (ACT), works from the premise that the avoidance of unpleasant private experiences (thoughts, feelings, bodily sensations) is ubiquitous yet may be pathogenic, resulting in treatment drop-out and further drug use.
METHODS
This Stage I pilot study developed and tested an ACT-based opioid detoxification behavioral therapy. Opioid dependent patients (N = 56) who were attending a licensed methadone clinic were randomized to receive either 24 individual therapy sessions of ACT or Drug Counseling (DC) in the context of a 6-month methadone dose reduction program.
RESULTS
While no difference was found on opioid use during treatment, 37% of participants in the ACT condition were successfully detoxified at the end of treatment compared to 19% of those who received DC. Fear of detoxification was also reduced across time in the ACT condition relative to DC.
CONCLUSION
This first study of ACT to assist opioid detoxification indicates promise. Research is needed to refine specific treatment strategies for this population to further strengthen effects.
Keywords: opiate, opioid dependence, opioid detoxification, methadone, opioid withdrawal, Acceptance and Commitment Therapy, mindfulness, behavior therapy
1. Introduction
The burden of heroin and other opioid use is substantial, including high rates of morbidity and mortality, health care and law enforcement costs, family discord and distress, and lost productivity (Becker et al., 2008; Clausen et al., 2009). Methadone and buprenorphine maintenance continue to be the most effective treatments for heroin and other opioid dependence, reducing heroin use and criminal activity, and improving social functioning ( Amato et al., 2004; Stotts et al., 2009a).
However, recent trends indicate that community-based treatment programs favor opioid detoxification over maintenance. For example, annual treatment admissions for opioid dependence increased by almost one-third from 1995 – 2005, and almost 40% of patients received detoxification (SAMSHA, 2010b). In another report, Injecting heroin abusers admitted for detoxification rose by 17%, while the proportion receiving opioid agonist substitution treatment decreased by 24% (Mannelli et al., 2009). Unfortunately, detoxification is associated with high rates of treatment drop-out and illicit opioid use (Chutuape et al., 2001; Magura and Rosenblum, 2001). To date, no detoxification method has been found to be very effective, with an average of 30% or fewer patients achieving opioid abstinence at the end of the detoxification program (Dunn et al., 2011; Gossop et al., 2001). Clearly, more effective detoxification procedures, particularly for outpatient settings are needed.
Unpleasant physical symptoms associated with opioid withdrawal (Latowsky, 1996), as well as fear of anticipated withdrawal symptoms (Eklund et al., 1997; Latowsky, 1996; Milby et al., 1994) have been hypothesized to play large roles in detoxification failure. In 1980, Milby and his colleagues (1980) identified among methadone maintenance clients an “iatrogenic detoxification phobia,” which can be reliably detected and was present in almost 30% of the sample examined. Prevalence rates in the general methadone maintenance (MM) population may be even higher as severely phobic clients are less likely to enter detoxification programs. Milby et al. (1994) found that clients with detoxification phobia were more likely to be in MM treatment longer and make fewer detoxification attempts. Hall et al. (1984) reported that detoxification-related anxiety increased as dose decreased. This anxious state appears to occasion avoidance behavior, which is evident in the high premature termination and relapse rates found in most detoxification programs.
More recently, experiential avoidance has been proposed as a mechanism by which a variety of poor psychological and behavioral outcomes result. Experiential avoidance is the phenomenon that occurs when a person is unwilling to experience particular unpleasant private experiences (thoughts, feeling, and physical sensations) and takes steps (e.g., thought suppression; drug use) to alter these experiences and/or the discomfort associated with them (Hayes et al., 1996). Evidence of experiential avoidance in opioid patients has been documented in recent studies. For example, individuals with chronic opioid use tend to have greater fear of anxiety and anxiety-related sensations (Lejuez et al., 2006), and MM patients have been shown to be less tolerant of distress than those who do not have substance use problems (Compton et al., 2001).
ACT may be an ideal treatment to target detoxification fear and experiential avoidance (Hayes et al., 1999). ACT is a contextual behavioral intervention that uses acceptance, mindfulness, and values-directed behavior change strategies in order to increase the breadth and diversity of a person’s behavioral responses in the presence of unpleasant private experiences (thoughts, feelings, and physical sensations), thereby decreasing reliance on experiential avoidance (Hayes et al., 2006). ACT strategies may increase a client’s willingness to tolerate withdrawal experiences and associated fears, thereby pre-empting the typical flight back to drug use at the first signs of withdrawal. ACT has been found to influence the impact of negative thoughts and feelings as well as problematic avoidance patterns in a wide range of psychological problems (Hayes et al., 2006), including substance use disorders (Gifford et al., 2004; Hayes et al., 2004; Luoma et al., in press; Smout et al., 2010).
Following the NIDA Behavioral Therapies Development program guidelines for the development and preliminary testing of a new behavioral therapy, this Stage I pilot study examined whether a newly developed ACT intervention (Stotts et al., 2009b) targeting relevant mechanisms (i.e., experiential avoidance, detoxification fear) would indicate promise for facilitating successful detoxification from methadone relative to individual drug counseling (Crits-Christoph et al., 1999; Schmitz et al., 2002). Primary outcomes included opioid abstinence and completion of the detoxification program, with a secondary focus on treatment mechanisms to inform further treatment development.
