Abstract
Objective
To examine the interaction of the cytokines interleukin-1 receptor antagonist (IL)-1Ra, IL-6 and IL-10 to predict preterm birth (PTB) in pregnant Hispanic women (n = 470).
Study Design
In this prospective study, demographic data were obtained prenatally and birth outcome data were obtained from the medical chart. Cytokines were measured from plasma obtained at 22 to 24 weeks gestation. Data analysis utilized logistic regression.
Result
PTB was predicted by level of IL-1Ra (odds ratio (OR) = 2.55; 95% confidence interval (CI) = 1.24, 5.24). The interaction between IL-1Ra and IL-6 and between IL-1Ra and IL-10 was significant (Wald = 4.01, P = 0.04 and Wald = 8.84, P<0.003, respectively) and was also predictive of PTB. As IL-1Ra levels increased while IL-10 levels were low, the probability of PTB greatly increased.
Conclusion
The interactions of select cytokines and cytokine receptor antagonists were associated with PTB. Future research should focus on the changes in cytokines during pregnancy to identify critical periods of change, and examine predictors of the cytokine response.
Keywords: pregnancy, prematurity, immune function
Introduction
Preterm birth (PTB), birth before 37 weeks gestation, is the leading cause of neonatal morbidity and mortality, with tremendous health and economic costs for infants, families and society.1 PTB accounts for as many as 75% of perinatal deaths in the United States. Surviving infants are at increased risk for serious long-term health consequences including cerebral palsy, chronic lung disease, neuromuscular disabilities, visual and hearing impairments, and motor, cognitive, behavioral, social-emotional and growth problems.2,3 The economic burden for this major public health issue in the United States is estimated to be $26.2 billion.4 PTB is thought to be a syndrome initiated by multiple pathological mechanisms, including inflammatory processes.5,6 However, typical indicators of infection such as elevated white blood count may not be present. In fact, cytokines, the biologic markers of inflammation, may be key indicators of the inflammatory pathway to PTB.4,7 Interleukin-1 (IL-1), and especially IL-1 beta (β) is often regarded as the most potent mediator of inflammation. Together with tumor necrosis factor-α, it stimulates the amnion, decidua and myometrium to produce prostaglandin.8 IL-6 stimulates production of acute phase proteins that accompany inflammatory disease and stimulate the production of enzymes needed for prostaglandin synthesis,8,9 while IL-8 has been posited to be important in cervical ripening.8 These pro-inflammatory cytokines, especially those that stimulate prostaglandin E2α, are believed to mediate the initiation of preterm labor.8,10 Conversely, IL-10 is thought to inhibit the synthesis of IL-1, thereby keeping the immune response in check.9 Additionally, increased transforming growth factor β assists in regulating progesterone, a hormone important for the maintenance of pregnancy.8 Moreover, IL-1 receptor antagonist (IL-1Ra) is a natural inhibitor of the pro-inflammatory effects of IL-1β, preventing IL-1β from sending signals to the cells; whenever IL-1β is produced, production of IL-1Ra occurs as well. Thus, IL-1Ra is considered as a marker of IL-1β production.11 Cytokines interact in a complex, non-linear way and can have both synergistic and antagonistic relationships with other cytokines.9,12,13 For example, IL-1 stimulates the production of IL-6 9 while IL-6 has an inhibitory effect on IL-1 production, providing important negative feedback.9 The synergistic relationship of IL1-β and IL-6 leads to an increase in the synthesis of oxytocin receptors in the myometrium.12,14 IL-10 inhibits the production of IL-1 and downregulates the expression of antigens by cells, which assists in keeping the immune response in check.9
Researchers examining the role of cytokines in PTB have used a variety of biological fluids including maternal and/or fetal blood, amniotic fluid, urine, cervical and/or vaginal secretions, and placental tissue. Maternal blood samples have shown the highest levels of IL-6, IL-1β and IL-8 associated with PTB.15–21 Others have reported increased levels of tumor necrosis factor-α, IL-12 and IL-18 in maternal blood in PTB.19 Increased levels of interferon γ with PTB have also been reported.22,23
However, the studies about levels of anti-inflammatory cytokines in maternal blood in relation to PTB are limited and the results are inconsistent. For example, Blanco-Quiros et al.24 reported increased levels of IL-10 with PTB, but Makhseed et al.23 found IL-10 levels decreased with PTB. In other studies, levels of transforming growth factor β-1 and granulocyte colony stimulating factor were increased with PTB.19,25
In addition, cytokine profiles are confounded by other variables. For example, higher levels of tumor necrosis factor-α and IL-1β in amniotic fluid were found in African American women experiencing PTB as compared with Caucasian women.15 Conversely, among Caucasian women experiencing PTB, IL-8 was higher than in African American women.26 In Hispanic women, acculturation has been recently implicated as predicting IL-10 levels and PTB.27 Maternal preeclampsia has been reported to be associated with elevated levels of IL-628 and women with gestational diabetes have been reported to have higher levels of IL-6 and lower IL-10 levels.25 Either overweight or obesity during pregnancy is another factor affecting cytokine levels and inflammation,29 as is age.30 Infections have also been implicated in the pathogenesis of PTB in conjunction with pro-inflammatory cytokines, particularly IL-6.31 Prior PTB increases the risk of, subsequent PTB32 possibly because of changes in cytokines and inflammation, and is thus, another potential confounder. Additionally, gravidity, or number of pregnancies, as well as social context, for example, marital status, that may increase psychological stress, needs to be considered when analyzing inflammation and cytokine levels.33
This study analyzed the interactions of two cytokines, IL-1Ra and IL-6, as well as interactions with IL-10, one of the major anti-inflammatory cytokines, in predicting PTB in Hispanic women, an understudied and ethnically at risk population.
