Abstract
Purpose
We describe our experience correcting a cohort of infants with contact lenses in the Infant Aphakia Treatment Study (IATS).
Materials and Methods
Fifty-seven infants 1 to 6 months of age were randomized to contact lens wear. An examination under anesthesia was performed at the time of enrollment and at approximately 1 year of age. A traveling examiner assessed visual acuity at approximately 1 year of age.
Results
Forty-two treated eyes (74 %) were fitted with silicone elastomer (SE) contact lenses; twelve eyes (21 %) with rigid gas permeable (RGP) contact lenses and three eyes (5%) wore both lens types. Median visual acuity was +0.80 logMAR in both lens type-wearing groups. The mean (± SD) keratometric power of the treated eyes was 46.3 ± 2.8 D at baseline and 44.6 ± 2.3 D at one year of age for a mean decrease of 0.2 ± 0.2 D/mo. Keratometric astigmatism of treated eyes was 1.98 ± 1.37 D at baseline and 1.62 ± 0.98 D at one year of age for a mean decrease of 0.05 ± 0.2 D/mo. The mean RGP lens base curve at baseline was 47.62 D ±2.62 D vs 47.00 D ± 3.50 D at 12 months after surgery. Children wearing SE lenses required a mean of 10.9 replacements (range 2–24) compared to 16.8 replacements (range 8–32) for children wearing RGP lenses. Three adverse events occurred.
Conclusions
Contact lenses were worn successfully with relatively few adverse events by a cohort of infants with unilateral aphakia. The visual acuity results were identical independent of the contact lens material or modality. RGP lenses needed replacement more often than SE lenses.
Keywords: contact lens, aphakia, cataract, infant
Introduction
Until the 1970s, it was generally believed that there was no means of restoring the vision in an eye with a unilateral congenital cataract.1 However, subsequent studies demonstrated that excellent visual results could be obtained with early surgical treatment coupled with optical correction with a contact lens and patching therapy of the fellow eye. 2–3 However, treatment results continue to be poor in some infants with unilateral congenital cataracts due to a delay in treatment or poor compliance with contact lens wear or patching therapy of the fellow eye.4–5 The Infant Aphakia Treatment Study was designed to compare the visual outcomes in children 1 to 6 months of age with a unilateral congenital cataract randomized to optical aphakic correction with contact lenses or an intraocular lens (IOL). Children randomized to IOL treatment had their residual refractive error corrected with spectacles. Children randomized to no IOL had their aphakia treated with a contact lens. In previous publications we have shown that the visual results are comparable for these two treatments at 1 year of age, but significantly more of the infants randomized to IOL implantation required additional intraocular surgeries.6–7 In this report, we compare the visual results achieved with silicone elastomer(SE) and rigid gas permeable (RGP) contact lenses as well as describing in more detail the clinical findings of the children randomized to contact lens wear.
Materials and Methods
The Infant Aphakia Treatment Study (IATS) is a multicenter, randomized, controlled clinical trial comparing intraocular lens and contact lens treatments after cataract surgery performed in children with a unilateral congenital cataract at 1 to 6 months of age. The study design, surgical technique, follow-up schedule, patching and optical correction regimens, evaluation methods, and patient characteristics at baseline have been reported in detail previously6 and are only summarized in this report. The complete protocol for the IATS can be viewed online at (http://www.sph.emory.edu/IATS). This study was approved by the institutional review boards of all the participating institutions and was in compliance with the Health Insurance Portability and Accountability Act. Informed consent was obtained from a parent or legal guardian of all patients prior to randomization. The off-label research use of the Acrysof SN60AT and MA60AC IOLs (Alcon Laboratories, Fort Worth, Texas) was covered by US Food and Drug Administration investigational device exemption # G020021.
Study Design
The main inclusion criteria were a visually significant congenital cataract (≥ 3 mm central opacity) in one eye and an age of 28 days to <210 days at the time of cataract surgery. Infants with a unilateral cataract due to persistent fetal vasculature (PFV) could be enrolled in the study as long as the PFV was not associated with visible stretching of the ciliary processes or involvement of the retina or optic nerve. The other main exclusion criteria were an acquired cataract, a corneal diameter <9 mm, a medical condition that might interfere with visual acuity testing, an intraocular pressure (IOP) ≥ 25 mmHg and prematurity (<36 gestational weeks). Patients were randomized to have either an IOL placed at the time of the initial surgery or to be left aphakic and corrected with a contact lens. Patients were examined at 1 day, 1 week, and 1, 3, 6, 9 and 12 months after surgery. Grating visual acuity was measured by a traveling examiner at 1 year of age (±2 months) using Teller Acuity Cards (Stereo Optical, Chicago, Illinois). Keratometry measurements were obtained at the time of initial cataract surgery and at an exam under anesthesia done approximately 2 weeks prior to the visual acuity assessment at 1 year of age; two keratometric readings within 1.0 D of each other were averaged. Refractive error was determined with cycloplegic retinoscopy. Caregiver diaries and telephone interviews were used to ascertain optical correction wearing time and patching time.