2. Methods
2.1 Participants
Participants were male and female opioid dependent patients currently taking prescribed methadone at a licensed facility and seeking detoxification in the Houston area. All participants met the following inclusion criteria: (1) 25 to 60 years old; (2) current opioid dependence according to DSM-IV criteria; (3) opioid use for at least 5 years; and (4) proof of current enrollment in a licensed methadone clinic.
Participants were excluded based on the following criteria: (1) medical condition or taking medication that would interfere with safe study participation; (2) current diagnosis of any other Axis I psychiatric disorder requiring medication or other additional treatment; (3) severe cognitive, neurological, and psychiatric impairment precluding cooperation with the study protocol; (4) current participation in other psychosocial therapy for substance abuse; (5) unwillingness of women of child-bearing age to use some means of contraception; or (6) impending incarceration.
All subjects provided written informed consent. The research protocol, consent form, and all assessment/advertising materials were reviewed and approved by the local institutional review board, the Committee for the Protection of Human Subjects (CPHS) of the University of Texas Medical School, Houston (Clinicaltrials.gov Identifier: NCT00439036).
2.2 Procedures
2.2.1 Recruitment and Setting
Participants were recruited directly from licensed methadone clinics as well as the general community via print and radio advertising. All study procedures were conducted at the Treatment Research Clinic (TRC) of the Center for Neurobehavioral Research on Addiction, University of Texas-Houston Health Science Center.
2.2.2 Study Design
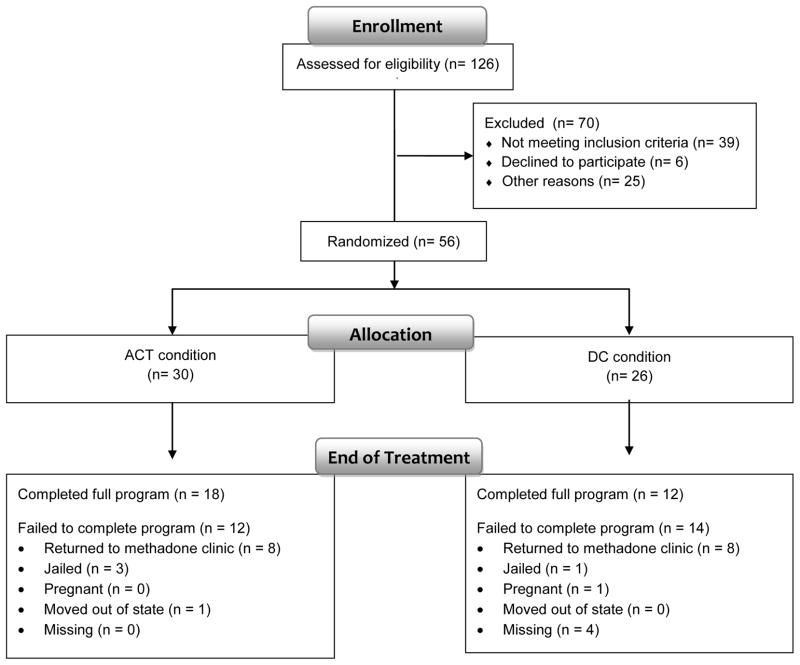
This study was a 24-week, randomized, controlled, parallel group pilot trial in which 56 subjects were randomized to one of two treatment conditions, an ACT-based opioid detoxification therapy or Drug Counseling. Both therapies were delivered in the context of a 5-month, linear, methadone dose reduction schedule. An urn randomization procedure run by a SAS program (Stout et al., 1994) was used to ensure even distribution of treatment groups with respect to gender and detoxification fear, based on the Detoxification Fear Survey Schedule-27 (Milby and Gurwitch, 1987) described below. Participants attended twice weekly clinic visits during treatment. At the end of 24 weeks all participants were contacted to complete end-of-treatment assessments on their final day or within the following week (Fig. 1).
Figure 1.
Study participant flow chart.
2.2.3 Methadone Dose Reduction Schedule
The 24-week detoxification program included a 4 week stabilization phase in which doses were adjusted based on client weight (1.2 mg/kg) followed by an 18 week dose reduction phase and 2 weeks at zero dose (Stotts et al., in preparation). Doses were reduced weekly in linear fashion over the 18 weeks in both groups. Participants were dosed twice per week at the TRC, with “take home” doses provided for the intervening days.
2.2.4 Acceptance and Commitment Therapy (ACT)
The protocol was adapted from previous ACT protocols for polysubstance abuse (Hayes et al., 2004) and methamphetamine use disorders (Smout et al., 2010), with modifications to reflect issues specific to methadone detoxification. The ACT protocol consisted of twenty-four, 50-minute sessions delivered weekly beginning in the stabilization period and continuing through the dose reduction period. ACT therapists were two masters-level clinicians who had been working at the TRC for at least two years conducting Cognitive-Behavioral Therapy (CBT) with cocaine and opioid dependent patients. Initial ACT training, including a 2-day workshop, weekly phone supervision for the first 6 months of the study, and yearly on-site, booster training sessions, were conducted by the fourth author, an expert national and international ACT trainer. Ongoing supervision, consisting of weekly audiotape review and supervision sessions, was provided by the first author as well as the post-doctoral fellow (third author).