Methods
Setting and participants
The Institutional Review Board at the primary universities approved the study and all participants provided signatures for informed consent. A convenience sample was used with non-consecutive sampling from 11 obstetrical sites. Participants from the Houston/Gulf Coast area were selected from a community clinic in Pasadena, Texas (n = 68). The majority of these participants were self-pay or state-funded. Participants from the San Antonio/South Texas area were selected from five private-practice physicians in south San Antonio (n = 172); the majority of these had Medicaid. In the Central Texas area, participants were selected from five Austin community clinics (n = 279) and were primarily self-pay or state-funded.
Data were collected between 2003 and 2007 from women at 22 to 24 weeks gestational age and birth outcomes were examined from the medical record. This gestation range was chosen as previous work has established this as an important time to measure biological factors associated with PTB.34 The mean gestational age at the time of data collection was 22.76 weeks (s.d. = 0.98). Before data collection, a power analysis based on estimates of 0.80 was conducted, indicating that a sample size of 470 women would be needed to detect moderate statistical effects with α<0.05. As a prelude to the current analysis that investigates PTB in logistic regression models, we conducted a sensitivity power analysis for a logistic regression model at the observed rate of PTB at 7% and the anticipated PTB rate of 12% to determine the smallest effect sizes that would be significant under these parameters. The power analysis for a sample size of 470, two-tailed α = 0.05 and power = 0.80, for a continuous-independent variable standardized in a z score format indicated that the minimal significant effect size that we could expect to observe was an odds ratio (OR) of 1.64 for a 7% PTB rate and 1.49 for a 12% PTB rate.
A number of criteria were used to select participants, including singleton intrauterine pregnancy at 22 to 24 weeks gestation confirmed by ultrasound and date of last menstruation, self-reported Hispanic ethnicity, ability to read or speak Spanish or English, and age between 14 and 40 years. Exclusion criteria were (1) known uterine or cervical abnormalities, (2) kidney disease, (3) heart disease, (4) autoimmune disorders, (5) diabetes requiring medication (that is, type 1 or 2 diabetes as diagnosed by the provider on the prenatal record) (6) asthma requiring use of steroid inhaler, (7) preeclampsia (as diagnosed on the prenatal record by the provider) at the time of data collection, (8) oral steroids 1 month before the time of enrollment, (9) congenital anomalies as determined by fetal ultrasound, (10) blood group isoimmunization, (11) active cervicovaginal bleeding or placenta previa or (12) major (Diagnostic and Statistical Manual of Mental Disorders Version IV) diagnosis of a mental disorder. After procurement of maternal and infant outcomes, we retained women with gestational diabetes in the database only if they were diet controlled (n = 18; 2 PTB); Participants with preeclampsia were incorporated in the analysis only if the diagnosis occurred after data collection at 22 to 24 weeks gestation (n = 33; 5 PTB).
Procedures
Research nurses reviewed prenatal records of possible participants to ascertain pregnancies at <22 weeks gestation. Once gestation of 22 to 24 weeks was determined, participants were recruited either before or after routine prenatal doctor visits. Risks and benefits of the study were described to potential participants. If they consented to be in the study, data collections were scheduled for a separate time from the prenatal visit with the provider.
At the time of data collection, the research nurses obtained demographic data and obstetrical history data from the participants’ charts. The nurses measured participants’ height and weight and calculated body mass index using participants’ pre-pregnancy weight, obtained from the chart. Local or systemic infections, including bacterial vaginosis were noted to control for infections that could affect cytokine levels. The nurses also drew 20 ml of venous blood, performed a speculum examination, and collected urine for drug screens and vaginal discharge on a microscope slide for later determination of bacterial vaginosis by Gram staining; the procedure has been previously described.35 The urine samples were later tested to establish whether cotinine or illicit drugs were present. Data collection appointments were between 1200 and 1400 h to control for diurnal variations in plasma cytokine levels.