Contact Lens Correction
Within one week following cataract surgery, patients randomized to the contact lens (CL) group were fitted with either a SE (Silsoft Super Plus; Bausch & Lomb, Rochester, New York) or a RGP (X-Cel Laboratories, Duluth, GA.) contact lens with a 2.0 D overcorrection to provide a near-point correction at 50 cm. Contact lenses were fitted in a clinical setting without the aid of general anesthesia. The initial lens type and wearing modality were chosen at the discretion of the contact lens professional at each study site. Contact lens professionals were certified by written examination for each investigational site. Patients were evaluated by both the surgeon and the IATS certified CL professional at each study visit. A patient was deemed to have failed contact lens wear if the lens was worn for fewer than 4 hours per day on average for a period of 8 consecutive weeks as defined by the IATS study protocol.
Contact Lens Fitting
Silsoft Super Plus contact lenses for pediatric aphakia (>20 diopters) are available with the following parameters: diameter, 11.3mm, base curves: 7.5 mm (45.00D), 7.7mm (43.75D), and 7.9mm (42.75D), optic zone of 7.0mm and powers ranging from +23.00D to +32.00D in 3D steps; the power closest to the target refractive error was selected. The Silsoft material has an oxygen permeability (Dk) value of 340, with oxygen transmissibility (Dk/t) of 58 at 0.61mm. [B+L, http://www.bausch.com/en_US/ecp/visioncare/product/softcontacts/silsoftplus_ecp.aspxaccessed08/18/2011]. If an accurate refraction could not be obtained initially, a 7.5mm BC/+32.0 D/11.3mm diameter Silsoft Super Plus lens was dispensed, and the lens power and fit were subsequently refined at the earliest opportunity. In cases in which a SE lens was not worn successfully, an RGP contact lens was fitted, and vice versa. RGP lenses were manufactured in Boston XO2 (hexafocon B, B+L, Rochester, NY) with an oxygen permeability (Dk) of 141, and a transmissibility of 28 at a nominal center lens thickness of 0.50mm.
A custom designed and standardized set of aspheric, high plus, lenticulated diagnostic lenses were used for RGP lens fitting. The diagnostic lenses have axial edge lifts of .11mm–.17mm. The lens design encompasses a mathematical relationship between the base curve and lens diameter. The base curve in millimeters plus 1.3mm equals the overall lens diameter. The size of the posterior optic zone equals the base curve radius in millimeters. If the diameter was increased, the anterior optic zone size was decreased to avoid center thicknesses greater than 0.50mm. However, the anterior optic zone diameter remained large enough for full pupil coverage in all gazes. The initial diagnostic lens base curve was selected by adding one diopter to the flat keratometric value. Ideally the lens fit would allow approximately 30 microns of tear power between the posterior lens surface and the cornea. Fluorescein dye was used to evaluate lens to cornea relationship, movement, centration and edge clearance. A change in lens-to-cornea relationship was primarily guided by fluorescein pattern evaluation. Lens powers were determined by retinoscopy over the diagnostic lens.
Application and removal training was provided to each caregiver along with a training video and written and verbal instructions that were confirmed at each study visit. Of the 42 SE patients, 28 wore a lens on a continuous wear schedule (7–21 nights), 6 wore daily wear, 3 both daily and continuous wear and 5 undocumented. Two patients wearing SE lenses had their routine lens care (removal, cleaning and/or exchange) performed by either the contact lens professional or the investigator at two clinical sites. Children wearing a GP material wore a lens on a daily wear basis.
Statistical Methods
The median visual acuity at 1 year of age for patients wearing RGP lenses was compared to that of patients wearing SE lenses using the Wilcoxon rank-sum test. A nonparametric test was used because of the skewed distribution of the data and because of the assignment of visual acuity values for patients with vision below the level detectable with Teller acuity cards (low vision, light perception, no light perception). Keratometric measurements were made at the time of cataract surgery and at approximately 1 year of age. Since the eligibility criteria for age was 1 to 6 months, the time interval between the keratometric measurements varied among patients and therefore the change in keratometric measurements was expressed as a rate (change per month). The mean keratometric power was compared between treated and fellow eyes at baseline and 1 year of age using a paired t test. The same test was used to compare the rate of change in keratometric power between treated and fellow eyes. The mean rate of change in keratometric power was compared between contact lens types using an independent group’s t test. A p-value <0.05 was deemed statistically significant.