The ACT-based treatment was designed to assist patients in managing the distressing physical and psychological experiences associated with methadone detoxification without resorting to their typical avoidant strategies, i.e., drug use. The overarching goals were to: (1) decrease experiential avoidance while increasing acceptance and willingness to experience unpleasant thoughts, feelings, and physical symptoms; and (2) to make commitments to engage in behaviors consistent with their chosen values or goals (rather than allowing negative thoughts, feelings or physical withdrawal symptoms determine behavior). Additional processes common to ACT interventions were also part of the protocol as described in detail elsewhere (Stotts et al., 2009b).
2.2.5 Drug Counseling (DC)
The DC intervention was identical in format to the ACT treatment, i.e., twenty-four, 50 minutes sessions delivered weekly during methadone dose reduction. DC therapists were two masters-level therapists employed at the TRC to conduct various behavior therapies with substance abuse patients enrolled in clinical trials. The DC approach followed the treatment manual by Schmitz and colleagues (Schmitz et al., 2001) and was designed to provide general education, support and encouragement for abstinence-oriented behaviors without using direct coping skills training. This DC protocol is not based on the 12-step model, however concurrent involvement in outside support programs was monitored and encouraged. DC was found to be equally effective as Relapse Prevention in a previous trial with cocaine dependent patients (Schmitz et al., 2001; Schmitz et al., 2002).
2.2.6 Treatment Integrity
All sessions were audiotaped for supervision and fidelity purposes. The first and third authors listened to each audiotape and provided feedback to prevent deviation and drift, bolstered by telephone supervision by Dr. Wilson for the ACT condition.
Audiotapes of 45 ACT and 52 DC sessions were randomly selected for adherence and competence coding, representing about 15% of all taped sessions. Coders for the ACT tapes were 2 clinical psychology graduate students with at least one year of ACT training and were supervised by the third author. DC sessions were also coded by a graduate student with previous experience in our substance abuse research-treatment clinic, and supervised by the first author. Coders were intensively trained, undergoing approximately 30 hours of didactic instruction, discussion, and practice.
For the ACT condition, the adherence coding manual was adapted from manuals and procedures used in previous ACT/substance abuse studies (Hayes et al., 2004; Luoma et al., in press). Single item ratings of overall therapist adherence to the ACT protocol and overall competence were further delineated through ratings of therapist use of six ACT processes (workability, willingness, values, committed action, defusion, and present moment focus), four processes contraindicated by ACT (promoting experiential avoidance, challenging cognitions, suggesting thoughts or feelings cause actions, any other cognitive therapy-consistent rationale), and one global item assessing any use of DC content. All items were rated on a five-point scale from 1 (not at all) to 5 (extensive). Ratings of overall adherence and competence averaged 4.3 (SD = .74) and 4.4 (SD = .66), respectively. Sessions typically included two or three ACT processes at a time, resulting in high scores for relevant ACT processes and low ratings for others. Thus, a high level of adherence would be reflected in moderate scores for specific ACT processes, lower ratings on contraindicated and DC items. ACT processes averaged 3.00 (1.2) with the highest scores on willingness (M=3.7, SD=1.2) and workability (M=3.4, SD=1.3) and the lowest on committed action (M=2.2, SD=1.1) and values (M=2.5, SD=1.3); contraindicated items and the DC item averaged 1.0 (0.0). The pattern of results suggests ACT therapists were adherent.
The DC coding measure was constructed similarly with overall adherence and competence items further delineated through three DC specific processes (explaining addiction as a disease; planning recovery; attending support groups) and two non-DC processes (challenging cognitions, identifying thoughts or feelings as mediating behavior) rated on a five-point scale from 1 (not at all) to 5 (extensive). Ratings of overall adherence and competence were similarly high for the DC condition, averaging 4.6 (.5) and 5.0 (.6), respectively. DC items averaged 3.2 (.8) and processes that were not part of the DC intervention averaged 1.0 (0.0). Results indicate that the DC therapists were similarly adherent to the protocol.
2.3 Assessments
Psychiatric Symptomatology
Axis I diagnoses were evaluated based upon criteria specified in the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) using the Structured Clinical Interview for DSM-IV (SCID: First et al., 1995). Each potential participant was also evaluated by a psychiatrist prior to randomization to ensure eligibility criteria were met.
Opioid Use
Qualitative urine toxicology screens for opioids were conducted twice per week by an off-site laboratory and confirmed by GC-Mass Spec procedures. Transfer to MM clinic during the study protocol was recorded by the research nurse. Drug Use History was measured using the Addiction Severity Index, 5th edition (McLellan et al., 1992).
Detoxification Fear
The presence and severity of detoxification fear was assessed monthly using the Detoxification Fear Survey Schedule (DFSS-27: Milby and Gurwitch, 1987). The DFSS-27 contains 27-items and measures the level of detoxification-related fear using a five-point Likert-type scale ranging from 0 (not disturbed at all) to 4 (very much disturbed), with good inter-item reliability in our sample (α = .96). Greater scores indicate greater levels of detoxification fear. The DFSS also comprises 2 similarly scored subscales: Fear of Relapse (α = .95) and Fear of Withdrawal (α = .96).
Avoidance and Inflexibility-Substance Use Specific
The Avoidance and Inflexibility Scale-Substance Use version (AIS: Gifford et al., 2004), measured monthly, is a 13-item measure designed to evaluate endorsement of avoidance strategies specifically related to drug use and withdrawal. High scores on this Likert-type scale indicate an avoidant stance toward unpleasant internal experiences related to drug use, e.g., negative thoughts, affect, or withdrawal symptoms necessarily lead to drug use, and efforts to quit are directed toward avoiding these negative internal experiences. Inter-item reliability was high (α = .96).
