Abstract
BACKGROUND
Romidepsin is a structurally unique histone deacetylase inhibitor FDA-approved for therapy of relapsed or refractory cutaneous T-cell lymphoma (CTCL). Localized electron beam radiation therapy (LEBT) is standard practice in the care of patients with chronically traumatized and painful lesions. Combinational therapy of those two modalities may be beneficial for the therapy of CTCL.
OBJECTIVES
To report observations on supportive LEBT utilized for isolated refractory lesions in patients on romidepsin.
METHODS
Observations during a phase 2 clinical trial sponsored by the National Cancer Institute (NCI 1312) examining the efficacy of romidepsin for patients with relapsed, refractory, or advanced CTCL, stage IB-IVA mycosis fungoides (MF) or Sézary syndrome. Skin responses were assessed by evaluation of five target lesions only. Patients with objective clinical responses in target lesions who had symptomatic non-target lesions were allowed limited localized radiation to isolated lesions for symptomatic relief. Patients who received localized radiation were not considered complete responders at any point.
RESULTS
Five patients with advanced MF (3 had stage IIB and 2 had stage IVA2) received localized electron beam radiation to symptomatic non-target lesions while on a protocol with romidepsin. None of these patients experienced additional or unexpected toxicity. Four of the five patients demonstrated fast and durable responses. We noted that significantly lower than standard doses of electron beam radiation effectively treated symptomatic lesions in these patients.
CONCLUSIONS
Electron beam therapy demonstrated significant responses at very low doses without additional toxicity in patients on protocol treatment with the histone deacetylase inhibitor romidepsin. This merits formal investigation in a clinical trial for potential synergy in patients with cutaneous T-cell lymphoma.
INTRODUCTION
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous group of epidermotropic non-Hodgkin lymphomas.1 Several Food and Drug Administration (FDA)-approved single agents are available for the treatment of CTCL; reported response rates range from 25% to 60%,2 highlighting the need to improve response rates and sustain remissions.
Localized electron beam radiation (LEBT) is an effective therapy for cutaneous lesions of CTCL because of the radiosensitivity of malignant lymphocytes and is used routinely as a part of the treatment algorithm for therapy at all stages of CTCL.2,3
Histone deacetylase (HDAC) inhibitors are a class of chemotherapeutics that target the deacetylase family of enzymes. Romidepsin (FK228, FR901226, depsipeptide), like other HDAC inhibitors, has been shown to induce cell cycle arrest in both G and G1 2/M phases and to induce apoptosis. 4 Monotherapy of CTCL with romidepsin has been characterized by vigorous clinical responses. Two recently completed phase 2 multi-centre clinical trials5,6 that examined the efficacy of romidepsin as monotherapy for patients CTCL supported FDA approval of the drug for clinical practice.
Herein, we present responses and safety profile of low dose electron beam radiation administered to symptomatic non-target lesions during romidepsin therapy in patients with objective clinical responses.
MATERIALS AND METHODS
In NCI 1312, ‘Phase II Trial of Depsipeptide in Patients with Cutaneous T-Cell Lymphoma and Relapsed Peripheral T-Cell Lymphoma’ (ClinicalTrials.gov identifier NCT00020436), romidepsin was administered as a 4-hour infusion at 14 mg/m2 on days 1, 8, and 15 of a 28-day cycle. The dose of infusion was lowered by 25%, if the patient had absolute granulocyte count ≥500/μl, but <1000μl, or platelet count ≥50,000/μl, but <75, 000/μl. The assessment of CTCL resolution were performed in all compartments including skin, lymph nodes, viscera, and blood and were efficacy endpoints in NCI1312. Disease in the skin or viscera was assessed by Response Evaluation Criteria In Solid Tumours (RECIST) criteria;7 up to five target skin lesions were selected and monitored for response. Lymph node disease and bone marrow involvement was assessed using International Working Group guidelines,8 and blood was assessed by flow cytometry. Patients with ongoing clinical response according to the RECIST criteria were allowed to have radiation to symptomatic non-target lesions. These patients received LEBT to isolated lesions as per protocol, allowing them to continue systemic therapy. Radiation therapy was not administered on days patients received romidepsin. No other topical or concurrent systemic therapy was allowed per protocol. Patients who received radiation while on protocol were not categorized as having a complete response at any time.