Assessment of PTB
Participant medical records were reviewed by the research nurses after birth for maternal and infant outcomes. To confirm accurate gestational age, an ultrasound at <20 weeks was used. PTB was defined as parturition before 37 weeks 0 days gestational age.
Cytokine analysis
After venipuncture, venous blood samples were immediately centrifuged at 2000 r.p.m. for 10 min. The plasma supernatant was then decanted and stored in polypropylene tubes at −80°C. Highly sensitive enzyme-linked immunosorbent assays for IL-1Ra, Il-6 and Il-10 levels were used from R&D Systems (Minneapolis, MN, USA). The minimal detectable level was >2.15 pg ml−1 for IL-1Ra, >0.01 pg ml−1 for IL-6 and >0.5 pg ml−1 for IL-10 using high sensitivity kits. Assay results were read spectrophotometrically using a μ-Quant Reader (Bio-Tek Instruments, Winooski, VT, USA). All assays were completed by the same laboratory technician who was blind to participant names and information. Each analyte had an interassay and intraassay variance <15%.
Data analysis
The distributional characteristics of the cytokines were examined before analysis to determine whether data transformations were necessary. Visual inspection of IL-1Ra and IL-6 indicated that the variables exhibited positive skew. A natural log transformation was applied to these variables, transforming both to reasonably normal distributions. The IL-10 distribution was characterized by a large number of zero values (22.1%), and the non-zero values exhibited a right-skewed distribution. Because there was a naturally occurring dichotomy in IL-10 and zero values cannot be transformed, we modeled IL-10 as a binary variable. For analysis, we standardized the log-transformed values of the IL-1Ra and IL-6 variables in a z score format to improve interpretation of coefficients in the logistic regression models (that is, the ORs can be interpreted as an increase in the probability of PTB per standard deviation change in the independent variables).
All data were analyzed using SAS statistical software (version 9.2, SAS Institute, Cary, NC, USA). Demographic and biological measures were analyzed descriptively using means for continuous variables and frequencies for categorical variables. A logistic regression model and estimated marginal effects were implemented using SAS PROC GLIMMIX (SAS statistical software). The dependent variable in the logistic regression model was PTB. The logistic regression model included all of the cytokines and all possible two-way interactions between the cytokines in a single model. In addition, the following covariates were included in the model: age (in years), body mass index, gravida, marital status, history of PTB, infection during pregnancy and preeclampsia.
Results
Sample characteristics
The study included 470 participants. Sample characteristics are presented in Table 1. The mean gestational age for the sample at the time of data collection was 22.76 weeks (s.d. = 0.98). Marital status was the only demographic with significant mean differences in IL-1Ra (F (1) = 7.42, P = 0.007). There was a significant difference between levels of gravida for IL-10 (F (3) = 3.32, P = 0.020). Tukey’s multiple comparisons tests indicated the mean difference of 2.08 was significantly different (confidence interval (CI) = 0.27, 3.93) between women who were pregnant for the first time versus women who were pregnant for the third time. The percentage of PTB was significantly higher for those with a previous PTB (χ2 (2) = 5.20, P = 0.023). Total infections during pregnancy before 24 weeks were not related to cytokine levels or percentages of PTB.
Table 1.
Raw percentages, means (standard deviations) by cytokine, and percentage PTB for each demographic characteristic
| Percent (n = 470) | Mean levels (s.d.) of IL-1Ra (pg ml−1) | Mean levels (s.d.) of IL-6 (pg ml−1) | Mean levels (s.d.) of IL-10 (pg ml−1) | Preterm birth % (n = 33) | |
|---|---|---|---|---|---|
| Age (in years) | |||||
| Younger than 18 | 6.0% | 400.06 (373.41) | 1.03 (0.91) | 2.47 (3.37) | 7.1% |
| 18 to 34 | 91.3% | 302.57 (314.48) | 1.74 (1.73) | 2.90 (5.58) | 7.2% |
| 35 or older | 2.8% | 392.30 (239.53) | 1.35 (0.73) | 1.82 (2.18) | 0.0 |
| Body mass index | |||||
| Normal | 49.4% | 266.96 (250.68) | 1.52 (1.71) | 3.47 (6.95) | 8.3% |
| Overweight | 33.2% | 330.65 (354.50) | 1.77 (1.65) | 2.43 (3.55) | 6.6% |
| Obese | 17.4% | 325.95 (209.24) | 1.71 (1.12) | 2.20 (2.88) | 2.8% |
| Gravida | |||||
| 1 Pregnancy | 27.6% | 273.79 (234.53) | 1.68 (1.92) | 2.21 (2.75)* | 8.6% |
| 2 Pregnancies | 30.2% | 309.34 (323.53) | 1.73 (1.83) | 2.60 (4.07) | 3.6% |
| 3 Pregnancies | 22.9% | 330.33 (408.49) | 1.74 (1.62) | 4.29 (9.46)* | 9.4% |
| 4 or More pregnancies | 19.2% | 353.61 (308.17) | 1.57 (1.10) | 2.40 (2.91) | 7.9% |
| Marital status | |||||
| Not married | 57.4% | 346.35** (378.68) | 1.74 (1.83) | 2.81 (4.85) | 6.7% |
| Currently married | 42.6% | 263.41 (198.81) | 1.61 (1.46) | 2.88 (6.10) | 7.5% |
| History of preterm birth | |||||
| No | 92.1% | 303.02 (289.05) | 1.70 (1.72) | 2.94 (5.59) | 6.2%* |
| Yes | 7.9% | 412.46 (556.19) | 1.60 (1.15) | 1.60 (1.78) | 16.2% |
| Infection during pregnancy | |||||
| No | 56.0% | 303.64 (324.13) | 1.78 (1.73) | 3.02 (6.09) | 8.1% |
| Yes | 44.0% | 324.39 (327.08) | 1.64 (1.71) | 2.49 (4.51) | 5.2% |
Abbreviations: IL-1Ra, interleukin-1 receptor antagonist; PTB, preterm birth.