Results
Study Population and Type of Contact Lens
There were 114 patients enrolled in the study with 57 patients randomized to contact lens treatment for aphakia. Among these 57 patients the median age at cataract surgery was 1.8 months (inter-quartile range = 1.1–3.1 months), 32 (56%) patients were female, and 49 (86%) patients were white. During the first 12 months after surgery, 42 (74%) patients wore SE lenses only on the treated eye, 12 patients (21%) wore RGP lenses only, and 3 patients (5%) wore both lens types. Only 3 of the 12 study sites fitted a RGP lens on a study patient. Thirty-seven of the eyes fitted with a SE lens wore a 7.5mm back optic zone radius (BOZR) with 11.3mm diameters. The remaining eight eyes were fitted with a flatter base curve. Five of these eight eyes were managed at one of the study sites.
The mean RGP lens BC at baseline was 47.62D ±2.62D. At 12 months after surgery, the mean RGP BC was 47.00D ±3.50D. Although our RGP sample size is small, the mean values determined in our study represent the initial RGP BC to be 1.62 D steeper than mean keratometric values at baseline and 1.87 D steeper at 12 months after surgery.
None of the patients randomized to contact lens wear was deemed a failure by study definition, and no secondary implants were performed during the first 12 months after cataract surgery.
Vision with Contact Lens Correction at 1 Year of Age
The visual acuity assessments were conducted by two masked, traveling examiners one of whom performed most of the examinations (72%). The median logMAR visual acuity of the treated eyes was +0.80 (Snellen equivalent 20/125) (interquartile range = 0.66–0.97). Very poor vision was present in the treated eye in 3 patients in the CL group (2 with pattern vision only detectable with the low vision card and 1 with light perception). The median logMAR visual acuity was 0.66 in the untreated eyes (Snellen equivalent 20/80–100)(interquartile range = 0.66–0.80).
There was no difference in median logMAR visual acuity between the 42 eyes wearing a SE lens only (median +0.80, interquartile range = 0.66–0.97) and the 12 eyes wearing a RGP lens only (median +0.80, interquartile range = 0.80–1.12 (p = 0.65).
Keratometric Findings
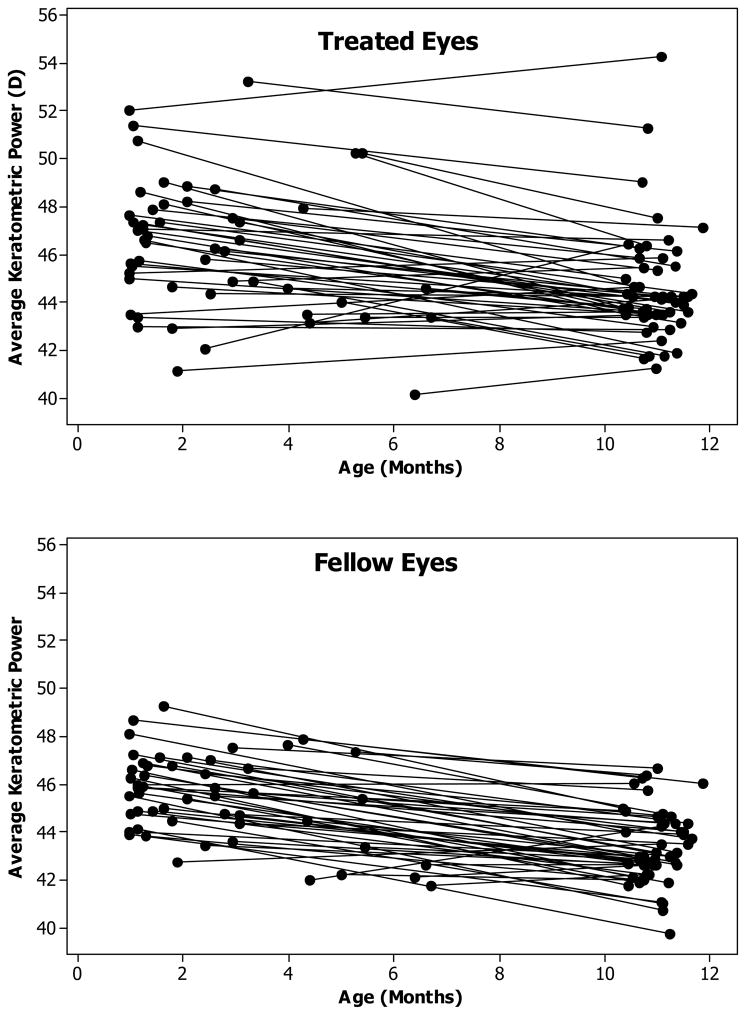
Keratometry measurements were available at both baseline and 1 year of age for 52 of the 57 contact lens patients. The mean time interval between baseline and 1 year of age was 8.5 months and varied from 4.1 to 10.4 months. At baseline, the treated eye was nearly 1.0 D steeper on average than the fellow eye (Treated Eye: 46.3 D, Fellow Eye: 45.4D, p = 0.009) (Table 1). The mean decrease in keratometric power per month was about 0.2 D for both the treated and fellow eyes (p = 0.56) resulting in a similar 1.0 D difference between the means of the treated and fellow eyes at 1 year of age (Treated Eye: 44.6 D, Fellow Eye: 43.4D, p = 0.001) (Table 1, Figure 1A and 1B).
