Abstract
Objective
Synovial inflammation, a feature of both osteoarthritis (OA) and meniscal injury, is hypothesized to be triggered in part via stimulation of Toll-like receptors (TLRs). We tested whether a TLR-2 or TLR-4 stimulating factor in synovial fluid (SF) from early knee OA patients with meniscal injury could lead to inflammatory activation of synoviocytes.
Methods
SF was obtained from patients with early OA cartilage damage undergoing arthroscopic meniscal procedures. SF was used to stimulate cell lines transfected with TLR-2 or TLR-4, and primary cultures of fibroblast-like synoviocytes (FLS). SF was used either alone or in combination with a TLR-2 stimulus (Pam3Cysk4) or a TLR-4 stimulus (LPS). In blocking experiments, SF was pre-incubated with anti-CD14 antibody.
Results
SF from these patients did not stimulate IL-8 release from TLR transfectants. Compared with SF on its own, SF (0.09–25%) in combination with TLR-2 or TLR-4 ligands resulted in significant augmentation of IL-8 release from both transfectants and primary FLS. Soluble CD14 (sCD14), a co-receptor for TLRs, was measured in early OA SF at levels comparable to advanced OA and rheumatoid arthritis. Blockade with anti-CD14 antibody abolished the ability of SF to augment IL-8 production in response to LPS, and diminished Pam3CysK4 responses.
Conclusions
SF augments FLS responses to TLR-2 and TLR-4 ligands. This effect was largely due to sCD14. Our results demonstrate that sCD14 in the setting of OA and meniscal injury sensitizes FLS to respond to inflammatory stimuli such as TLR ligands.
Keywords: Osteoarthritis, inflammation, fibroblast-like synoviocytes, synovitis
Introduction
Many patients with osteoarthritis (OA) or joint injury develop low-grade synovitis, and there is growing evidence that synovitis impacts symptoms (1–3) and rate of cartilage degeneration (4) in patients with OA. Synovial infiltration by macrophages and lymphocytes (5, 6) have been demonstrated in both early and advanced stage OA (7,8). Moreover, infiltrates occur in patients with a history of joint injury undergoing arthroscopic surgery for meniscal tears, even in the absence of radiographic evidence of OA (9). However, the molecular stimulus for synovitis in the setting of OA or meniscal injury is as yet unclear.
Initial inflammatory responses to infection are mediated by receptors for Pathogen-Associated Molecular Patterns (PAMPs), including Toll-like receptors (TLRs). Cell-surface TLRs include TLR-2 and TLR-4; TLR-2 binds lipopeptides from prokaryotic cell walls, and TLR-4 recognizes LPS from gram-negative bacteria. Binding of ligands to TLRs initiates intra-cellular signaling that results in inflammatory gene transcription. Recent studies in various models of tissue injury and repair (10), including ischemia/ reperfusion (11), non-infectious lung injury (12), and femoral fracture (13), have implicated TLRs in inflammatory responses after non-infectious injury. In these settings, TLRs may be triggered by endogenous danger signals (Damage-Associated Molecular Patterns, DAMPs) produced by tissue injury and cellular stress.
DAMPs which interact with TLRs, particularly TLR-2 and TLR-4, include extracellular matrix components such as low-molecular weight fragments of hyaluronan (LMW-HA) (14), tenascin C (TnC)(15), and cellular products high-mobility group box-1 (HMGB-1) (16) and heat-shock protein 96 (Hsp 96)(17). TnC and Hsp96 activate synovial fibroblasts via TLRs in the setting of rheumatoid arthritis (RA) (15, 17). Both DAMPs and TLRs are expressed in OA joint tissues, including synovial membrane and cartilage (18). Murine chondrocyte nitric oxide and MMP-13 production was diminished in response to LMW-HA and HMGB-1 in TLR-2/TLR-4 double knock-out mice (19), suggesting a role for TLRs in chondrocyte catabolic responses. The importance of TLR responses in fibroblast-like synoviocytes (FLS) are less clear in OA and joint injury, as most studies have focused on FLS responses in RA. However, synovial fibroblasts from both OA and RA patients respond to TLR ligands with increased chemokine production (20), an activity that has obvious implications for development of synovitis in both inflammatory and “non-inflammatory” arthritis.
Given the strong association between joint injury and risk for OA development and progression (21), we hypothesized that endogenous activation of TLRs stimulates inflammatory responses in patients with OA or joint injuries (22). We anticipated that endogenous TLR ligand(s) present in synovial fluid (SF) from patients with OA could lead to inflammatory activation of FLS. We collected synovial fluids (SF) from patients undergoing arthroscopic surgery for meniscal tears with early-stage OA cartilage damage. We then tested the ability of SF to induce inflammatory cytokine production in two in vitro systems: (i) TLR-negative cell lines transfected with TLR-2 or TLR-4, and (ii) primary FLS cultures.
Materials & Methods
Reagents
Culture media, antibiotics, and additives were from Invitrogen (Carlsbad, CA). LPS from Escherichia coli 0111:B4 (a TLR-4 ligand) and Pam3CysK4 (a TLR-2 ligand) were purchased from Invivogen (San Diego, CA), and recombinant CD14 (rCD14) from Peprotech (Rocky Hill, NJ).