P<0.05.
P<0.005.
In addition to the characteristics shown in Table 1, there were 33 preeclamptics noted at delivery in the total sample. There were no significant differences in cytokine levels between participants who were diagnosed with preeclampsia and those who were not. Therefore, we kept the women who delivered PTBs due to preeclampsia in the analysis (n = 5). There were also 18 women with gestational diabetes noted at delivery. The levels of cytokines did not significantly differ between participants who had diabetes and those who did not, so data from these participants who delivered preterm (n = 2) were also retained in the analysis.
There were 33 PTBs in the sample, with 28 spontaneous and five medically indicated due to preeclampsia. In all, 8 (24%) of the 33 preterm infants were <34 weeks gestation. Except for one mother using an opiate who delivered a term low birth weight infant, the use of drugs did not influence birth outcomes. In addition, the number of smokers was very low (n = 3) and smoking was thus not used as a covariate.
We analyzed several demographic variables for differences in PTB. The sample had 58.3% (n = 274) participants who had lived in the United States <10 years with a PTB rate of 5.1%, versus those participants who had lived in the United States >10 years (41.7%, n = 196) who had a 9.7% PTB rate. We found similar results when examining whether the participants were foreign born or US born, with a 5% PTB rate for the foreign born participants (68% of the sample, n = 320), and 10% (31.8% of the sample, n = 150) for the US born participants.
Demographic and biological characteristics
We examined missing data patterns before analysis. Among the 470 participants, 4 (0.8%) were missing data on IL-10, leaving 466 participants with complete data on the variables of primary interest. We elected not to impute values for missing data since there was not sufficient data to do. To assess any potential bias due to the excluded cases, we conducted analyses without covariates, allowing us to make use of all of the eligible cases.
To limit the number of covariates and avoid an oversaturated model, we examined each of the demographic and biological characteristics individually as predictors of PTB in logistic regression models. The intent of the analysis was to provide the most conservative assessment of the relationship between each of these variables and PTB on our outcome of primary interest, because their unique effects could potentially be obscured in a multiple predictor model. Among the covariates we included in our final model, only history of PTB was a significant predictor of PTB (χ2 = 4.79 (1468), P = 0.029, OR = 2.91). However, we also included premature rupture of membrane as a covariate; there was only one full-term birth mother and seven PTB mothers who had premature rupture of membrane. To rule out the possibility that including premature rupture of membrane in the model may have influenced our primary findings, we analyzed the data for only those who did not experience premature rupture of membrane. The IL-1Ra × IL-6 interaction was not significant (P = 0.159); however, the IL-1Ra × IL-10 interaction was significant (P = 0.003). We also analyzed the data for only those with preeclampsia and with a history of PTB to determine if these variables affected the results. When removing 33 cases with preeclampsia (5 of which resulted PTB in this study), the IL-1Ra × IL-6 interaction was marginally significant (P = 0.072) and the IL-1Ra × IL-10 interaction was significant (P = 0.003). When removing 37 cases with a history of PTB (6 of which resulted in PTB in this study), the IL-1Ra × IL-6 interaction was marginally significant (P = 0.068) and the IL-1Ra × IL-10 interaction was significant (P = 0.001). Thus, the P-values were slightly reduced in these subsamples and removing five and seven cases of PTB from the analysis reduced power by 5 and 9%, respectively. There were 13 cases of positive bacterial vaginosis who had PTB by Nugent’s criteria and these were taken into account as the covariate infection during pregnancy in all the statistics presented.