Table 1.
Average Central Keratometric Power (D) at Baseline and 1 Year of Age for Treated and Fellow Eyes of 52 Patients Treated with a Contact Lens
| Follow-up Visit | Treated Eye* | Fellow Eye* | Difference* | p-value** | 95% CI† |
|---|---|---|---|---|---|
| Baseline | 46.3 (2.8) | 45.4 (1.8) | 0.9 (2.3) | 0.009 | 0.2–1.5 |
| Age 1 Year | 44.6 (2.3) | 43.4 (1.5) | 1.2 (2.5) | 0.001 | 0.5–1.9 |
| Change Per Month§ | −0.2 (0.2) | −0.2 (0.2) | 0.02 (0.3) | 0.56 | −0.06–0.1 |
Values are mean (standard deviation)
The p-value for the paired t test comparing the means of the treated and fellow eyes.
The 95% Confidence Interval for the difference between the means of the treated and fellow eyes.
The change was calculated as (Follow-up – Baseline) and therefore negative values indicate a decrease in average central keratometric power.
Figure 1.
Longitudinal changes in mean keratometry values. Plots of the baseline and follow-up values versus age for the treated (top) and fellow (bottom) eyes. The lines connect the values of individual eyes.
The mean decrease in keratometric power per month was smaller for treated eyes wearing RGP lenses (0.10 D, n=12, SD=0.28) compared to eyes wearing SE lenses (0.22, n=39, SD= 0.23) but this difference was not statistically significant (p = 0.15).
Corneal Astigmatism
The mean (± SD) keratometric astigmatism in the treated eyes at baseline was 1.98 ±1.37 D (n = 57, range = 0–6.25 D) (Table 2). The mean baseline flat meridian was 45.38 ±2.66 D, (range = 39.50–52.12 D) and the mean baseline steep meridian was 47.36 ±2.91 D (range = 40.75–54.37 D). At 1 year of age, the mean keratometric astigmatism in the treated eyes was 1.62 ±0.98D (n = 52, range = 0–5.0D). The mean flat meridian at one year of age was 43.78 ±2.34 D (range = 40.25–53.62 D) and the mean steep meridian at one year of age was 45.40 ±2.34 D (range = 42.0–54.87 D). The mean decrease in keratometric astigmatism per month was 0.05 ± 0.2 D/mo.
Table 2.
Keratometric Astigmatism at Baseline and 1 Year of Age
| Keratometric Astigmatism (D) | Baseline (n=57) n (%) |
1 Year of Age (n = 52) n (%) |
|---|---|---|
| 0.0–0.5 | 5 (9%) | 7 (13%) |
| 0.6–1.0 | 13 (23%) | 10 (19%) |
| 1.1–1.5 | 5 (9%) | 14 (27%) |
| 1.6–2.0 | 13 (23%) | 7 (13%) |
| 2.1–2.5 | 7 (12%) | 7 (13%) |
| 2.6–3.0 | 6 (11%) | 3 (6%) |
| > 3.0 | 8 (14%) | 4 (8%) |
Contact Lens Replacements
All contact lenses were provided by the study at no cost to the patient. During the first 12 months after surgery, replacement lenses were provided (n = 59) for 45 eyes (76%) wearing SE and 14 eyes (24%) wearing RGP lenses. Two patients are included in both groups as they were refit during the first 12 months after surgery with the alternative lens material and required lens replacements. Each time a lens was lost, a new over refraction determined or fitting parameter adjusted, two new lenses were provided to the patient.
For the SE wearing group, a total of 490 lenses were provided for 45 patients during the first 12 months after surgery. The average number of lenses provided per patient was 10.9 (range, 2–24). If one assumes an average retail cost of $175 per lens, eleven lenses equates to $1925. For the RGP wearing group, a total of 236 lenses were provided for 14 patients. The average number of lenses per patient was 16.8 (range, 8–32). If one assumes an average retail cost of $75 per lens, seventeen lenses equates to $1275.