SF and serum samples
Patients were recruited from orthopedic practices at Rush University Medical Center (Chicago, IL) and the Hospital for Special Surgery (New York, NY), under Institutional Review Board (IRB) approved protocols at both institutions; all patients gave informed consent. Patient groups were as follows: 1) Early OA (meniscectomy): Patients undergoing arthroscopic meniscectomy for treatment of meniscal tears were recruited from practices of two orthopedic surgeons (C. B-J., and N. V.) at Rush University Medical Center. SF and serum was obtained at the time of surgery and banked for the Rush Knee Arthritis and Meniscal Injury Repository. Early-stage OA cartilage change was confirmed in these patients by intra-operative inspection and degree of degradation scored using the Outerbridge system (23). 2) Advanced OA: Patients undergoing total knee replacement (TKR) were recruited at the Hospital for Special Surgery. Diagnosis of OA was made by the operating surgeon. All advanced patients had intra-operative evidence of full-thickness diffuse cartilage loss (24). 3) RA: SF from patients undergoing TKR at the Hospital for Special Surgery with a clinical diagnosis of RA was included for comparison of sCD14 levels. 4) Asymptomatic donors: SF was obtained from ten asymptomatic organ donors with no documented history of joint disease via the Gift of Hope Organ & Tissue Donor Network (Elmhurst, IL). Specimens were collected within 24 hours of death, and collected with IRB approval. Characteristics of patients are shown in Table 1.
Table 1.
Characteristics of patient groups included in sCD14 analysis of SF
| Early OA (Meniscectomy) |
Advanced OA | RA | Asymptomatic Donors |
|
|---|---|---|---|---|
| N = | 30 | 7 | 6 | 10 |
|
sCD14 in SF (µg/ml) |
2.10 (1.54–2.36) |
2.29 (1.48–3.36) |
1.70 (1.55–1.94) |
0.72 (0.55–1.43) |
|
Age (Median, IQR+) |
56 (47–62) |
69 (55–77) |
67 (51–72) |
64 (52–74) |
|
BMI (Median, IQR) |
29.5 (27–35) |
26.6 (24.3–35.5) |
NR^ | NR |
|
Gender (% Male) |
50% | 71% | 16% | 40% |
|
Race (% Caucasian) |
76% | 100% | 50% | 60% |
|
Outerbridge grade (# of pts) |
grade 1 = 1 grade 2 = 11 grade 3 = 10 grade 4* = 8 |
grade 4# = 7 | NR | NR |
|
K-L grade (# of pts) |
grade 0 = 1 grade 1 = 6 grade 2 = 10 grade 3 = 4 grade 4 = 0 missing = 9 |
NR | grade 0 = 0 grade 1 = 0 grade 2 = 1 grade 3 = 3 grade 4 = 1 missing = 1 |
NR |
Interquartile range
NR = Not recorded
All grade 4 lesions in this group were focal cartilage defects.
Patients that did not have diffuse grade 4 (full-thickness) cartilage wear were excluded from the advanced OA group.
Synovial tissues for FLS culture
Synovial membranes were obtained from five asymptomatic organ donors via the Gift of Hope Donor Network. Cartilage degeneration in these donors was assessed and graded using the modified Collins score (grade 0 = normal cartilage, to grade 4 = full thickness cartilage erosion of > 30%) by an experienced reader (25). Characteristics of these donors are presented in Table 2.
Table 2.
Characteristics of tissue donors from which FLS cultures were established
| Donor # | Age | Gender | Cartilage score * (tibia/femur) |
|---|---|---|---|
| 5263 | 65 | F | 2/3 |
| 5264 | 19 | M | 0/0 |
| 5265 | 59 | F | 2/1 |
| 5271 | 55 | M | 2/3 |
| 5272 | 38 | F | 1/1 |
Cartilage degeneration and structural joint integrity was scored according to the modified Collin’s grading system (25). Briefly, grade 0 represents normal cartilage and grade 4, greater than 30% of articular surface eroded to bone.
Cell Culture
Transfected human embryonic kidney 293 (HEK-293) cells expressing TLR constructs were a gift from Dr. R.W. Finberg (University of Massachusetts Medical School, Worcester, MA) (26). HEK-293 cells transfected with TLR-4 alone served as negative control for cells co-transfected with TLR-4 and myeloid differentiation factor-2 (MD-2); MD-2 is required for LPS signaling via TLR-4. HEK-293 cells transfected with CD14 alone served as negative control for cells co-transfected with TLR-2 and the co-receptor CD14. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT) and 0.5 µg/ml Puromycin (TLR-2 transfectants) or 0.5 mg/ml Geneticin (TLR-4 transfectants). Control experiments confirmed specificity of this system, as LPS only stimulated TLR-4/MD2 transfected cells while Pam3CysK4 only stimulated TLR-2/CD14 transfected cells.
FLS cultures were established from synovial biopsies by enzymatic digestion (27). Tissues were treated for 90 minutes with 1 mg/ml of pronase (Calbiochem, Darmstadt, Germany) followed by overnight digestion with 1 mg/ml of type I collagenase (Worthington Biochemical, NJ). Dissociated cells were resuspended in DMEM supplemented with 10% FBS, antibiotics, non-essential amino acids, sodium pyruvate, sodium bicarbonate, L-glutamine, and 0.5 µM β-mercaptoethanol. Select FLS at passage 4 were immunostained with FITC conjugated anti-human CD14 antibody (clone 61D3, eBioscience, San Diego, CA) or an isotype control, and analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Cultures were negative for CD14 at passage 4, indicating depletion of CD14+ synovial macrophages. FLS were used between passages 4 and 9.
In vitro stimulation with SF and TLR ligands
10 SF specimens were tested for endotoxin contamination (Pyrochrome™ endotoxin assay, Associates of Cape Cod, East Falmouth, MA). Only one contained measurable endotoxin activity, and this specimen was excluded from further experiments. HEK-293 transfectants (2 × 104 cells/well) or FLS (3 × 103 cells/well) were seeded in 96 -well tissue culture plates. After 24 hours, cells were stimulated with indicated concentrations of patient SF, LPS, Pam3CysK4, rCD14 alone or in combination for 6 or 18 hours. Supernatants were collected for IL-8 and IL-6 measurement by ELISA (R & D Systems, Minneapolis, MN). These cytokines are known products of FLS, and are induced by TLR stimulation in monocytes. Total RNA was isolated from cell lysates after 6 hour stimulations using the RNeasy® Mini RNA Isolation Kit (Qiagen, Germany). In blockade experiments, anti-human CD14 (mAb clone MEM-18, Abcam, Cambridge, MA) was added to SF at 37°C and incubated 1 hour prior to use as stimuli.