Logistic regression model
Before fitting the logistic regression model, we assessed for collinearity in the independent variables. The highest correlation observed among the predictors was r = 0.49 for age and gravida; the second largest correlation was r = 0.25 for age and marital status. The largest correlation between cytokines was the point-biserial correlation between IL-1Ra and the binary version of IL-10 at r = 0.14. The correlations were below the diagnostic collinearity cutoffs of r = 0.9.36
Model results are presented in Table 2. Because we were concerned with the ratio of non-PTB cases to PTB cases, we examined the two critical interactions in smaller models that included the variables in the interaction and two covariates, age and body mass index, that exhibited significant relationships to one or more of the cytokines. The interaction between IL-1Ra and IL-6 remained significant in the smaller model (χ2 = 3.91, P = 0.048) as did the interaction between IL-1Ra and IL-10 (χ2 = 8.96, P = 0.003).
Table 2.
Results of logistic regression
| Estimate | Standard error | Wald χ2 | χ2 Probability | |
|---|---|---|---|---|
| Intercept | −1.93 | 1.85 | ||
| IL-1 | 5.80 | 1.79 | 10.44 | 0.001 |
| IL-6 | 0.69 | 0.63 | 1.20 | 0.274 |
| IL-1 × IL-6 | 0.59 | 0.28 | 4.52 | 0.033 |
| IL-10 | 1.33 | 0.97 | 1.88 | 0.170 |
| IL-1 × IL-10 | −5.81 | 1.80 | 10.41 | 0.001 |
| IL-6 × IL-10 | −0.22 | 0.68 | 0.11 | 0.744 |
| Maternal age | −0.03 | 0.06 | 0.21 | 0.649 |
| Maternal BMI | −0.10 | 0.05 | 3.63 | 0.057 |
| Infection during pregnancy | −0.12 | 0.48 | 0.06 | 0.801 |
| History of preterm birth | 1.37 | 0.71 | 3.79 | 0.052 |
| Gravida | −0.05 | 0.22 | 0.04 | 0.837 |
| Gestational age (days) at which the blood was drawn | 0.10 | 0.12 | 0.71 | 0.400 |
| Marital status | 0.60 | 0.50 | 1.44 | 0.231 |
| Preeclampsia | 0.93 | 0.68 | 1.90 | 0.168 |
Abbreviations: BMI, body mass index; IL-1, interleukin-1.
IL-1Ra was the only cytokine that had a significant main effect in the model; risk of PTB increased as the level of IL-1Ra increased. The interaction between IL-6 and IL-10 was not significant. However, there was a significant effect for the IL-1Ra and IL-6 interaction. Predictive values for the interaction are presented in Figure 1. The interaction between IL-1Ra and IL-10 was also significant, indicating that mothers with high IL-1Ra and non-detectable IL-10 were at greatest risk for PTB. Predictive values for the interaction are presented in Figure 2. Following recommendations from Hosmer and Lemeshow,37 we elected to not report ORs for variables involved in interactions as the exponent of the coefficient. Rather, we report ORs at the means of the other variables involved in interactions. The OR for IL-1Ra to predict PTB was 2.55 (95% CI = 1.24, 5.24), the OR for IL-6 was 1.45 (95% CI = 0.93, 2.28) and the OR for IL-10 was 2.52 (95% CI = 0.50, 12.78). The observed power for these ORs was >0.99 for IL-1Ra, 0.50 for IL-6 and >0.99 for IL-10.
Figure 1.
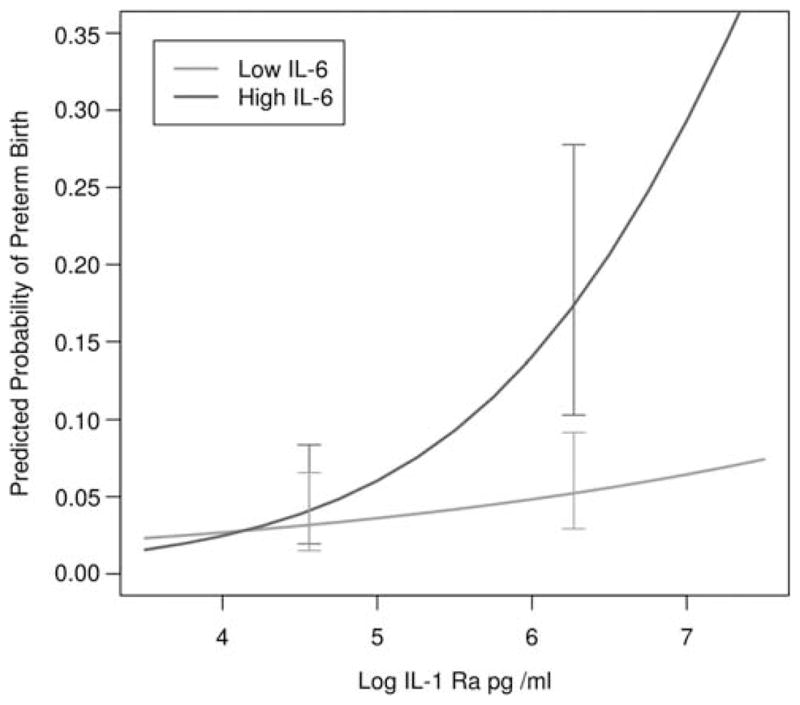
Predicted probabilities of preterm birth as a function of interleukin-1 receptor antagonist (IL-1Ra) and IL-6. Predicted results in the figure are based on a model in which we dichotomized IL-6, to estimate the standard error of the difference for points on a continuous variable. IL-6 was dichotomized at the 80th percentile, which was representative of the point on the IL-6 continuum where significant differences emerged. Predicted probabilities are generated from the logistic regression model. Error bars represent half of a confidence interval (CI) on each line so that non-overlapping bars represent significant differences.