Three adverse events occurred in our study population. The diagnosis for the events was corneal abrasion, bacterial keratitis and corneal opacity presumed to be related to a tight fitting lens. All three patients wore a SE lens on an overnight basis. All three adverse events resolved without sequelae.
Discussion
We found the same median logMAR grating acuities in eyes wearing SE and RGP contact lenses at one year of age. The majority of the patients wore SE contact lenses. The median retail cost of replacing SE lenses was higher than for RGP. None of the children developed complications related to the use of contact lenses that resulted in permanent loss of vision. The corneas of eyes wearing SE lenses flattened more than eyes wearing RGP lenses.
The median logMAR grating acuity of the aphakic eyes at age 1 year is similar to that reported by others for infants with unilateral aphakia corrected with contact lenses.8 Allen and coworkers reported the logMAR acuity at 8 years of age was +1.05 (Snellen equivalent 20/225) for children undergoing lensectomies between 4–6 weeks of age, +1.23 (Snellen equivalent 20/340) when the lensectomies was performed between 6–8 weeks of age and +1.44 (Snellen equivalent 20/550) when the lensectomies was performed after 8 weeks of age.9 Autrata and coworkers reported a logMAR acuity of +0.58 (Snellen equivalent 20/76) at 5 years of age in a cohort of children who underwent a unilateral lensectomies when 3.1 months of age.10 The median logMAR grating acuity we measured in the untreated phakic eyes is similar to the median monocular visual acuity in normal 12-month-old phakic children.11 Since the visual acuity of children improves during early childhood, we anticipate the acuity will improve in these children over time.12
None of the patients randomized to contact wear had a secondary IOL implanted. In another series, 30% of aphakic children have been reported to discontinue contact wear.13
The majority of eyes (70%) in this study were fitted with SE lenses. The use of SE lenses for pediatric aphakia is well documented, and is the lens of first choice for most pediatric contact lens practitioners due to its ease of fitting, high power availability and excellent oxygen permeability14–18. Although the SE lens is available up to +32D powers, it is limited to 3D steps in power. Additionally, only three base curves are available with powers greater than 20 D with the steepest BC (7.5mm) equaling 45.00D, which is flatter than the average corneal curvature in our study population. Nearly 90% of the eyes wearing SE lenses in our series were fitted with 7.5mm BCs, which was also the default BC selection. One study site fitted 5 eyes with a flatter BC than the 7.5 mm option, which is unusual for this patient population.19
The use of RGP contact lenses for correction of infantile aphakia has been well recognized.20–23 However, only 3 of the 12 study sites fitted RGP lenses on 12 eyes. Although our RGP sample size is small, the mean values determined in our study represent the initial RGP BC to be 1.62 D steeper than mean keratometric values at baseline and 1.87 D steeper at 12 months after surgery. This steep cornea-lens relationship is probably a reason for high lens loss. The contact lens practitioners fitting RGP lenses usually make changes to the BC based on fluorescein pattern evaluations in a clinical setting. This evaluation may be performed with a portable slit lamp, hand held burton lamp or LED cobalt flashlight.24 RGP lenses require more time and expertise to fit, which is likely why they are used less often to correct pediatric aphakia.
The visual acuity data obtained at one year of age fails to provide convincing evidence regarding which lens material is the best option for managing infantile aphakia. Although RGP lenses are customizable to fully correct the refractive error, and SE lenses were limited in availability to 3D steps in this study, the visual outcomes were the same at one year of age.
The infant cornea is typically steeper in curvature than the adult cornea. With age, the globe’s radius enlarges and the corneal curvature eventually flattens to an adult mean of approximately 43 D. The mean corneal curvature at birth has been reported to range from 47 to 48.50 D.25–26 Moore reported that the mean corneal curvature flatten at a faster rate in aphakic than normal eyes.27 We did not find a difference in the rate of corneal flattening between the normal fellow eyes and the aphakic eyes in our study, although the aphakic eyes had steeper corneal curvatures at both the time of cataract surgery and at age 1 year.
Our study compared keratometric data obtained at the time of surgery to data obtained after an average of 8.5 months of contact lens wear. The mean rate of decrease in keratometric power was less in eyes that wore RGP lenses (0.10 D/mo) compared to eyes wearing SE lenses (0.22 D/mo), although this difference was not statistically significant (p = 0.15). This difference may arise from an orthokeratology effect secondary to the relatively flat SE lenses. It may also be that the RGP lens may prevent the cornea from flattening, particularly when fitted steeper than the mean corneal curvature. Longitudinal studies after discontinuing RGP wear may help to elucidate the effect of RGP lenses on corneal curvature.