Real-time polymerase chain reaction (qPCR) measurement of IL-8, TLR-2 and TLR-4
mRNA levels of IL-8, TLR-2 and TLR-4 were determined by real-time PCR. Total RNA was reverse transcribed using iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA). iQ™ SYBR Green Supermix (Bio-Rad, Hercules, CA) was used for PCR, and reactions carried out on a Bio-Rad CFX™ 96 real time system. The following human primers were used: IL-8 forward 5’ATGACTTCCAAGCTGGCCGTGGCT 3’, reverse 5’ TCTCAGCCCTCTTCAAAAACTTCTC 3’. TLR-2 forward 5’ TGGATGGTGTGGGTCTTGG 3’, reverse 5’ AGGTCACTGTTGCTAATGTAGG 3’. TLR-4 forward 5’ GCCCTGCGTGGAGGTGGTTC 3’, reverse 5’ TCCAGAAAAGGCTCCCAGGGCT 3’. GAPDH forward 5’ CAACGGATTTGGTCGTATT 3’, reverse 5’ GATGGCAACAATATCCACTT 3’. After normalizing Ct values to GAPDH, expression levels were calculated relative to media controls (28).
Flow cytometry and Immunostaining of TLR expression in FLS cells
For flow cytometric analysis, FLS were non-enzymatically dissociated, resuspended in PBS containing 1% BSA and 0.05% sodium azide, and immunostained with either PE- conjugated mAbs against human TLR-2 (clone TL2.1), TLR-4 (clone HTA125), or a PE-conjugated isotype control (Mouse IgG2a, all mAbs from eBioscience, San Diego, CA). Analysis was performed on a FACSCanto II flow cytometer (BD Flow Cytometry Systems, San Jose, CA). For visualization of immunostaining, FLS cells plated in 6-well tissue culture plates were grown to 80–90% confluence and stained with biotinylated anti-TLR4 mAb (Clone HTA 125, Abcam, MA) followed by Alexa Fluor 488 labeled streptavidin (Invitrogen, CA). Cells were visualized under a 40× water-immersion objective. A two-photon laser (Chameleon Ultra; Coherent Inc, Santa Clara, CA) was used for excitation. Images of representative fields were acquired with PrairieView software (Prairie Technologies) and fluorescence densities were analyzed with Imaris software (Bitplane Inc.).
Measurement of sCD14 in SF and serum
SF was centrifuged at 500g to remove cell debris prior to storage at −80°C. SF and serum specimens were thawed, diluted 1: 200, and sCD14 measured utilizing the Human CD14 DuoSet™ (R & D Systems, Minneapolis, MN).
Statistical analyses
Data was analyzed using Graphpad Prism (v 5.0, La Jolla, CA). Cytokine levels from in vitro experiments were compared between groups using one-way ANOVA. If a p-value less than 0.05 was obtained, Tukey’s post-test was applied. For comparison of sCD14 levels between patient groups, Kruskal-Wallis ANOVA with Dunn’s multiple comparison post- test was applied. Association between sCD14 levels and FLS IL-8 release was tested with Spearman’s correlation.
Results
Most SF from early-OA patients do not directly stimulate HEK-293 cells transfected with TLR-2 or TLR-4
To test whether a TLR-4 or TLR-2 activating factor could be detected in SF specimens, we utilized HEK-293 cells transfected with TLR-4 and the cofactor MD2, TLR-4 alone, TLR-2 and the cofactor CD14, or CD14 alone. In total, we screened 31 SF specimens at 25% concentration from both early (n = 23) and advanced OA (n=8, total knee replacement) patients for the ability to stimulate IL-8 production from transfectants. None stimulated CD14 or TLR-2/CD14 transfected cell lines, and only one reproducibly stimulated cells transfected with TLR-4 (data not shown). As this specimen was in limited supply, the stimulatory capacity could not be further characterized. SF concentrations above 25% caused cell death indicated by trypan blue staining, and could not be analyzed.
Effects of OA SF on IL-8 production from HEK-293 transfectants in response to TLR-2 and TLR-4 ligands
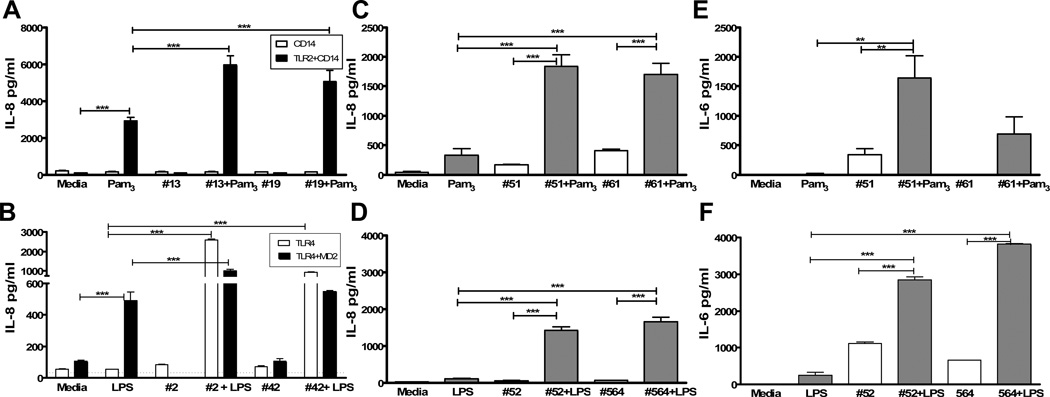
We next stimulated HEK-293 transfectants with the TLR-4 ligand LPS or the TLR-2 ligand Pam3CysK4, alone or in combination with SF. LPS and Pam3CysK4 stimulated co-transfected cells as expected (Figure 1A and 1B). 25% SF alone did not stimulate IL-8 production from either set of transfectants, but addition of SF to control ligands significantly increased levels of IL-8 compared to either Pam3CysK4 (Fig. 1A) or LPS (Fig. 1B) alone.