Withdrawal
Severity of the opioid withdrawal syndrome was measured weekly using the Subjective Opioid Withdrawal Scale (SOWS; Handelsman et al., 1987). The SOWS contains 16 symptoms which are rated on intensity using a scale of 0 (not at all) to 4 (extremely), with greater scores suggesting greater subjective opioid withdrawal experiences. Inter-item reliability was high (α = .94).
HIV/HCV Risk Behavior
The Blood Borne Virus Transmission Risk Assessment Questionnaire (BBVTRAQ; Fry et al., 1998) was used to assess several behavioral practices (e.g, sexual, injecting) related to the transmission of viruses not uncommon in the opioid-dependent population. Participants rated the frequency with which they engaged in each of 34 behaviors in the past month. Inter-item reliability was sufficient (α = .81).
2.4 Data Analysis
Statistical analyses utilized SAS 9.2.1 (Inc., 2008) and STATA v. 11.0 (StataCorp) and followed an intention-to-treat model. Due to the pilot nature of this study, descriptive data as well as results of traditional significance testing are reported. Sociodemographic, session attendance, and treatment completion comparisons were evaluated using t-tests or contingency tables with chi-square testing. Examination of end of treatment (EOT) success used exact logistic regression due to small cell sizes. EOT success was defined as having an opioid negative urine drug screen at the EOT assessment without having re-enrolled in a methadone clinic. Failure was defined as either submitting an opioid positive urine drug screen at EOT or re-enrollment in a methadone clinic. We are not suggesting that returning to a methadone clinic equates to failure as this is highly preferable to returning to heroin or other opioid use and is associated with positive outcomes. Our goal, however, was to assist methadone maintenance patients to gradually reduce their dose and be abstinent of all opioids, including methadone, by the end of the program. Hence, our definition of success and failure reflect that goal. In this analysis, participants who were missing (n = 4), in jail (n = 4), pregnant (n = 1) or had moved out of state (n = 1) were all coded as treatment failures. Sensitivity analyses investigating the impact of missing data failed to change the reported conclusions.
Evaluation of longitudinal data used generalized linear mixed models. Bootstrapping was needed to address the violation of the assumption of normality for the Pearson Residuals in the logistic mixed models for opioid use and HIV/HCV risk behavior as a function of time, treatment and the interaction of time and treatment: k = 1000 boot-strapped samples provided more appropriate estimates for standard errors. Small sample size and non-normal boot-strapped distributions recommended the use of a bias-corrected confidence interval. Standard logarithmic transformations were adequate remedies for violations of distributional assumptions for mixed models analyzing hypothesized treatment mechanisms. Appropriate modeling of longitudinal trajectories for treatment mechanisms often required linear and quadratic trends. Model fitting proceeded as discussed by Raudenbush and Bryk (2002) focusing first on fitting the temporal function of the longitudinal data followed by examination of treatment main effects and treatment by time interactions. Due to the pilot nature of this work, treatment mechanisms were graphed for visual inspection for hypothesis-generation purposes.
3. Results
3.1 Sociodemographics
Sample demographics suggest comparability across treatment conditions (Table 1). Years of reported alcohol use appeared to be higher in the control condition, however this finding was not statistically significant after correction for multiple testing.
Table 1.
Sociodemographics
| ACT Condition | Drug Counseling | |
|---|---|---|
| N | 30 | 26 |
| Male, % (n) | 57% (17) | 69% (18) |
| Race, % (n) | ||
| White | 77% (23) | 88% (23) |
| Black | 13% (4) | 4% (1) |
| Hispanic | 10% (3) | 8% (2) |
| Employed, % (n) | ||
| Full-Time | 33% (10) | 35% (9) |
| Part-Time | 13% (4) | 12% (3) |
| Unemployed | 53% (16) | 54% (14) |
| Relationship Status, % (n) | ||
| Single | 40% (12) | 54% (14) |
| Divorced | 20% (6) | 23% (6) |
| Separated | 10% (3) | 8% (2) |
| Widowed | 0% (0) | 4% (1) |
| Married | 30% (9) | 12% (3) |
| Age, M (SD) | 40.3 (10.7) | 39.4 (8.7) |
| Education, M (SD) | 12.9 (1.6) | 13.1 (1.7) |
| Drug Use Indices (past 30 days) | ||
| Heroin, number of days, M (SD) | 2.0(5.6) | 0.2(1.0) |
| Other opiates, number of days, M (SD) | 1.0(2.4) | 0.2(0.5) |
| Methadone, number of days, M (SD) | 26.5(8.3) | 27.8(6.3) |
| Alcohol Use (Any), number of days, M (SD) | 1.9(4.4) | 2.0(4.8) |
| Marijuana, number of days, M (SD) | 2.0(5.1) | 3.9(9.1) |
| Cocaine, number of days, M (SD) | 0.5(0.7) | 1.1(3.1) |
| Drug Use Indices (lifetime) | ||
| Heroin, years, M (SD) | 6.7(7.8) | 10.7(11.5) |
| Other opiates, years, M (SD) | 3.9(3.7) | 5.6(8.7) |
| Methadone, years, M (SD) | 5.0(4.9) | 6.6(7.0) |
| Alcohol Use (Any), years, M (SD) | 6.3(6.9) | 14.6(13.4) |
| Marijuana, years, M (SD) | 7.9(8.3) | 7.9(12.1) |
| Cocaine, years, M (SD) | 3.4(4.5) | 6.1(8.6) |
| Total Number of Sessions Attended, M (SD) | 14.2 (6.6) | 15.3 (6.4) |
All testing used a Bonferroni-adjusted p-value of p ≤ 0.003, and all comparisons were non-significant. For ASI indices, n = 28 for the ACT condition and n = 24 for the Drug Counseling condition.