We found that the cataractous eyes had steeper corneal curvatures than the fellow eyes. Trivedi and Wilson28 also reported that the corneas of cataractous eyes of children with unilateral cataracts were significantly steeper than the corneas of the fellow eye at birth to six months of age.
We found that on average the keratometric astigmatism for the contact lens wearing cohort decreased by 0.05 D/mo. This is consistent with another report that noted a reduction of astigmatism in children during the first year of life.29
The average annual contact lens replacement costs ranged from $1275 for RGP wearers, to $1925 for silicone elastomer lens wearers, which represents approximately 17 lenses/year for RGP and 11 lenses/year for SE contact lens wearers. Although RGP lenses were replaced more often than SE lenses, their unit cost is less than SE lenses. These costs may be greater than those encountered in general practice due to the study design, which called for two lenses to be ordered for all parameter changes to ensure that a duplicate spare lens would always be available.
Contact lens professionals had the option to fit HEMA-based soft hydrogel lenses if necessary, however none exercised that option. Custom designed high oxygen flux soft silicone hydrogel lenses are now available on the U.S. market. However, they were not approved at the time of our initial study design, so were not included in this study. Silicon hydrogel materials for infantile aphakia are the preferred soft material in Australia.30
Ultraviolet radiation exposure damages the human eye, and has been reported to be associated with a variety of ocular disorders.31–33 The aphakic eye is potentially more vulnerable to retina changes related to UV exposure since the crystalline lens filters UV light. SE contact lenses do not provide UV protection, whereas RGP contact lenses may be ordered with UV blockers. This is a theoretical advantage for RGP lenses over SE lenses. However, there are no data to show whether this difference is clinically meaningful.
Summary
Contact lenses for the optical correction of infantile unilateral aphakia provide a safe and effective treatment. The visual outcomes at 1 year of age between the silicone elastomer contact lens group and rigid gas permeable contact lens group were identical with median recorded visual acuities of logMAR +0.80 for each group.
Acknowledgments
Supported by National Institutes of Health Grants U10 EY13272 and U10 EY013287 and in part by NIH Departmental Core Grant EY06360 and Research to Prevent Blindness, Inc., New York, New York
We would like to thank Bausch & Lomb and X-Cel Contacts for providing contact lenses used in this study at a reduced fee.
Appendix. The Infant Aphakia Treatment Study Group
Administrative Units and Participating Clinical Centers Clinical Coordinating Center (Emory University): Scott R. Lambert, MD (Study Chair); Lindreth DuBois, MEd, MMSc (National Coordinator)
Data Coordinating Center (Emory University): Michael Lynn MS (Director), Betsy Bridgman, BS; Marianne Celano PhD; Julia Cleveland, MSPH; George Cotsonis, MS; Carey Drews-Botsch, PhD; Nana Freret, MSN; Lu Lu, MS; Seegar Swanson; Thandeka Tutu-Gxashe, MPH
Visual Acuity Testing Center (University of Alabama, Birmingham): E. Eugenie Hartmann, PhD (Director); Clara Edwards; Claudio Busettini, PhD; Samuel Hayley
Steering Committee: Scott R. Lambert, MD; Edward G. Buckley, MD; David A. Plager, MD; M. Edward Wilson, MD; Michael Lynn, MS; Lindreth Dubois, Med MMSc; Carolyn Drews-Botsch, PhD; E. Eugenie Hartmann, PhD; Donald F. Everett, MA
Contact Lens Committee: Buddy Russell, COMT; Michael Ward, MMSc
Participating Clinical Centers (In order by the number of patients enrolled):
Medical University of South Carolina; Charleston, South Carolina (14): M. Edward Wilson, MD; Margaret Bozic, CCRC, COA
Harvard University; Boston, Massachusetts (14): Deborah K. Vanderveen, MD; Theresa A. Mansfield, RN; Kathryn Bisceglia Miller, OD
University of Minnesota; Minneapolis, Minnesota (13): Stephen P. Christiansen, MD; Erick D. Bothun, MD; Ann Holleschau, B.A.; Jason Jedlicka, OD; Patricia Winters, OD; Jacob Lang, O.D.