Figure 1.
SF augments responses to TLR-2 (Pam3CysK4, A, C, E), and TLR-4 (LPS, B, D, F) ligands in both HEK-293 transfected cell lines (A & B) and primary FLS cultures (C–F). Cells were stimulated as described with 25% SF (listed by specimen #), Pam3 (Pam3CysK4 100–500ng/ml, A, C, E), LPS (100ng/ml, B, D, F), or SF + Pam3 or LPS in duplicate or triplicate wells. For A, white bars represent HEK-293 cells transfected with CD14, black bars are cells transfected with both TLR-2 and CD14. Similarly, for B, white bars represent HEK-293 cells transfected with TLR-4, and black bars are cells transfected with both TLR-4 and MD-2. After 18 hours, IL-8 was measured by ELISA in culture supernatants. C–F: FLS cells were stimulated similarly to the HEK-293 cells. C & D: FLS IL-8 production and E & F: IL-6 production after 18 hours. Results shown are representative of 2–3 separate experiments. *** p<0.001, ** p<0.01.
OA SF augments IL-8 and IL-6 production by FLS in response to both TLR-2 and TLR-4 ligands
To determine whether SF impacts cells within the joint, we established primary fibroblast-like synoviocyte (FLS) cultures. Similar to responses of HEK-293 cells, SF (25%) from OA patients significantly increased production of IL-8 in response to both Pam3CysK4 (Figure 1C) and LPS (Figure 1D). SF also augmented IL-6 production in response to these ligands (Figure 1E and 1F). In total, twenty-three separate OA SF specimens (twenty-two from early OA and one from an advanced OA patient) were tested in combination with LPS and six in combination with Pam3CysK4. All augmented LPS responses and five of six augmented responses to Pam3CysK4.
OA SF may contain factors which can directly induce IL-8 production, such as IL-1β or TNF-α (29). In control experiments (not shown), we tested whether FLS were responsive to these two cytokines. Exposure to IL-1β (1 or 10 ng/ml), but not TNFα, induced release of IL-8 from FLS cells. However, we previously reported that SF IL-1β was only detected in one of eighteen early knee OA patients (29) and measured at < 1pg/ml. Although OA SF may contain other unknown factors which can directly induce IL-8 production, of twenty-three samples tested, only four (#61 Fig. 1C, #52 and #564 Fig. 1F, and #83 not shown) induced IL-8 or IL-6 without addition of LPS or Pam3CysK4. Of these four, two (#61 and #83) could be attributed to IL-8 in SF itself. Sufficient SF from #564 was not available for cytokine analysis, while the fourth specimen (#52) induced twice the IL-6 in SF alone. With exception of this one sample, augmentation of cytokine release from FLS in response to SF + TLR ligands could not be attributed to a direct effect of SF in the majority of cases.
To determine whether cells remaining in SF could explain the effect, three control assays were performed (data not shown). First, SF was treated with 250 U/ml hyaluronidase (Sigma,St. Louis, MO) for 10 minutes to reduce viscosity prior to centrifugation to remove cells. Second, we filtered SF through a 0.45µm filter prior to use. These two treatments had no effect on our results. Finally, we incubated unfiltered SF with LPS alone for 18 hours in the absence of FLS. No IL-8 production above baseline was observed. Therefore, effects could not be attributed to cells supplied by SF.
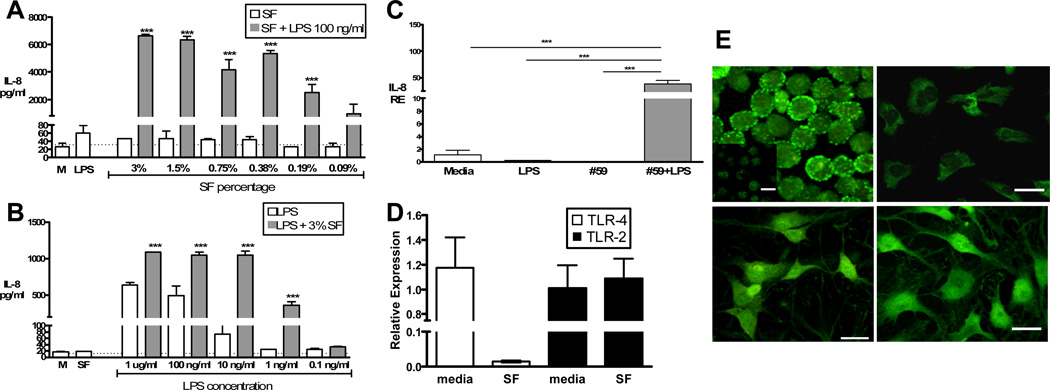
Titration of SF in combination with LPS
FLS were stimulated with two-fold dilutions of SF from 25% down to less than 1%, in combination with LPS (100 ng/ml). There was a dose-related decrease in IL-8 production in response to LPS, but concentrations as low as 0.09% were still effective (Figure 2A). Next, 10-fold dilutions of LPS from 1ug/ml to 0.01ng/ml were used alone or with 3% SF. LPS alone induced IL-8 release at higher concentrations (Figure 2B), and addition of SF augmented this response. At low LPS concentrations (1ng/ml), IL-8 production was only observed with addition of SF. This potentiating effect of SF was also observed at the transcriptional level (Figure 2C), measured by real-time qPCR.
Figure 2.