Figure 2.
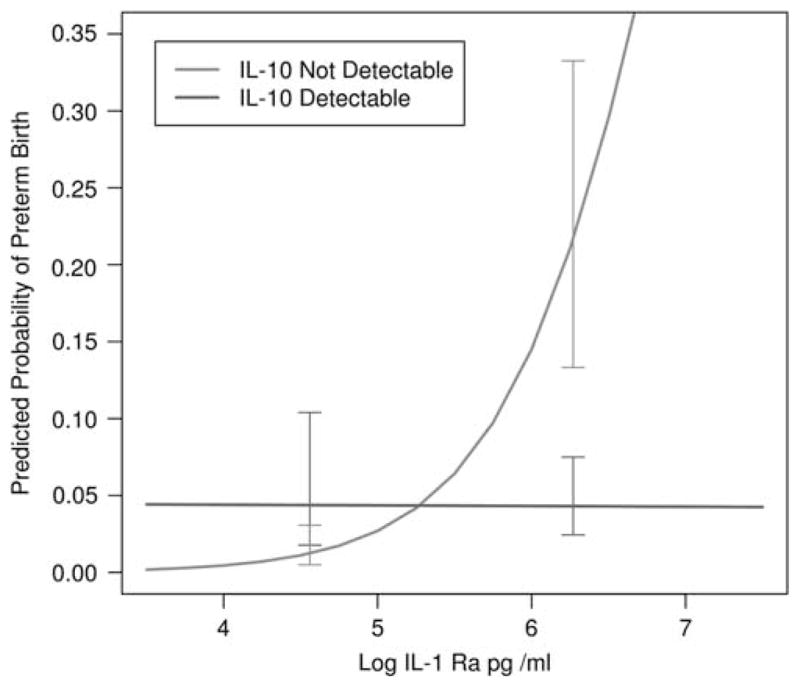
Predicted probabilities of preterm birth as a function of interleukin-1 receptor antagonist (IL-1Ra) and IL-10. Predicted probabilities are generated from the logistic regression model. Error bars represent half of a confidence interval (CI) on each line so that non-overlapping bars represent significant differences.
Discussion
Our results indicate that in pregnant Hispanic women aged 14 to 40 years, at 22 to 24 weeks gestation, IL-1Ra with IL-6 had a direct interaction while IL-1Ra interacted inversely with IL-10 to predict PTB. The finding that IL-1Ra, as a marker of the biological activity of IL-1β, predicts PTB is consistent with several other studies.11,18,19,21 In the study of 17 inflammatory markers,19 high serum levels of IL-1β and IL-6 as well as other pro-inflammatory cytokines were associated with increased risk of PTB. Moreover, Vogel et al.38 found that in women with a history of PTB, IL-6 was a marker of recurrent PTB. In our study, women who had a history of PTB had lower levels of IL-6 than those who did not; however, the difference was not significant. Our results are consistent with the idea that IL-1β may stimulate the production of IL-6.9 However, the interaction between IL-1Ra and IL-6 was only weakly significant and is likely to be an example of a synergistic relationship that may be linked to oxytocin receptor synthesis in the myometrium of the uterus.14
It is important to note in interpreting the results that changes in the pro- and anti-inflammatory cytokines in women with PTB potentially represent a subset of the women who deliver before 37 weeks. Moreover, multiple cytokines likely affect cells at the same time. Our data suggested an interaction of cytokines and cytokine receptor antagonists that may be related to the biological response. For example, the finding that the risk of PTB increases as IL-1Ra increases and when IL-10 is low may be an example of how the cytokines work antagonistically on the cells at the same time. Our results are consistent with previous studies that showed that in the relative absence of anti-inflammatory cytokines, the risk of labor is increased.39
It warrants discussion that the PTB rate out of this sample was relatively low (7%). However, there were differences in the PTB rate by whether the participant had been born in the United States or lived in the United States >10 years. These data suggest that the risk of PTB increased the longer a woman lived in the United States and was exposed to American culture. This risk warrants further investigation as to possible causal mechanisms. Moreover, our findings are lower than the national PTB rate for Hispanics (12.2%) and much lower than the PTB rates for African Americans (18.1%).40
Our results are consistent with the results of prior studies that examined possible multifactorial causes of PTB. Romero et al.41 described the cytokine network as redundant, indicating that the blockade of any single factor of a cytokine is insufficient to prevent PTB. Anti-inflammatory cytokines such as IL-10 decrease the inflammatory response and are thought to disturb the cascade of pro-inflammatory cytokines; thus, IL-10 is thought to be an important cytokine in safeguarding pregnancy.41 Although IL-10 did not have a significant main effect on PTB in our study, as the results of earlier studies suggest, this finding may be associated with a biological bias toward Th1, or pro-inflammatory cytokines, and a less favorable predisposition to the Th2, or anti-inflammatory cytokines. That is, it may be that it is the overabundance of pro-inflammatory cytokines in association with low levels of anti-inflammatory cytokines that is important in the cascade leading to PTB.23
One limitation of our study is that it was cross-sectional in design. Therefore, we could not examine changes in the cytokines over pregnancy to identify critical periods in the prediction of PTB. Longitudinal studies of pregnancy biomarkers such as cytokines are evolving;42 results indicate that a decrease in pro-inflammatory cytokines and an increase in counter-regulatory cytokines, particularly IL-10, are optimal for maintenance of pregnancy. Further study, however, is needed to clarify the trajectory of maternal inflammation during pregnancy to predict key periods of risk for PTB and to identify key times to initiate intervention. Another limitation was the use of a convenience sample, possibly increasing the risk of selection bias.