RESULTS
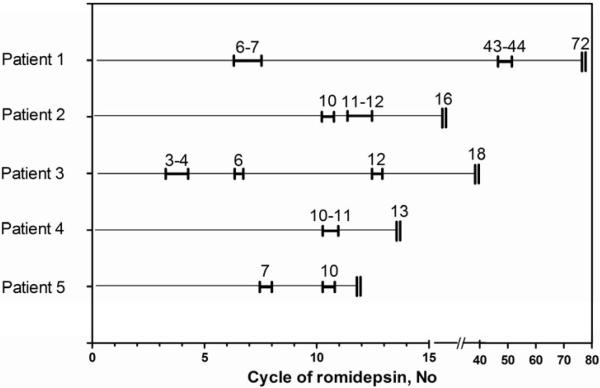
Five patients, including 3 from the first report of 71 patients enrolled on NCI 1312 and 2 who enrolled subsequently, received LEBT as supportive care to non-target lesions that were painful or otherwise symptomatic compromising their quality of life, while significant and durable clinical responses were otherwise demonstrated. Four of the five patients achieved a partial response (PR) to romidepsin therapy with an average reduction of 41% (range from 34% to 72%) in target lesion size by RECIST prior to radiation therapy. Despite evidence of clinical benefit, there were non-target lesions that were chronically traumatized, inflamed, and caused severe pain. Because the overall response rate on the skin was based primarily on target lesions, low dose LEBT to such lesions did not interfere with response assessment. LEBT to symptomatic lesions was administered according to Fig. 1 and Table 1 with significant improvement or complete resolution of the irradiated lesions (Fig. 2). Patient 1 remains on intermittent treatment with romidepsin now 10 years after study enrolment. Patient 3 was removed from study after 24 months of therapy with romidepsin and while off study, the patient resumed treatment with romidepsin and has remained in clinical remission for 2 years. Three patients developed disease progression outside the irradiated areas and romidepsin was discontinued. No adverse reactions were observed following irradiation, and there was no evidence of a radiation recall phenomenon in subsequent cycles.
Figure 1.
Time of administration of LEBT during the course of romidepsin. Five patients with symptomatic lesions received LEBT. Thin lines across for each patient represent a course of therapy with time of LEBT administration bracketed. A double vertical line at the end shows when disease progression was declared, or for patient 1, therapy with romidepsin stopped.
Table 1.
Percentages of the body surface area of irradiated region in comparison with initial involvement of cutaneous T-cell lymphoma at enrollment and regimen of irradiation
| Patient | Age/Race/Sex | Stage | On- study TBSA |
BSA irradiated |
Type of lesions |
Cycle | Energy | Dose per fraction |
No. of fractions |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 41/W/M | IIB | 16% | 2.5% | plaques | 6-7 | 9 MeV | 180 cGy | 20 |
| 2.0% | plaques | 43-44 | 6 MeV | 180 cGy | 20 | ||||
|
| |||||||||
| 2 | 41/W/F | IVA2 | 91.5% | 8.6% | papules | 10 | 6 MeV | 600 cGy | 4 |
| 9.7% | papules | 11 | 6 MeV | 400 cGy | 6 | ||||
| 10.7% | plaques | 12 | 6 MeV | 400 cGy | 8 | ||||
|
| |||||||||
| 3 | 61/W/F | IIB | 0.8%a | 0.3% | tumors | 3 | 6 MeV | 400 cGy | 12 |
| 0.1 | tumors | 4 | 6 MeV | 400 cGy | 4 | ||||
| 0.1 | tumors | 6 | 6 MeV | 400 cGy | 6 | ||||
| 0.01 | tumor | 12 | 6 MeV | 400 cGy | 2 | ||||
|
| |||||||||
| 4 | 34/AA/F | IVA2 | 82% | 6% | plaques | 10 - 11 |
6 MeV | 180 cGy | 10 |
|
| |||||||||
| 5 | 42/W/M | IIB | 36.5%b | 2.9% | tumors | 7 | 6 MeV | 200 cGy | 5 |
| 2.0% | plaques | 10 | 6 MeV | 200 cGy | 5 | ||||
TBSA, Total Body Surface Area for patches and plaques only; BSA, Body Surface Area;
All CTCL lesions were tumors.