Cells were stimulated as described with A: SF in decreasing percentages alone (white bars) or with LPS 100ng/ml (grey bars); or B: LPS in decreasing concentrations alone (white bars) or with 3% SF (grey bars), all in duplicate. M = Media control, Dotted line = ELISA limit of detection. *** p <0.001 compared to both SF or LPS alone. C: IL-8 mRNA levels (RE = Relative Expression) measured by qPCR after six hour stimulation with 3% SF #59 with or without 100ng/ml LPS. *** p <0.001. D: mRNA levels of TLR-4 (white) and TLR-2 (black) after six hour stimulation with or without 3% SF #59. Expression levels were calculated relative to media control. Results in A–D are representative of two separate experiments. E: Two-photon images of TLR-4 staining. Top left: Positive control, TLR-4 staining of HEK-TLR4 transfectants (isotype control staining shown in inset). Top right: Unstimulated FLS culture stained with isotype control mAb (mean fluorescence intensity, MFI arbitrary units = 61.4+/− 30.0). Bottom left: TLR-4 staining of unstimulated FLS (MFI = 173.0 +/− 25.8). Bottom right: TLR-4 staining of FLS stimulated with 3% SF (MFI = 179.7 +/− 21.9). White scale bars = 20mm.
SF does not upregulate TLR expression in FLS cells
Unknown SF factors could upregulate TLR expression, thereby augmenting responses to ligands. We compared TLR-2 and TLR-4 mRNA levels in unstimulated and SF stimulated FLS. Exposure to SF (3%) did not increase but rather tended to decrease TLR-4 mRNA levels, and had no effect on TLR-2 mRNA levels (Figure 2D). After correcting for background staining with istoype control, cell surface staining analyzed by flow cytometry was consistent with the mRNA expression pattern (TLR-2: Mean fluorescence intensity (MFI) untreated = 1098, treated = 1054; TLR-4: MFI untreated = 788, treated = 584). Given the high background of flow cytometry samples, we visualized immunostaining of TLR-4 using two-photon microscopy. As demonstrated in Figure 2E, Mean Fluorescence Intensity (MFI, arbitrary units) of TLR-4 staining in cells exposed to 3% SF (179.7 +/− 21.9) was comparable to unstimulated cells (173.0 +/− 25.8).
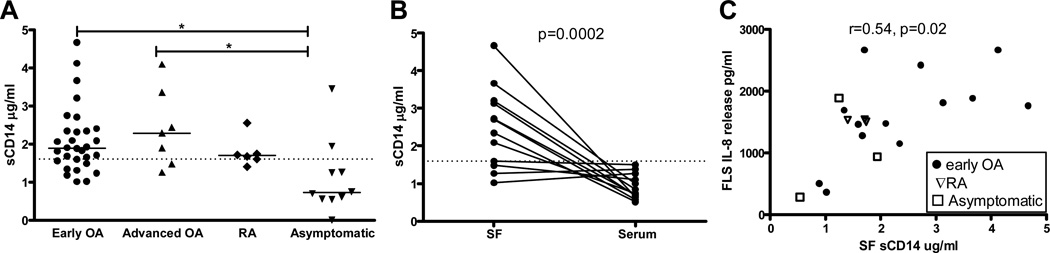
sCD14 levels in SF from patients with OA
Heat-treating SF at 98°C for ten minutes prior to use destroyed its ability to augment LPS responses in FLS (data not shown), indicating a heat-denaturable factor was responsible. One soluble protein found in SF from RA patients (30) that can interact with both TLR-2 and TLR-4 is sCD14 (31, 32). We measured sCD14 in SF from thirty patients with early OA; characteristics of these patients are shown in Table 1. Levels of sCD14 in these patients ranged from 1–5 µg/ml (Figure 3A), comparable to levels in advanced OA and RA. Median levels in SF from 10 asymptomatic postmortem donors without a history of joint disease (Figure 3A) were 0.72 µg/ml (Interquartile range 0.55–1.4), lower than the patient groups (p < 0.01). sCD14 was then measured in paired SF and serum from twelve early OA patients. In 8 of 12 patients, SF levels were higher than sera (Figure 3B). sCD14 concentration in an age-matched, pooled normal human serum specimen was 1.6µg/ml.
Figure 3.
SF soluble CD14 (sCD14) levels in early OA patients are (A) comparable to levels observed in advanced OA and Rheumatoid Arthritis, (B) elevated in SF compared with serum, and (C) correlate with SF augmentation of FLS LPS response. A: SF sCD14 in early OA patients undergoing meniscectomy (n=30), advanced OA patients undergoing total knee replacement (n=7) and RA patients undergoing TKR (n=6), as well as asymptomatic organ donors without known joint disease (n=10) were measured by ELISA (see Table 1 for patient characteristics). *P< 0.05 (Kruskal-Wallis) compared with asymptomatic donors. B: In early OA patients undergoing meniscectomy, SF levels were higher than in paired serum specimens (Mann-Whitney p=0.0002). Dotted line = level in pooled, normal human serum. C: FLS were stimulated for 18 hours as described in Materials and Methods with LPS (100ng/ml) + SF (0.2%) from patients with early OA (n=12, circles), RA (n=3, triangles), and asymptomatic controls (n=3, squares). IL-8 levels measured in supernatants after stimulation correlated with sCD14 levels in the SF (Spearman rho = 0.54, p = 0.02).
FLS response to SF + LPS correlates with sCD14 concentration in SF
To determine if SF sCD14 concentration correlated with magnitude of IL-8 release in vitro, we tested 18 SF specimens (12 early OA, 3 RA, and 3 asymptomatic controls) in a single experiment. 0.2% SF was used, as titration experiments indicated low concentrations of SF were sufficient. IL-8 release from FLS cells exposed to SF + LPS correlated strongly (Spearman ρ = 0.54, p = 0.02) with SF sCD14 concentrations (Figure 3C), and was not disease specific. The lowest FLS response and lowest sCD14 concentration was measured in the only specimen (asymptomatic control) with no structural joint or cartilage damage (Collins grade 0).