3.2 Treatment Completion and Session Attendance
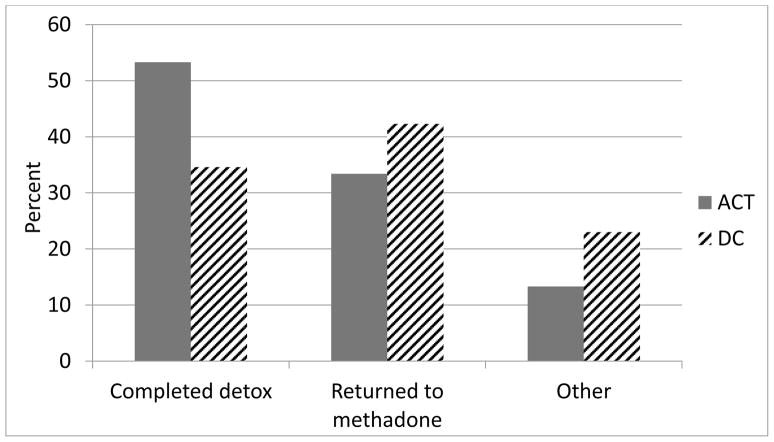
Participants were considered treatment completers if they presented to the clinic at least once in the final 2 weeks of the program at which point they were receiving no methadone. While not statistically significant, 60% of the ACT group completed the detoxification program compared to 46.2% in the DC condition, X2 (N = 56) = 1.1, p = .3). A more detailed account of dispositional status at the end of treatment is depicted in Figure 2. No differences were found between groups on number of treatment sessions attended, t (54) = 0.63, p = 0.53. On average, participants attended 14.7 of the 24 sessions.
Figure 2.
Percentage of participants by group who Completed detox (participants who completed the detoxification program and did not immediately return to the methadone clinic); Returned to methadone (participants who returned to a methadone clinic during or at the end of treatment), or; Other (participants who went to jail, moved out of the state, became pregnant or were missing). For illustrative purposes, all participants who returned to a methadone clinic were included in the “Returned to methadone” category even if they attended the final weeks of the detoxification program; therefore numbers differ slightly from Figure 1.
3.3. Opioid use
During Treatment
Analysis of the probability of opioid positive urines as a function of time, treatment and the interaction of time and treatment revealed an effect for time (R.R. 1.01, 95% CI 1.00–1.02), indicating increased opioid use across the detoxification period. However, no effect was found for treatment (R.R. 1.14, 95% CI 0.44–2.91) nor the interaction of time and treatment (R.R. 1.00, 95% CI 0.99–1.01).
EOT Success
With regard to success at the end of treatment as defined above (Section 2.4), 36.7% of the ACT group were successful compared to 19.2% of the DC group, X2 (N = 56) = 1.80, p = .17 (R.R. = 1.91, 95% CI 0.76–4.77).
3.4 Treatment Mechanisms: Treatment, Treatment × Time Effects
AIS
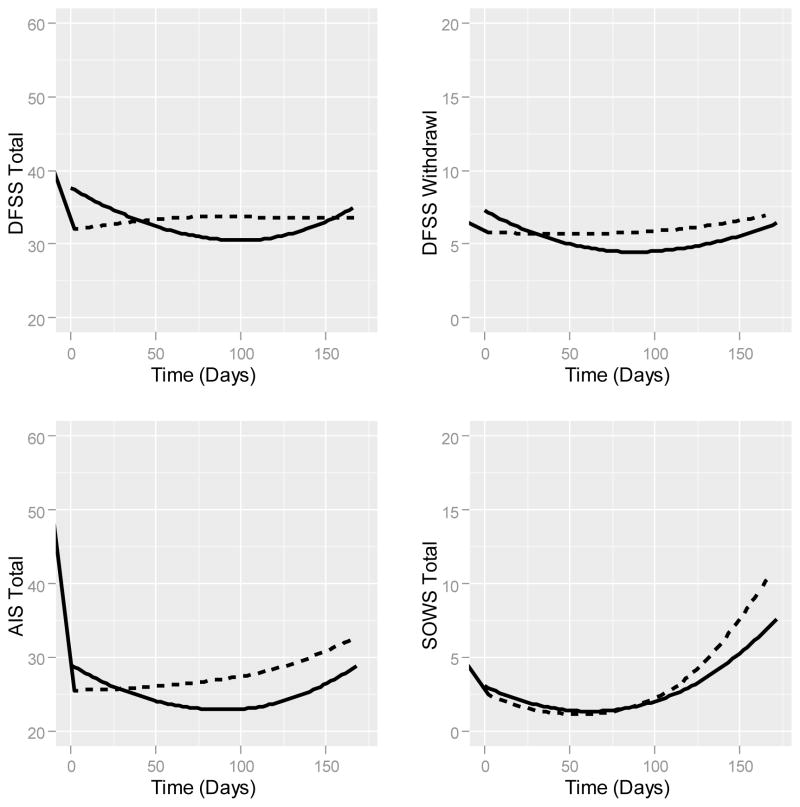
Modeling of substance use-specific avoidance and inflexibility changes, after controlling for lower order effects, demonstrated a quadratic trend (F(1,51) = 100.85, p ≤ 0.0001), ), reflecting a reduction in the use of avoidance strategies toward experiences related to drug use, followed by an increase at the end of the dose reduction period when withdrawal symptoms are at their worst. Neither the treatment (F(1,176) = 0.04, p = 0.83) nor the time by treatment (F(1,176) = 1.76, p = 0.18) interaction achieved statistical significance. (Fig. 3).