Cleveland Clinic; Cleveland, Ohio (10): Elias I. Traboulsi, MD; Susan Crowe, BS, COT; Heather Hasley Cimino, OD
Baylor College of Medicine; Houston, Texas (10): Kimberly G. Yen, MD; Maria Castanes, MPH; Alma Sanchez, COA; Shirley York
Oregon Health and Science University; Portland, Oregon (9): David T Wheeler, MD; Ann U. Stout, MD; Paula Rauch, OT, CRC; Kimberly Beaudet, CO, COMT; Pam Berg, CO, COMT
Emory University; Atlanta, Georgia (9): Scott R. Lambert, MD; Amy K. Hutchinson, MD; Lindreth Dubois, Med, MMSc; Rachel Robb, MMSc; Marla J. Shainberg, CO
Duke University; Durham, North Carolina (8): Edward G. Buckley, MD; Sharon F. Freedman, MD; Lois Duncan, BS; B.W. Phillips, FCLSA; John T. Petrowski, OD
Vanderbilt University: Nashville, Tennessee (8): David Morrison, MD; Sandy Owings COA, CCRP; Ron Biernacki CO, COMT; Christine Franklin, COT
Indiana University (7): David A. Plager, MD; Daniel E. Neely, MD; Michele Whitaker, COT; Donna Bates, COA; Dana Donaldson, OD
Miami Children’s Hospital (6): Stacey Kruger, MD; Charlotte Tibi, CO; Susan Vega
University of Texas Southwestern; Dallas, Texas (6): David R. Weakley, MD; David R. Stager Jr M.D.; Joost Felius, PhD; Clare Dias, CO; Debra L. Sager; Todd Brantley, OD
Data and Safety Monitoring Committee: Robert Hardy, PHD (Chair); Eileen Birch, PhD; Ken Cheng, MD; Richard Hertle, MD; Craig Kollman, PhD; Marshalyn Yeargin-Allsopp, MD (resigned); Cyd McDowell; Donald F. Everett, MA
Medical Safety Monitor: Allen Beck, MD
Footnotes
Proprietary interests: none
References
- 1.Costenbader FD, Albert DG. Conservatism in the management of congenital cataract. AMA Arch Ophthalmol. 1957;58:426–430. doi: 10.1001/archopht.1957.00940010438018. [DOI] [PubMed] [Google Scholar]
- 2.Beller R, Hoyt CS, Marg E, et al. Good visual function after neonatal surgery for congenital monocular cataracts. Am J Ophthalmol. 1981;91:559–65. doi: 10.1016/0002-9394(81)90053-2. [DOI] [PubMed] [Google Scholar]
- 3.Frey T, Friendly D, Wyatt D. Re-evaluation of monocular cataracts in children. Am J Ophthalmol. 1973;76:381–8. doi: 10.1016/0002-9394(73)90495-9. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz B, Worle J. Visual results in congenital cataract with the use of contact lenses. Graefes Arch Clin Exp Ophthalmol. 1991;229:123–32. doi: 10.1007/BF00170543. [DOI] [PubMed] [Google Scholar]
- 5.Cheng KP, Hiles DA, Biglan AW, Pettapiece MC. Visual results after early surgical treatment of unilateral congenital cataracts. Ophthalmology. 1991;98:903–10. doi: 10.1016/s0161-6420(91)32203-6. [DOI] [PubMed] [Google Scholar]
- 6.Lambert SR, Buckley EG, Drews-Botsch C, et al. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 128:21–7. doi: 10.1001/archophthalmol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert SR, Buckley E, Drews-Botsch C, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: Grating acuity and adverse events at age 1 year. Arch Ophthalmol. 2010;128:810–818. doi: 10.1001/archophthalmol.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ainsworth JR, Cohen S, Levin AV, Rootman DS. Pediatric cataract management with variations in surgical technique and aphakic optical correction. Ophthalmology. 1997 Jul;104:1096–101. doi: 10.1016/s0161-6420(97)30179-1. [DOI] [PubMed] [Google Scholar]
- 9.Allen RJ, Speedwell L, Russell-Eggitt I. Long-term visual outcome after extraction of unilateral congenital cataracts. Eye. 2010 Jul;24:1263–7. doi: 10.1038/eye.2009.295. [DOI] [PubMed] [Google Scholar]
- 10.Autrata R, Rehurek J, Vodickova K. Visual results after primary intraocular lens implantation or contact lens correction for aphakia in the first year of age. Ophthalmologica. 2005;219:72–9. doi: 10.1159/000083264. [DOI] [PubMed] [Google Scholar]
- 11.Mayer DL, Beiser AS, Warner AF, et al. Monocular acuity norms for the Teller Acuity Cards between ages one month and four years. Invest Ophthalmol Vis Sci. 1995;36:671–685. [PubMed] [Google Scholar]
- 12.Chen YC, Hu AC, Rosenbaum A, Spooner S, et al. Long Term Results of Early Contact Lens Use in Pediatric Unilateral Aphakia. Eye Contact Lens. 2010;36:19–25. doi: 10.1097/ICL.0b013e3181c6dfdc. [DOI] [PubMed] [Google Scholar]
