Abstract
The dried flower buds of Magnolia sp. are widely used as herbal medicines because of their anti-inflammatory, anti-malarial and anti-platelet activities. Here, we found that veraguensin and galgravin, lignan compounds derived from Magnolia sp., dose-dependently inhibited osteoclast formation in co-cultures of bone marrow cells and osteoblastic cells. These compounds also inhibited receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclast differentiation in RAW264.7 cells and bone marrow macrophages. In the RANKL-induced signaling pathway, veraguensin and galgravin reduced p38 phosphorylation and suppressed the expression of c-Fos, a key transcription factor for osteoclastogenesis. Veraguensin and galgravin also inhibited osteoclastic pit formation, which was accompanied by decreased mature osteoclast viability. In conclusion, these results indicate that veraguensin and galgravin can inhibit bone resorption and may offer novel compounds for the development of drugs to treat bone-destructive diseases such as osteoporosis.
Keywords: Veraguensin., Galgravin, RANKL, Osteoclast, p38, c-Fos
Introduction
Bone homeostasis represents a balance between osteoclastic bone resorption and osteoblastic bone formation. Excessive bone resorption by osteoclasts is involved in the pathogenesis of several lytic bone diseases, including osteoporosis, hypercalcemia, rheumatoid arthritis, tumor metastasis, periodontitis and Paget’s disease (Rodan and Martin 2000). Osteoclasts are formed from hematopoietic precursors of the monocyte/macrophage lineage. Receptor activator of nuclear factor κB (RANK) ligand (RANKL), a member of the tumor necrosis factor family, plays crucial roles in osteoclast differentiation, function and survival (Boyle et al. 2003; Feng 2005; Takahashi et al. 1999; Suda et al. 1999). Osteoclast precursors express RANK, which recognizes RANKL expressed by osteoblastic cells through cell–cell interactions. The precursor cells then differentiate into osteoclasts in the presence of macrophage colony-stimulating factor (M-CSF). A soluble decoy receptor for RANKL, osteoprotegerin (OPG) blocks osteoclastogenesis by inhibiting the RANKL–RANK interaction. Mature osteoclasts also express RANK, and RANKL supports the survival and regulate bone-resorbing activity of osteoclasts. Therefore, RANKL signaling may offer a therapeutic target for preventing and treating bone diseases, such as osteoporosis.
To discover new types of antiresorptive agents, we screened for natural agents that inhibit the differentiation and function of osteoclasts. We found that two compounds, veraguensin and galgravin, from the flower buds of Magnolia sp. were able to inhibit osteoclastogenesis. The dried flower buds of Magnolia biondii Pamp., Magnolia fargesii and Magnolia kobus are components of “Xin-Yi”, a widely used traditional Chinese medicine, and of “Shin-I”, a traditional Japanese medicine (Lim et al. 2009). Veraguensin and galgravin have been reported to exhibit anti-inflammatory (da Silva Filho et al. 2004) and anti-malarial (da Silva Filho et al. 2008) and anti-platelet (Chen et al. 1993) activities. However, their effects on bone resorption have not been reported. In the present study, we investigated the effects of veraguensin and galgravin on the differentiation and function of osteoclasts.
Materials and methods
Reagents
Dried flower buds of Magnolia sp. were obtained from Tochimoto Tenkaido Co., Ltd. (Osaka, Japan). Fast red-violet LB salt and naphthol AS-MX phosphate were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). Recombinant soluble RANKL (sRANKL) was purchased from PeproTech EC Ltd. (London, UK). PD98059 was purchased from Calbiochem (La Jolla, CA). 1α,25-Dihydroxyvitamin D3 [1α,25-(OH)2D3] and prostaglandin E2 (PGE2) were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Anti-c-Jun N-terminal kinase (JNK), anti-phospho-JNK, anti-p38, anti-phospho-p38 and anti-IκB rabbit polyclonal antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). Anti-NFATc1 mouse polyclonal antibodies were purchased from Affinity Bio Reagents Co., Ltd. (Rockford, IL, USA). Anti-c-Fos and anti-actin mouse polyclonal antibodies were purchased from Sigma Chemical Co. (St. Louis, MO). All other chemicals and reagents were of analytical grade.
Isolation and identification of veraguensin and galgravin
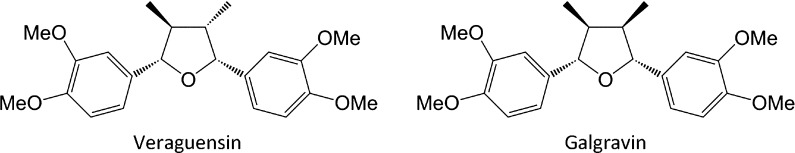
The dried flower buds of Magnolia sp. (500 g) were extracted with methanol (5 L) for 2 weeks. The evaporated methanol extract (76.60 g) was dissolved in distilled water, and successively fractionated with ethyl acetate (55.78 g), hexane (17.05 g) and 90% methanol (37.44 g). The 90% methanol-eluted fraction was applied on a silica gel open column (BW silica gel; BW-820 M, Fuji Silysia Chemical Ltd., Aichi, Japan) and eluted with hexane and ethyl acetate (9:1, 8:2, 7:3 and 1:0 v/v). The 7:3 hexane and ethyl acetate fraction was crystallized and filtered, and then separated using a reverse phase preparative high-performance liquid chromatography system equipped with a COSMOSIL Cholester Waters column [mobile phase, 50% AcCN (v/v); size, 20 × 250 mm; flow rate in the mobile phase, 5 mL/min]. The structures of the active compounds (veraguensin and galgravin) were confirmed by 1H nuclear magnetic resonance (NMR) (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectra obtained on a Fourier-transform NMR system (JEOL, Japan) and by mass spectrometry. The chemical structures are shown in Fig. 1.