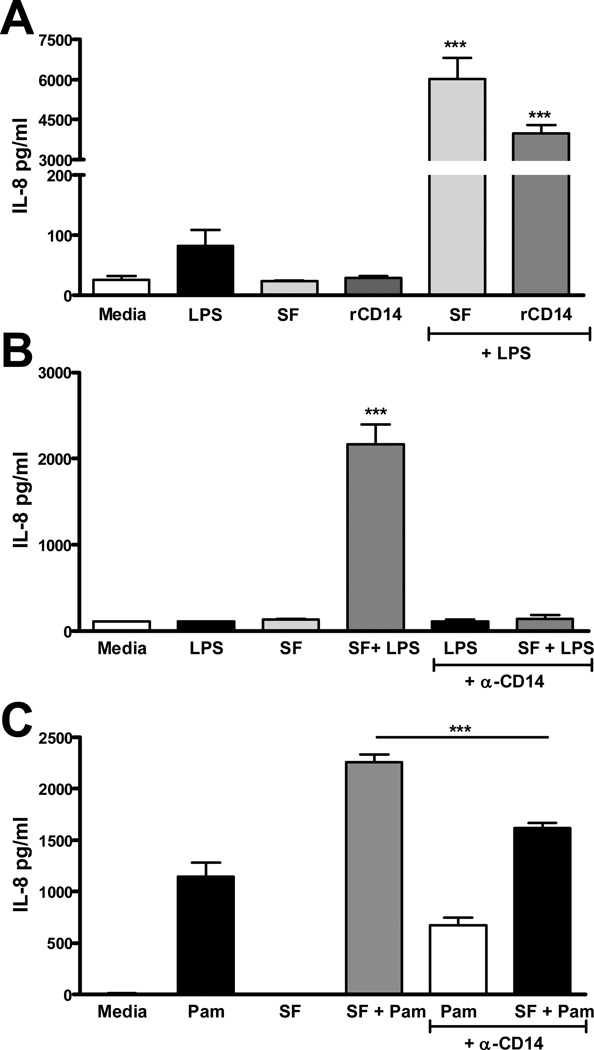
rCD14 reproduces, and anti-CD14 blocks, the potentiating effect of SF on LPS responses in FLS cultures
To test whether CD14 augments LPS responses in FLS, rCD14 was used in place of SF, at levels comparable to 25% SF (0.5 µg/ml). rCD14 in combination with 100 ng/ml LPS induced 40-fold higher IL-8 release than LPS alone, and approached levels stimulated by SF + LPS (Figure 4A). Next, SF (3%) was pre-incubated with a 10-fold greater molar equivalent of anti-CD14 mAb prior to use. This treatment abolished SF augmentation of LPS responses, and diminished SF augmentation of Pam3CysK4 responses in FLS cultures (Figures 4B and 4C).
Figure 4.
Addition of recombinant CD14 augments response of FLS to LPS (A) and incubation with anti-CD14 abrogates SF augmentation of both the LPS (B) and Pam3CysK4 (C) response. In A, primary FLS were stimulated as described with LPS 100ng/ml, 25% SF#21, rCD14 0.5ug/ml (expected concentration in 25% SF), SF + LPS, or rCD14 + LPS. Results are representative of two experiments. *** p<0.001 compared with all other groups. In B and C 1.5% SF#59, 100ng/ml LPS (B) or Pam3CysK4 (C) was pre-incubated with monoclonal anti-CD14 (clone MEM-18) prior to incubation with FLS cells. The anti-CD14 itself had no effect on IL-8 on its own (data not shown). *** p<0.001.compared to all other groups (B), or as indicated (C).
Discussion
Osteoarthritis leads to chronic joint symptoms and disability, and low-grade inflammation within the synovial membrane is associated with pain (2), and contributes to progression of cartilage loss (4). We found synovitis associated with symptoms in patients undergoing arthroscopic surgery for meniscal tears, even in the absence of radiographic OA (9). The majority of these patients had degenerative type meniscal tears and intra-operative evidence of early cartilage damage, consistent with the view that meniscal tears may be a sign of early-stage “pre-radiographic” disease in many patients (33). Close to one million arthroscopic meniscal procedures are performed every year in the US (34). Therefore, patients indicated for these procedures represent a population in which mediators involved in early stages of OA can be explored. We hypothesized that soluble TLR ligands present in SF of these patients are responsible for activating FLS. To test this hypothesis, we screened SF using in vitro stimulation of TLR-2 and 4 expressing cells (HEK-293 transfectants). Although we did not detect a TLR-agonist in these patients, SF consistently enhanced TLR-transfectant and primary FLS responses to classical TLR-2 and TLR-4 ligands (Figure 1).
Very low concentrations of SF (<0.1%) enhanced IL-8 in response to LPS in FLS cultures, and SF allowed primary FLS to respond to LPS at concentrations 100-fold lower (1ng/ml) than in the absence of SF (100 ng/ml) (Figure 2 A&B). This was not due to a direct effect of SF on cytokine production, nor to upregulation of TLR expression in FLS (Figure 2 D&E). As SF augmented responses to both TLR-2 and TLR-4 ligands (Pam3CysK4 and LPS) we assessed sCD14 in SF. CD14 is a co-receptor for TLR-2 and TLR-4 on various cell types, particularly macrophage lineage cells. It transfers LPS to the TLR-4 complex, and enhances LPS-mediated signaling in macrophages (35). Similarly, CD14 facilitates TLR-2 ligand recognition by receptor complexes (32). It is expressed on monocytes as a glycophosphatidyl inositol (GPI)-anchored membrane protein (36), but is also found in soluble form. Whether SF sCD14 levels correlate with fluid monocyte counts could not be answered in this study, as fluid specimens were centrifuged and frozen prior to analysis. Future work is necessary to determine cellular sources of sCD14 in early OA patients, but higher levels in SF compared to serum suggests local production in the joint.