- 13.Mittelviefhaus H, Mittelviefhaus K, Gerling J. Etiology of contact lens failure in pediatric aphakia. Indications for intraocular lenses? Ophthalmologe. 1998;95:207–12. doi: 10.1007/s003470050263. [DOI] [PubMed] [Google Scholar]
- 14.Cutler SI, Nelson LB, Calhoun JH. Extended wear contact lenses in pediatric aphakia. J Pediatr Ophthalmol Strabismus. 1985;22:886–91. doi: 10.3928/0191-3913-19850501-03. [DOI] [PubMed] [Google Scholar]
- 15.Nelson LB, Cutler SI, Calhoun JH, et al. Silsoft extended wear contact lenses in pediatric aphakia. Ophthalmology. 1985;92:1529–31. doi: 10.1016/s0161-6420(85)33825-3. [DOI] [PubMed] [Google Scholar]
- 16.Levin AV, Edmunds SA, Nelson LB, et al. Extended wear contact lenses for the treatment of pediatric aphakia. Ophthalmology. 1988;95:1107–13. doi: 10.1016/s0161-6420(88)33052-6. [DOI] [PubMed] [Google Scholar]
- 17.Aasuri MK, Venkata N, Preetam P, et al. Management of pediatric aphakia with silsoft contact lenses. CLAO J. 1999;25:209–12. [PubMed] [Google Scholar]
- 18.Ozbed Z, Durak I, Berk TA. Contact lens in the correction of childhood aphakia. CLAO J. 2002;28:28–30. [PubMed] [Google Scholar]
- 19.De Brabander J, Kok JH, Nuijts RM, et al. A practical approach to and long-term results of fitting silicon contact lenses in aphakic children after congenital cataract. CLAO J. 2002;28:31–5. [PubMed] [Google Scholar]
- 20.McQuaid K, Young TL. Rigid Gas Permeable Contact Lens Changes in the Aphakic Infant. CLAO J. 1998;24:36–40. [PubMed] [Google Scholar]
- 21.Amos CF, Lambert SR, Ward MA. Rigid gas permeable contact lens correction of aphakia following congenital cataract removal during infancy. J Pediatr Ophthalmol Strabismus. 1992;29:243–5. doi: 10.3928/0191-3913-19920701-13. [DOI] [PubMed] [Google Scholar]
- 22.Shaughnessy MP, Ellis FJ, Jeffrey AR, et al. Rigid gas-permeable contact lenses are safe and effective means of treating refractive abnormalities in the pediatric population. CLAO J. 2001;27:195–201. [PubMed] [Google Scholar]
- 23.Saltarelli DP. Hyper oxygen-permeable rigid contact lenses as an alternative for the treatment of pediatric aphakia. Eye Contact Lens. 2008;34:84–93. doi: 10.1097/ICL.0b013e31811eadaa. [DOI] [PubMed] [Google Scholar]
- 24.Sindt CW. Spectrum. Jan, 2010. Fitting infants and young children with GP Lenses CL. [Google Scholar]
- 25.Inagaki Y. The rapid change of corneal curvature in the neonatal period and infancy. Arch Ophthalmol. 1986;104:1026–7. doi: 10.1001/archopht.1986.01050190084044. [DOI] [PubMed] [Google Scholar]
- 26.Asbell PA, Chiang B, Somers ME, et al. Keratometry in children. CLAO J. 1990;16:99–102. [PubMed] [Google Scholar]
- 27.Moore BD. Mensuration data in infant eyes with unilateral congenital cataracts. Am J Optom Physiol Opt. 1987;64:204–210. doi: 10.1097/00006324-198703000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Triedi RH, Wilson ME. Keratometry in pediatric eyes with cataract. Arch Ophthalmol. 2008;126:38–42. doi: 10.1001/archophthalmol.2007.22. [DOI] [PubMed] [Google Scholar]
- 29.Mutti DO, Mitchell GL, Jones LA, et al. Refractive astigmatism and the toricity of ocular components in human infants. Optom Vis Sci. 2004;81:753–61. doi: 10.1097/00006324-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay RG, Chi JT. Contact Lens Management of Infantile Aphakia. Clin Exp Optom. 2010;93(1):3–14. doi: 10.1111/j.1444-0938.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 31.Young RW. The family of sunlight-related eye diseases. Optom Vis Sci. 1994;71:125–44. doi: 10.1097/00006324-199402000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Ooi JL, Sharma NS, Papalkar D, et al. Ultraviolet fluorescence photography to detect early sun damage in the eyes of school-aged children. Am J Ophthalmol. 2006;141:294–8. doi: 10.1016/j.ajo.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Cejkova J, Stipek S, Crovska J, et al. UV rays, the prooxident/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Res. 2004;53:1–10. [PubMed] [Google Scholar]