Patient had tumors occupying additional 10% of TBSA
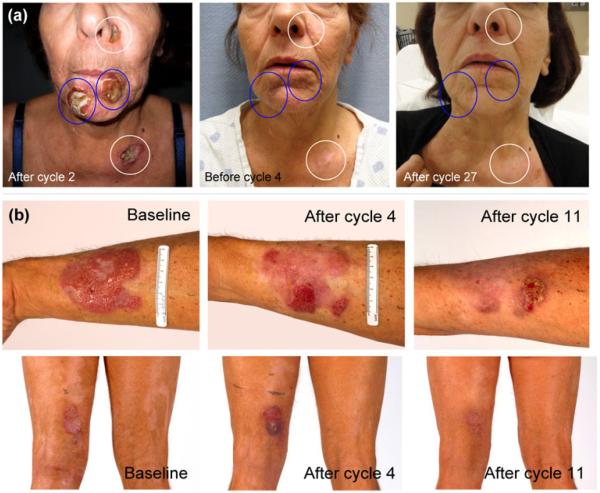
Figure 2.
(a) Patient 3. Tumors treated with romidepsin (white circles) or romidepsin and localized electron beam therapy (LEBT) (blue circles). Sustained clinical remission with complete resolution of tumors. (b) Patient 5. Lesions at baseline and after 4 or 11 cycles of romidepsin. Upper panels: forearm plaque with response to romidepsin alone. Lower panels: posterior thigh plaque, which became ulcerated but cleared with irradiation with 10 Gy in 5 fractions during cycle 7.
DISCUSSION
We described the clinical experience in five patients who received low dose LEBT to symptomatic non-target lesions during the romidepsin clinical trial NCI1312. Four of five patients experienced rapid and durable clinical responses at the radiotherapy site, suggesting that in vitro reports of synergy between romidepsin and LEBT may be clinically relevant.9 Use of LEBT was only a supportive care measure and was not formally built in or optimized as part of the clinical trial. Importantly, there was no additional or unexpected toxicity observed at the sites of irradiation at the doses provided.
Recently, Kamstrup et. al. explored the possibility of using lower radiation doses for total skin electron beam therapy, which could limit toxicity and allow this treatment to be repeated for long-term disease control.10 Low-dose total skin electron beam as monotherapy produced only short-lived responses in this cohort. However, there are significant preclinical data suggesting that HDAC inhibitors, especially romidepsin, may show synergy when used with electron beam or other therapeutic modalities such as ultraviolet irradiation. Radiosensitization seems to be a class effect of HDAC inhibitors,11 and the radiosensitizing activity of romidepsin has been demonstrated in vitro in human squamous carcinoma, gastric adenocarcinoma, and colon carcinoma cell lines.9,12
Several mechanisms have been proposed to explain this sensitization. Because the sites of active transcription are generally more sensitive to radiation, relaxation of chromatin due to HDAC inhibition may lead to increased sensitivity.13 Alternatively, cumulative cell cycle effects may play a role; romidepsin at therapeutic concentrations arrested lymphoma cell lines primarily in G1,14 whereas ionizing radiation led to G2/M arrest.15 Downregulation or increased acetylation of DNA repair proteins has been proposed as a mechanism of reduced radiation-induced DNA double-stand break repair, and the observation of prolonged phosphorylated histone H2AX is consistent with that hypothesis.
We have shown that LEBT can be safely used with romidepsin and was effective across all doses including very low doses of radiation. Due to the fact that our observations were not done in systematic or controlled manner, we cannot conclude whether there is a synergy of radiation and HDAC inhibitor therapy based on our data. However, taken together with demonstrated synergy in vitro, our clinical observations of durable clinical responses in the patients receiving very low doses of radiation are suggestive of such synergy. Therefore, prospective randomized clinical trial to evaluate low doses of EBT during HDAC therapy to formally evaluate their synergy and to establishing the therapeutic schedule and to assess their efficacy is warranted.
SUMMARY.
What’s already known about this topic? In vitro studies reported synergy between romidepsin and electron beam therapy due to radiosensitization of malignant lymphocytes by HDAC inhibitors.
What does this study add? Our observations show that standard of care low-dose localized electron beam therapy for treatment of cutaneous T cell lymphoma can be administered safely and effectively while a patient is receiving romidepsin. There was no additional or unexpected toxicity observed.