Three lines of evidence support a role for SF sCD14 in mediating FLS responses to TLR-2 or TLR-4 ligands in our assays. First, SF sCD14 concentrations correlated with IL-8 release in response to SF + LPS (Figure 3C). Second, substitution of rCD14 in place of SF recapitulated SF effects (Figure 4A). Addition of rCd14 to LPS did not fully restore FLS IL-8 release to levels seen with SF, so we cannot rule out a role for additional factors supplied by SF such as endogenous TLR ligands or other cytokines in a few samples. Finally, anti-CD14 mAb abolished the effect of SF on LPS responses, and diminished PamCys3K4 responses. Similar to membrane-anchored CD14 (mCD14), sCD14 facilitates LPS-induced leukocyte activation in vitro (31). In endothelial cells (37) sCD14 increased sensitivity to LPS; high levels of LPS stimulated cells but low-levels required the presence of sCD14 similar to our observations in FLS cultures exposed to SF (Figure 2). In endothelial cells, expression of mCD14 was still required to respond to low-levels of LPS even in the presence of sCD14 (37). In our study, FLS were mCD14 negative by flow cytometry prior to use in stimulation assays, although we can’t rule out low-level expression.
sCD14 is found in RA SF, and rCD14 added to LPS augments intercellular adhesion molecule-1 (ICAM-1) expression by RA FLS cultures (30). The present study demonstrates that SF sCD14 is not disease specific, as it was also found in patients with early and advanced OA, RA, and asymptomatic donors (Figure 3A). Regardless of diagnosis, sCD14 concentration correlated with SF augmentation of FLS IL-8 production induced by LPS. Although immunosuppressives used by RA patients may have lowered levels in this group, sCD14 levels measured in our RA patients were comparable to previous reports (30). Our asymptomatic organ donors are not ideal controls given the variable presence of cartilage degeneration and potential degradation post-mortem. Still, sCD14 levels detected in SF from ten donors collected within 24 hours of death were significantly lower than in patients. SF from three of these donors were tested in FLS assays, and were also able to augment LPS responses. Interestingly, the lowest response (and lowest sCD14 concentration) was measured in SF from the only control with normal cartilage (Collins grade 0). It is possible that structural joint damage, irrespective of mechanism of damage, influences the level of sCD14 found in SF.
Definitive proof that sCD14 plays an important role in OA pathogenesis will need future experimentation using disease models. In the current investigation, sCD14 in SF allowed FLS to respond to lower levels of TLR-2 and TLR-4 ligands, but did not by itself stimulate cytokine production. We speculate sCD14 in SF may play a role in sensitizing cell populations within the joint to TLR stimuli, not only in inflammatory arthritis, but also in OA as well. If the threshold for synovial inflammatory response to TLR-2 and TLR-4 ligands is lowered by increasing amounts of sCD14 in SF, then inflammation may be triggered more easily when TLR ligands are produced. This might be expected in three scenarios: (i) Increased production of endogenous ligands is expected during periods of increased matrix turnover episodically in chronic arthritis, or after a joint injury (a meniscal tear or ligament injury). In this scenario, SF sCD14 might impact development of effusion, synovitis, and symptomatic flares in both OA and RA patients. (ii) The association of crystal deposition and OA has been recently reviewed (38). Both uric acid and calcium pyrophosphate have been demonstrated to activate TLR pathways. “Sensitizing” of synoviocyte TLR responses by increased levels of sCD14 in OA may increase the inflammatory response to these crystals. (iii) Finally, both OA and RA are reported risk factors for septic arthritis (39). Although immunosuppressive medications likely impact risk in RA patients, sCD14 may be an additional risk factor in both RA and OA. sCD14 may lower the threshold for, or increase severity of TLR responses to infectious agents.
FLS responses described in this study were observed in vitro, so it is not clear whether effects of SF sCD14 are functional in vivo. SF concentrations over 25% induced cell death, so the impact of 100% SF could not be determined. The viscosity of SF likely limits diffusion or availability of oxygen and media components important for cell survival in monolayer cultures. This also limits our ability to detect TLR agonist activity which might be in low concentrations. Still, our culture system was an attempt to partially recreate the joint environment in vitro, and we found that SF exerted an augmenting effect on TLR responses at least in part via sCD14. More work is needed to characterize additional SF factors that may influence FLS responses to TLR ligands, and determine effects of SF sCD14 on other cell types that respond to TLR stimuli in vivo.
In summary, the current investigation supports a role for sCD14 in potentiating FLS responses to TLR-2 and TLR-4 ligands in both early and advanced OA patients. As a similar role for CD14 has been suggested in RA, our work suggests similarity in TLR activation requirements of FLS in these two different arthritides. Our findings do not specifically address initiation of disease, but are consistent with reports supporting a role for local inflammation even in early stages of OA. We suspect differences between extent and severity of inflammation in OA and RA may be due to differences in adaptive inflammatory responses, as more pronounced autoantibody production and systemic inflammation are more characteristic of RA. CD14 has recently been demonstrated to participate in signaling and intracellular trafficking of other TLRs including TLR-3, TLR-7 and TLR-9 (40, 41), thus the effect of SF on other TLR ligands including candidate endogenous ligands needs to be explored.
Acknowledgements
The authors would like to acknowledge the following individuals for their contributions: The patients for their donation of synovial fluids for this study; Dr. R.W. Finberg for the gift of the HEK-293 transfectants; Joel Block, MD for critical review of the manuscript; Najia Shakoor, MD for performing the Kellgren-Lawrence scoring for the meniscectomy patients; Mukesh Ahuja, MS for his assistance in clinical data collection; Scott Rodeo and Eva Asomugha for collection of the end-stage OA synovial fluid specimens at HSS; Julia Kurko, MD for assistance with the flow cytometry; and Arkady Margulis, MD for coordination of the post-mortem tissue and fluid donations from the Gift of Hope and the Collins grading. In addition, we would like to express our appreciation to the Gift of Hope Organ and Tissue Donation Network, and the donors’ families for access to the post-mortem specimens.