The major significance of the findings presented here is that cytokines operate in concert with each other. Studying single cytokines or even examining only all of one class of cytokines will not give the complete picture needed to understand relationships leading to PTB. Key areas for future research are examining the interactions among cytokines during pregnancy, including baseline or early pregnancy measurement, the balance of pro-inflammatory and anti-inflammatory cytokines, and the ways in which this balance or imbalance may affect pregnancy outcomes. Once predictors of pregnancy outcomes are identified, levels of various cytokines at particular time intervals may prove to be useful markers of pregnancy maintenance.
Acknowledgments
This research was funded by NIH/NINR R01 07891.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193(3):626–635. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 2.Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21 (2):123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- 3.Ashton D. Prematurity—infant mortality: the scourge remains. Ethn Dis. 2006;16(2 Suppl 3):S3-58–62. [PubMed] [Google Scholar]
- 4.Behrman RE, Butler AS. Preterm Birth Causes, Consequences, and Prevention. Institute Medicine of the National Academies, National Academies Press; Washington DC: 2006. [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001;15(3):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn S. Cytokines in the perinatal and neonatal periods: selected aspects. J Perinat Neonatal Nurs. 2008;22(3):187–190. doi: 10.1097/01.JPN.0000333918.15081.86. [DOI] [PubMed] [Google Scholar]
- 9.Corwin EJ. Understanding cytokines part I: physiology and mechanism of action. Biol Res Nurs. 2000;2(1):30–40. doi: 10.1177/109980040000200104. [DOI] [PubMed] [Google Scholar]
- 10.Formby B. Immunologic response in pregnancy. Its role in endocrine disorders of pregnancy and influence on the course of maternal autoimmune diseases. Endocrinol Metab Clin North Am. 1995;24(1):187–205. [PubMed] [Google Scholar]
- 11.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13(4–5):323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 12.Farina L, Winkelman C. A review of the role of proinflammatory cytokines in labor and noninfectious preterm labor. Biol Res Nurs. 2005;6(3):230–238. doi: 10.1177/1099800404271900. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP. Nonlinear systems in medicine. Yale J Biol Med. 2002;75(5–6):247–260. [PMC free article] [PubMed] [Google Scholar]
- 14.Friebe-Hoffmann U, Chiao JP, Rauk PN. Effect of IL-1beta and IL-6 on oxytocin secretion in human uterine smooth muscle cells. Am J Reprod Immunol. 2001;46(3):226–231. doi: 10.1034/j.1600-0897.2001.d01-6.x. [DOI] [PubMed] [Google Scholar]
- 15.Lyon D, Cheng C-Y, Howland L, Rattican D, Jallo N, Pickler R, et al. Integrated review of cytokines in maternal, cord, and newborn blood: part I—associations with preterm birth. Biol Res Nurs. 2010;11(4):371–376. doi: 10.1177/1099800409344620. [DOI] [PubMed] [Google Scholar]
- 16.Murtha AP, Greig PC, Jimmerson CE, Herbert WN. Maternal serum interleukin-6 concentration as a marker for impending preterm delivery. Obstet Gynecol. 1998;91(2):161–164. doi: 10.1016/s0029-7844(97)00602-9. [DOI] [PubMed] [Google Scholar]
- 17.Murtha AP, Greig PC, Jimmerson CE, Roitman-Johnson B, Allen J, Herbert WNP. Maternal serum interleukin-6 concentrations in patients with preterm premature rupture of membranes and evidence of infection. Am J Obstet Gynecol. 1996;175(4 Part 1):966–969. doi: 10.1016/s0002-9378(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 18.Torbe A, Czajka R, Kordek A, Rzepka R, Kwiatkowski S, Rudnicki J. Maternal serum proinflammatory cytokines in preterm labor with intact membranes: neonatal outcome and histological associations. Eur Cytokine Netw. 2007;18(2):102–107. doi: 10.1684/ecn.2007.0092. [DOI] [PubMed] [Google Scholar]