Figure 3.
Fitted values for generalized linear mixed models are plotted with lowess smoothing for each hypothesized treatment mechanism over time. DFSS = Detoxification Fear Survey Schedule; AIS = Avoidance and Inflexibility Scale; SOWS = Subjective Opiate Withdrawal Scale. Solid line (-----) = Acceptance and Commitment Therapy condition; Dashed line (- - - - -) = Drug Counseling condition.
DFSS
Controlling for lower order effects, evaluation of changes in detoxification fear using the total score, demonstrated a quadratic by treatment interaction effect (F(1,227) = 5.01, p = 0.02; Fig. 3). Inspection of simple effects of time for the ACT condition indicated that for every additional week in treatment the DFSS Total Score decreased (b = −0.413, 95% CI: −0.74 to −0.08), and that for every additional week of treatment this rate of change slowed (b = 0.006, 95% CI: 0.004 to 0.008). Examination of simple effects of time for the DC condition failed to identify either a linear (b = −0.042, 95% CI: −0.495 to 0.411) or quadratic (b = 0.003, 95% CI: −0.0001 to 0.005) change over time. Controlling for lower order effects, the model of the DFSS Fear of Relapse Subscale demonstrated a quadratic (F(1,225) = 12.43, p = 0.00) trend, but failed to find an effect for treatment (F(1,225) = 0.13, p = 0.71) or the interaction of treatment by time (F(1,225) = 0.45, p = 0.50). The DFSS Fear of Withdrawal Subscale model reflected the pattern observed in the DFSS Total Scale model. Controlling for lower order effects, an interaction of treatment with the quadratic effect of time (F(1,225) = 27.55, p ≤ 0.01) emerged. Evaluating the simple effects of time within the ACT condition indicated that for every additional week in treatment the DFSS Withdrawal Scale decreased (b = −0.070, 95% CI: −0.133 to −0.007), but that this rate of change slowed over time (b = 0.0030, 95% CI: 0.002 to 0.003). The control condition demonstrated no such linear (b = 0.031, 95% CI: −0.050 to 0.07) or quadratic (b = 0.00039, 95% CI: −0.00037 to 0.00117) effects of time. Thus, the ACT condition was associated with greater decreases in detoxification fear, particularly withdrawal, across treatment, however fear increased toward the end of the detoxification period as withdrawal symptoms peaked.
SOWS
Linear (F(1,51) = 18.35, p ≤ 0.0001) and quadratic trends (F(1,51) = 1659.55, p ≤ 0.0001) also occurred for the Subjective Opioid Withdrawal Symptoms Scale. However, analyses failed to find effects for treatment (F(1,840) = 0.63, p = 0.43) or the treatment by time interaction (F(1,840) = 2.21, p = 0.14).
3.5 HIV/HCV Risk Behavior
BBVTRAQ
Analysis of HIV/HCV risk behavior as a function of time, treatment and the interaction of time and treatment failed to reveal an effect for time (b = 0.0008, 95% CI −0.023–0.022), treatment (b = 0.11, 95% CI −0.33–0.62) or the interaction of time and treatment (b = −0.01, 95% CI −0.04–0.02).
4. Discussion
Outpatient opioid detoxification is highly challenging. This stage I pilot trial tested a novel behavioral therapy based on ACT, targeting experiential avoidance and detoxification fear related to the detoxification process. ACT was compared to an equally intensive individual drug counseling therapy with equally competent therapists. While many of the findings were not statistically significant, perhaps due to sample size, it is notable that program completion rates were almost 15% higher, and success rates at the end of treatment were nearly doubled in the ACT group. In addition, fear of detoxification, particularly fear of withdrawal, was reduced over time in the ACT condition, although the progressive reduction in fear slowed toward the end of the detoxification period.
While difficult to compare across opioid detoxification studies due to differing dosing strategies, lengths and settings of detoxification, and detoxification agents (e.g., buprenorphine, lofexidine), participants in our ACT treatment had comparable if not higher rates of success (37%) relative to recent outpatient opioid detoxification studies using agents purportedly easier to detoxify from than methadone. For example, the end of treatment success rate for outpatient detoxification with buprenorphine/naloxone in a multi-center trial was 29% (Ling et al., 2005). Lintzeris et al. (2002) reported 21% opioid negative urine drug screens at the end of a shorter term taper. In the methadone detoxification arm of Gruber et al. (2008), only about 9% were opioid-free following a 21-day detoxification regimen. A recent review of 28 buprenorphine detoxification studies reported that a median of 30% of patients were opioid negative at the end of treatment across studies (range: 22 – 51%; Dunn et al., 2011). While recognizing that our success rates, as in other studies, were far from ideal, relative to our control group as well as previous studies the ACT treatment suggests promise as an adjunct to opioid detoxification.