Supported by: National Institute of Arthritis, Musculoskeletal and Skin Diseases 1K08AR057859-01 (CRS); Junior Career Development Award jointly sponsored by the American College of Rheumatology and the Association of Specialty Professors (CRS); Rush University Translational Science Consortium New Investigator Award (CRS).
Footnotes
Disclosures:
Rush University and Hospital for Special Surgery have filed a patent application for biomarkers in Osteoarthritis on behalf of CRS.
References
- 1.Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of knee osteoarthritis: the MOST study. Ann Rheum Dis. 2010;70(5):805–811. doi: 10.1136/ard.2010.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(10):1033–1040. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252(3):772–780. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakkas LI, Scanzello C, Johanson N, Burkholder J, Mitra A, Salgame P, et al. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin Diagn Lab Immunol. 1998;5(4):430–437. doi: 10.1128/cdli.5.4.430-437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenberg DL, Egan MS, Cohen AS. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982;9(2):204–209. [PubMed] [Google Scholar]
- 7.Pearle AD, Scanzello CR, George S, Mandl LA, DiCarlo EF, Peterson M, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15(5):516–523. doi: 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scanzello CR, McKeon B, Swaim BH, Dicarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: Molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63(2):391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 11.Kaczorowski DJ, Nakao A, Mollen KP, Vallabhaneni R, Sugimoto R, Kohmoto J, et al. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. 2007;84(10):1279–1287. doi: 10.1097/01.tp.0000287597.87571.17. [DOI] [PubMed] [Google Scholar]
- 12.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16(8):693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 13.Mollen KP, Levy RM, Prince JM, Hoffman RA, Scott MJ, Kaczorowski DJ, et al. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83(1):80–88. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- 14.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279(17):17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 15.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 16.van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31(3):280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, 2nd, Tak PP, et al. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182(8):4965–4973. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HA, Cho ML, Choi HY, Yoon CS, Jhun JY, Oh HJ, et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54(7):2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 19.Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010;62(7):2004–2012. doi: 10.1002/art.27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierer M, Rethage J, Seibl R, Lauener R, Brentano F, Wagner U, et al. Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J Immunol. 2004;172(2):1256–1265. doi: 10.4049/jimmunol.172.2.1256. [DOI] [PubMed] [Google Scholar]
- 21.Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009;35(3):579–590. doi: 10.1016/j.rdc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20(5):565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 23.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 24.Scanzello CR, Umoh E, Pessler F, Diaz-Torne C, Miles T, Dicarlo E, et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage. 2009;17(8):1040–1048. doi: 10.1016/j.joca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5(1):23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 26.Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, et al. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100(5):1860–1868. [PubMed] [Google Scholar]
- 27.Rosengren S, Boyle DL, Firestein GS. Acquisition, culture, and phenotyping of synovial fibroblasts. Methods Mol Med. 2007;135:365–375. doi: 10.1007/978-1-59745-401-8_24. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Scanzello CR, Umoh E, Pessler F, Diaz-Torne C, Miles T, Dicarlo E, et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage. 2009;17(8):1040–1048. doi: 10.1016/j.joca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Yu S, Nakashima N, Xu BH, Matsuda T, Izumihara A, Sunahara N, et al. Pathological significance of elevated soluble CD14 production in rheumatoid arthritis: in the presence of soluble CD14, lipopolysaccharides at low concentrations activate RA synovial fibroblasts. Rheumatol Int. 1998;17(6):237–243. doi: 10.1007/s002960050041. [DOI] [PubMed] [Google Scholar]
- 31.Hailman E, Vasselon T, Kelley M, Busse LA, Hu MC, Lichenstein HS, et al. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156(11):4384–4390. [PubMed] [Google Scholar]
- 32.Nakata T, Yasuda M, Fujita M, Kataoka H, Kiura K, Sano H, et al. CD14 directly binds to triacylated lipopeptides and facilitates recognition of the lipopeptides by the receptor complex of Toll-like receptors 2 and 1 without binding to the complex. Cell Microbiol. 2006;8(12):1899–1909. doi: 10.1111/j.1462-5822.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 33.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60(3):831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;(11):1–25. [PubMed] [Google Scholar]
- 35.Ostuni R, Zanoni I, Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell Mol Life Sci. 2010;67(24):4109–4134. doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd-Jones KL, Kelly MM, Kubes P. Varying importance of soluble and membrane CD14 in endothelial detection of lipopolysaccharide. J Immunol. 2008;181(2):1446–1453. doi: 10.4049/jimmunol.181.2.1446. [DOI] [PubMed] [Google Scholar]
- 38.Nowatzky J, Howard R, Pillinger MH, Krasnokutsky S. The role of uric acid and other crystals in osteoarthritis. Curr Rheumatol Rep. 2010;12(2):142–148. doi: 10.1007/s11926-010-0091-4. [DOI] [PubMed] [Google Scholar]
- 39.Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010;375(9717):846–855. doi: 10.1016/S0140-6736(09)61595-6. [DOI] [PubMed] [Google Scholar]
- 40.Baumann CL, Aspalter IM, Sharif O, Pichlmair A, Bluml S, Grebien F, et al. CD14 is a coreceptor of Toll-like receptors 7 and 9. J Exp Med. 2011;207(12):2689–2701. doi: 10.1084/jem.20101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HK, Dunzendorfer S, Soldau K, Tobias PS. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity. 2006;24(2):153–163. doi: 10.1016/j.immuni.2005.12.012. [DOI] [PubMed] [Google Scholar]