- 19.Vogel I, Goepfert AR, Thorsen P, Skogstrand K, Hougaard DM, Curry AH, et al. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J Reprod Immunol. 2007;75(2):133–140. doi: 10.1016/j.jri.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Grischke EM, Schwab S, Hettinger S, Loibl S, Aulmann M, et al. Predictive value of serum interleukin-6 and -8 levels in preterm labor or rupture of the membranes. Acta Obstet Gynecol Scand. 2000;79(8):667–672. [PubMed] [Google Scholar]
- 21.Hasegawa K, Furuichi Y, Shimotsu A, Nakamura M, Yoshinaga M, Kamitomo M, et al. Associations between systemic status, periodontal status, serum cytokine levels, and delivery outcomes in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2003;74(12):1764–1770. doi: 10.1902/jop.2003.74.12.1764. [DOI] [PubMed] [Google Scholar]
- 22.Curry AE, Vogel I, Drews C, Schendel D, Skogstrand K, Flanders WD, et al. Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2007;86(9):1103–1110. doi: 10.1080/00016340701515423. [DOI] [PubMed] [Google Scholar]
- 23.Makhseed M, Raghupathy R, El-Shazly S, Azizieh F, Al-Harmi JA, Al-Azemi MMK. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reprod Immunol. 2003;49(5):308–318. doi: 10.1034/j.1600-0897.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 24.Blanco-Quiros A, Arranz E, Solis G, Villar A, Ramos A, Coto D. Cord blood interleukin-10 levels are increased in preterm newborns. Eur J Pediatr. 2000;159(6):420–423. doi: 10.1007/s004310051299. [DOI] [PubMed] [Google Scholar]
- 25.Kuzmicki M, Telejko B, Zonenberg A, Szamatowicz J, Kretowski A, Nikolajuk A, et al. Circulating pro- and anti-inflammatory cytokines in Polish women with gestational diabetes. Horm Metab Res. 2008;40(8):556–560. doi: 10.1055/s-2008-1073166. [DOI] [PubMed] [Google Scholar]
- 26.Menon R, Merialdi M, Lombardi SJ, Fortunato SJ. Differences in the placental membrane cytokine response: a possible explanation for the racial disparity in preterm birth. Am J Reprod Immunol. 2006;56(2):112–118. doi: 10.1111/j.1600-0897.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 27.Wommack JC, Ruiz RJ, Marti CN, Stowe RP, Brown CEL, Murphey C. Interleukin-10 predicts preterm birth in acculturated hispanics. Biol Res Nurs. 2011 doi: 10.1177/1099800411416225. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 29.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci. 2010;65A(4):429–433. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(s1):16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 32.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195(3):643–650. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Beeckman K, De Putte SV, Putman K, Louckx F. Predictive social factors in relation to preterm birth in a metropolitan region. Acta Obstet Gynecol Scand. 2009;88(7):787–792. doi: 10.1080/00016340902974007. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz RJ, Fullerton J, Brown CE, Dudley DJ. Predicting risk of preterm birth: the roles of stress, clinical risk factors, and corticotropin-releasing hormone. Biol Res Nurs. 2002;4(1):54–64. doi: 10.1177/1099800402004001007. [DOI] [PubMed] [Google Scholar]
- 35.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox J, editor. Regression Diagnostics. Sage Publications; London: 1991. [Google Scholar]
- 37.Hosmer DW, Lemeshow S. Probability and Statistics Series: Applied Logistic Regression. 2. Vol. 354. John Wiley and Sons, Inc; New York: 2000. [Google Scholar]
- 38.Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):516–525. doi: 10.1111/j.0001-6349.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 39.Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47(2):87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 40.National Center for Health Statistics. Final Natality Data. [cited 15 May 2011]. Available from: http://www.marchofdimes.com/peristats.
- 41.Romero R, Espinoza J, Gonçalves L, Kusanovic J, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, et al. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 2010;53(2):170–177. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