Acknowledgments
Financial Disclosure of the Authors: Drs. Piekarz, Bates, and Geskin were clinical investigators in a clinical trial involving romidepsin. The clinical trial was supported by a Cooperative Research and Development Agreement between the National Cancer Institute and Gloucester Pharmaceuticals. Drs. Akilov and Grant have no conflicts of interest to declare.
Funding/Support: This work was supported by SPORE NIH 5P50CA121973-03 Project 5 (to L.G.). Financial support for the editorial assistance was provided by Celgene Corporation.
Footnotes
Publisher's Disclaimer: These articles have been accepted for publication in the British Journal of Dermatology and are currently being edited and typeset. Readers should note that articles published below have been fully refereed, but have not been through the copy-editing and proof correction process. Wiley-Blackwell and the British Association of Dermatologists cannot be held responsible for errors or consequences arising from the use of information contained in these articles; nor do the views and opinions expressed necessarily reflect those of Wiley-Blackwell or the British Association of Dermatologists
REFERENCES
- 1.Dummer R, Assaf C, Bagot M, et al. Maintenance therapy in cutaneous T-cell lymphoma: who, when, what? Eur J Cancer. 2007;43:2321–9. doi: 10.1016/j.ejca.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Geskin LJ. Cutaneous T-Cell Lymphoma (Mycosis Fungoides and Sezary Syndrome) In: Kaushansky K, Lichtman MA, Beutler E, et al., editors. Williams Hematology. 8 edn. The McGraw-Hill Companies; 2010. pp. 1595–608. [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology . In: Non-Hodgkin’s Lymphomas. Zelenetz AD, Abramson JS, editors. Vol. 1. Vol. 2012. National Comprehensive Cancer Network, Inc.; pp. 91–102. 2012. [DOI] [PubMed] [Google Scholar]
- 4.Ueda H, Nakajima H, Hori Y, et al. Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968, on Ha-ras transformed NIH3T3 cells. Biosci Biotechnol Biochem. 1994;58:1579–83. doi: 10.1271/bbb.58.1579. [DOI] [PubMed] [Google Scholar]
- 5.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–7. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–91. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Horning SJ, Coiffier B, et al. NCI Sponsored International Working Group Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Jung M, Dritschilo A, et al. Enhancement of radiation sensitivity of human squamous carcinoma cells by histone deacetylase inhibitors. Radiat Res. 2004;161:667–74. doi: 10.1667/rr3192. [DOI] [PubMed] [Google Scholar]
- 10.Kamstrup MR, Specht L, Skovgaard GL, et al. A prospective, open-label study of low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 2008;71:1204–7. doi: 10.1016/j.ijrobp.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Camphausen K, Tofilon PJ. Inhibition of histone deacetylation: a strategy for tumor radiosensitization. J Clin Oncol. 2007;25:4051–6. doi: 10.1200/JCO.2007.11.6202. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Adachi M, Zhao X, et al. Histone deacetylase inhibitors FK228, N-(2-aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)amino-methy]lbenzamide and m-carboxycinnamic acid bis-hydroxamide augment radiation-induced cell death in gastrointestinal adenocarcinoma cells. Int J Cancer. 2004;110:301–8. doi: 10.1002/ijc.20117. [DOI] [PubMed] [Google Scholar]
- 13.Cho HJ, Kim SY, Kim KH, et al. The combination effect of sodium butyrate and 5-Aza-2′-deoxycytidine on radiosensitivity in RKO colorectal cancer and MCF-7 breast cancer cell lines. World J Surg Oncol. 2009;7:49. doi: 10.1186/1477-7819-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasakawa Y, Naoe Y, Inoue T, et al. Effects of FK228, a novel histone deacetylase inhibitor, on human lymphoma U-937 cells in vitro and in vivo. Biochem Pharmacol. 2002;64:1079–90. doi: 10.1016/s0006-2952(02)01261-3. [DOI] [PubMed] [Google Scholar]
- 15.Palayoor ST, Macklis RM, Bump EA, et al. Modulation of radiation-induced apoptosis and G2/M block in murine T-lymphoma cells. Radiat Res. 1995;141:235–43. [PubMed] [Google Scholar]