Growing evidence has highlighted the impact of emotional and cognitive processes in substance use ( Lejuez et al., 2006) and the potential utility of acceptance-based intervention for substance-related problems (Gifford et al., 2004; Hayes et al., 2004; Luoma et al., in press; Smout et al., 2010). Negative affect and fear have been documented in association with methadone tapering and have been recommended as a focus of clinicians (Calsyn et al., 2006; Latowsky, 1996). High anxiety and fear about the withdrawal experience often lead to treatment drop-out and relapse. Thus, in this first iteration of the ACT treatment for methadone detoxification we chose to target detoxification fear (Milby et al., 1980; Milby et al., 1994), as well as the ACT-based mechanisms of avoidance and inflexibility in responding in the presence of negative private events. Results suggested that our ACT treatment differentially impacted detoxification fear relative to DC. Impact on the targeted ACT mechanisms, however, was less, which may have weakened our effects.
To glean information beyond p-values, data were graphed for exploratory hypothesis generation. Responses over time between the groups on the detoxification fear measure were visually different as well as statistically reliable, with ACT participants reporting a decrease in fear during most of the treatment. Visual inspection of data from the substance use specific avoidance measure (AIS), however, also suggested differences in the predicted direction. A differential decrease in avoidance and inflexibility in the ACT group relative to the DC group was observed, particularly in the second half of treatment. Both groups increased in avoidance though as doses neared zero and withdrawal symptoms peaked at the end of the detoxification period. Synthesizing the data from this pilot trial, future iterations of the ACT opioid detoxification treatment perhaps need to incorporate stronger methods for increasing the acceptance of and flexibility around unpleasant detoxification experiences, particularly at the end of detoxification. More intensive, group-based methods with fewer, longer duration sessions (e.g., workshops) may be more effective (Gregg et al., 2007; Luoma et al., in press) and should be pursued.
Conclusions must be tempered by limitations of this pilot study. Most notably, the sample size in this initial study of a novel treatment is small, increasing instability of obtained results and, of course, making statistical significance difficult to achieve. This is a common challenge in the early stages of behavioral treatment development which may require alternative statistical procedures, e.g., Bayesian statistics (Green et al., 2009; Wijeysundera et al., 2009), which allow for calculation of treatment effect probabilities. While our overall adherence and competence ratings were high, therapists implemented some processes less often than others which could have dampened results. There was also only one coder per audiotape which could have introduced bias to our therapy fidelity ratings. Finally, the therapy training time was greater in the ACT condition due to the complexity of the treatment, thus potentially creating an allegiance bias.
We believe our results suggest promise for an ACT-based treatment as an adjunct to detoxification. Further development and refinement of the intervention is clearly needed, however, to isolate critical mechanisms and treatment strategies to increase the strength of its effect throughout the detoxification period. Although employed with increasing frequency, outpatient opioid detoxification remains an unsupported treatment strategy, necessitating the identification and development of novel and effective pharmacological and behavioral strategies to improve success. ACT should be further investigated as a novel behavioral therapy for this purpose.
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIDA Grant DA019436 and P50DA009262; the NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributers
Dr. Stotts conceived the principal aim of the study with the assistance of Drs. Grabowski and Schmitz. Dr. Masuda and Dr. Wilson assisted is the therapy development and implementation. Drs. Moeller and Grabowski supervised implementation of the study in the Treatment Research Clinic. Drs. Green and Northrup conducted data analyses and assisted with the interpretation of the results. All authors contributed to and have approved the final manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Substance Abuse Mental Health Services Administration. Office of Applied Studies. Treatment Episode Data Set Admissions (TEDS-A), 1992 - present. 2010b Retrieved online at: http://wwwicpsrumichedu/icpsrweb/SAMHDA/series/00056/studies/25221?sortBy=7(8202010)
- Amato L, Davoli M, Ferri M, Gowing L, Perucci CA. Effectiveness of interventions on opiate withdrawal treatment: an overview of systematic reviews. Drug Alcohol Depend. 2004;73:219–226. doi: 10.1016/j.drugalcdep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Calsyn DA, Malcy JA, Saxon AJ. Slow tapering from methadone maintenance in a program encouraging indefinite maintenance. J Subst Abuse Treat. 2006;30:159–163. doi: 10.1016/j.jsat.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, three-, and six-month outcomes after brief inpatient opioid detoxification. Am J Drug Alcohol Abuse. 2001;27:19–44. doi: 10.1081/ada-100103117. [DOI] [PubMed] [Google Scholar]
- Clausen T, Waal H, Thoresen M, Gossop M. Mortality among opiate users: opioid maintenance therapy, age and causes of death. Addiction. 2009;104:1356–1362. doi: 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Salloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry. 1999;56:493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxifcation duration and clinical treatment outcomes: a review. Drug Alcohol Depend. 2011;119:1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund C, Hiltunen AJ, Melin L, Borg S. Abstinence fear in methadone maintenance withdrawal: a possible obstacle for getting off methadone. Subst Use Misuse. 1997;32:779–792. doi: 10.3109/10826089709039377. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID -I/P, Version 2.0) Biometric Research Department, Psychiatric Institute; NY: 1995. [Google Scholar]
- Fry C, Rumbold G, Lintzeris N. The Blood Borne Virus Transmission Risk Assessment Questionnaire (BBVTRAQ): Administration and Procedures Manual. Turning Point Alcohol and Drug Centre; Melbourne: 1998. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behav Therapy. 2004;35:689–705. [Google Scholar]
- Gossop M, Marsden J, Stewart D, Treacy S. Outcomes after methadone maintenance and methadone reduction treatments: two-year follow-up results from the National Treatment Outcome Research Study. Drug Alcohol Depend. 2001;62:255–264. doi: 10.1016/s0376-8716(00)00211-8. [DOI] [PubMed] [Google Scholar]
- Green CE, Moeller FG, Schmitz JM, Lucke JF, Lane SD, Swann AC, Lasky RE, Carbonari JP. Evaluation of heterogeneity in pharmacotherapy trials for drug dependence: a Bayesian approach. Am J Drug Alcohol Abuse. 2009;35:95–102. doi: 10.1080/00952990802647503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg JA, Callaghan GM, Hayes SC, Glenn-Lawson JL. Improving diabetes self-management through acceptance, mindfulness, and values: a randomized controlled trial. J Consult Clin Psychol. 2007;75:336–343. doi: 10.1037/0022-006X.75.2.336. [DOI] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend. 2008;94:199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Loeb PC, Kushner M. Methadone dose decreases and anxiety reduction. Addict Behav. 1984;9:11–19. doi: 10.1016/0306-4603(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44:1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. Guilford Press; New York, NY US: 1999. [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Bissett R, Piasecki M, Batten SV, Gregg J. A preliminary trial of twelve-step facilitation and acceptance and commitment therapy with polysubstance-abusing methadone-maintained opiate addicts. Behav Therapy. 2004;35:667–688. [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, Strosahl K. Experimental avoidance and behavioral disorders: a functional dimensional approach to diagnosis and treatment. J Consult Clin Psychol. 1996;64:1152–1168. doi: 10.1037//0022-006x.64.6.1152. [DOI] [PubMed] [Google Scholar]
- Latowsky M. Improving detoxification outcomes from methadone maintenance treatment: the interrelationship of affective states and protracted withdrawal. J Psychoactive Drugs. 1996;28:251–257. doi: 10.1080/02791072.1996.10472486. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Paulson A, Daughters SB, Bornovalova MA, Zvolensky MJ. The association between heroin use and anxiety sensitivity among inner-city individuals in residential drug use treatment. Behav Res Ther. 2006;44:667–677. doi: 10.1016/j.brat.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, Brigham G, Harrer J, Reid M, Muir J, Buchan B, Orr D, Woody G, Krejci J, Ziedonis D. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100:1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N, Bell J, Bammer G, Jolley DJ, Rushworth L. A randomized controlled trial of buprenorphine in the management of short-term ambulatory heroin withdrawal. Addiction. 2002;97:1395–1404. doi: 10.1046/j.1360-0443.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- Luoma JB, Kohlenberg BS, Hayes SC, Fletcher L. Slow and steady wins the race: a randomized clinical trial of acceptance and commitment therapy targeting shame in substance use disorders. J Consult Clin Psychology. doi: 10.1037/a0026070. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Rosenblum A. Leaving methadone treatment: lessons learned, lessons forgotten, lessons ignored. Mt Sinai J Med. 2001;68:62–74. [PubMed] [Google Scholar]
- Mannelli P, Patkar AA, Peindl K, Gorelick DA, Wu LT, Gottheil E. Very low dose naltrexone addition in opioid detoxification: a randomized, controlled trial. Addict Biol. 2009;14:204–213. doi: 10.1111/j.1369-1600.2008.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Milby JB, Garrett C, Meredith R. Iatrogenic phobic disorders in methadone maintenance treated patients. Int J Addict. 1980;15:737–747. doi: 10.3109/10826088009040051. [DOI] [PubMed] [Google Scholar]
- Milby JB, Gurwitch RH, et al. Assessing pathological detoxification fear among methadone-maintenance patients - the DFSS. J Clin Psychol. 1987;43:528–538. doi: 10.1002/1097-4679(198709)43:5<528::aid-jclp2270430517>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Milby JB, Hohmann AA, Gentile M, Huggins N, Sims MK, McLellan AT, Woody G, Haas N. Methadone maintenance outcome as a function of detoxification phobia. Am J Psychiatry. 1994;151:1031–1037. doi: 10.1176/ajp.151.7.1031. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models. 2. Sage Publications; Thousand Oaks: 2002. [Google Scholar]
- SAS Institute, Inc. SAS 9.2. Cary, NC: 2008. [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Sayre SL, DeLaune KA, Rhoades H, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-alcohol dependent patients. Drug Alcohol Depend. 2002;66 (Suppl):S158. [Google Scholar]
- Smout MF, Longo M, Harrison S, Minniti R, Wickes W, White JM. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abuse. 2010;31:98–107. doi: 10.1080/08897071003641578. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata: Release 11. Statacorp LP; College Station, TX: [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009a;10:1727–1740. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Masuda A, Wilson K. Using Acceptance and Commitment Therapy during methadone dose reduction: rationale, treatment description, and a case report. Cogn Behav Pract. 2009b;16:205–213. doi: 10.1016/j.cbpra.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Mooney M, Herin DV, Moody DE, Schmitz JM, Grabowski J. Opioid maintenance pharmacotherapy and discontinuation: pharmacological and behavioral therapy strategies. Experimental and clinical psychopharmacology in preparation. [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Wijeysundera D, Austin P, Hux J, Beattie W, Laupacis A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol. 2009;62:13–21.e15. doi: 10.1016/j.jclinepi.2008.07.006. [DOI] [PubMed] [Google Scholar]