Fig. 1.
Structures of veraguensin and galgravin
Animals
Male ddY mice aged 4–6 weeks (SLC Inc., Shizuoka, Japan) were housed in an air-conditioned room with a 12 h light/dark cycle at a temperature of 23 ± 3 °C and humidity of 55 ± 5%. Mice were given free access to food (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan) and water. This experimental animal study was approved by and conducted in accordance with the guidelines of the Animal Experiment Committee of Chubu University.
Osteoclast differentiation in co-cultures of bone marrow cells (BMCs) and osteoblastic cells
BMCs were obtained from the long bones of 4–6-week-old male ddY mice. In the co-culture system, BMCs were cultured with UAMS-32 cells on 96-well plates in α-Minimal Essential Medium (MEM) containing 10% fetal bovine serum (FBS), and 10−6 M PGE2. After the co-culture, the cells were fixed with 10% formalin for 10 min, ethanol for 1 min, and then dried.
Osteoclast formation from RAW264.7 cells and bone marrow macrophages (BMMs)
RAW264.7 cells were seeded in 96-well plates (3 × 103 cells/well) and cultured in the presence of RANKL (50 ng/mL) for 3 days. Mature osteoclasts were formed in RAW264.7 cell cultures in the presence of RANKL and PD98059 (20 μM) for 4 days. To obtain BMMs, BMCs were cultured for 1 day in α-MEM containing 10% FBS and 50 ng/mL M-CSF in 60-mm dishes. After culture for 1 day, non-attached cells were collected and used as BMMs. In the BMM culture system, BMMs were cultured in 96-well plates in the presence of 50 ng/mL M-CSF for 3 days, treated with 100 ng/mL RANKL, and cultured for a further 3 days. The cells were fixed with 10% formalin for 10 min, ethanol for 1 min, and then dried.
Osteoclast pit formation
To obtain mature osteoclasts, 1 × 107 BMCs and 1 × 106 UAMS-32 cells were co-cultured in collagen gel-coated 100-mm plates for 5–6 days in α-MEM containing 10% FBS, 10−8 M 1α,25(OH)2D3 and 10−6 M PGE2. The plates were treated with collagenase and viable cells were harvested for use in subsequent experiments. For resorption pit assays, aliquots of the crude mature osteoclast preparations were placed on dentin slices in 96-well plates (Suda et al. 1997). After preincubation for 2 h, the dentin slices were transferred to 48-well plates (1 dentin slice/well) containing 0.3 mL of α-MEM supplemented with 10% FBS, and cultured with or without veraguensin and galgravin for 48 h. Resorption pits on the dentin slices were visualized after staining with hematoxylin. The number of resorption pits on each slice was counted under a microscope.
Cell viability assay
The reduction activity of (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was determined as an indicator of living cells using a MTT assay kit (Roche Diagnostics, Mannheim, Germany). Absorbance of MTT formazan was measured at 570 nm using a microplate reader and cell viability was shown as the percentage absorbance relative to the control.
Tartrate-resistant acid phosphatase (TRAP) assay
Osteoclast formation was evaluated by measuring TRAP activity and counting the number of TRAP-positive multinucleated cells. Fixed cells were incubated in 50 mM citrate buffer (pH 4.6) containing 10 mM tartrate and 5 mM p-nitrophenylphosphate for 30 min. The reaction was stopped by adding 0.1 M NaOH. TRAP activity was evaluated by measuring absorbance at 405 nm using a microplate reader (Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan). TRAP staining was performed as described previously (Hasegawa et al. 2010). TRAP-positive multinucleated cells with three or more nuclei were counted.
Western blot analysis
Cells were lysed with RIPA buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 50 mM β-glycerophosphate, 1% NP-40, 1 mM Na3VO4 and 1× protease inhibitor cocktail). The extracted proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene fluoride membranes. The membranes were incubated with primary antibodies against JNK, phospho-JNK, p38 mitogen-activated protein kinase (MAPK), phospho-p38 MAPK, IκB, c-Fos, NFATc1 and β-actin, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Immunocomplexes were visualized by enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Amersham, Buckinghamshire, UK).
Statistical analysis
SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all analyses. All data are presented as means ± standard deviation. Differences between groups were compared by using Dunnett’s t test. p < 0.05 was considered statistically significant.
Results
Veraguensin and galgravin inhibited osteoclast differentiation in co-cultures of BMCs and osteoblastic UAMS-32 cells
To assess the effects of veraguensin and galgravin on osteoclast differentiation, we first established co-cultures of mouse BMCs and osteoblastic UAMS-32 cells. Veraguensin and galgravin showed no or very weak inhibitory effects on TRAP activity and cell viability in cells cultured in the presence of 1α,25(OH)2D3 (Fig. 2a, b). However, the number of multinucleated osteoclasts was decreased by veraguensin and galgravin in a dose-dependent manner at concentrations of 30–100 μM (Fig. 2b, c). To clarify whether these compounds affected the osteoclast precursors or osteoblastic cells, we assessed cell viability and mRNA expression of RANKL and OPG, which regulate osteoclast differentiation, in osteoblastic UAMS-32 cells. However, neither cell viability nor mRNA expression was changed by veraguensin and galgravin (data not shown). These results suggest that veraguensin and galgravin suppressed osteoclast formation by directly affecting osteoclast progenitors, but not osteoblasts.
Fig. 2.
Effects of veraguensin and galgravin on osteoclast differentiation in co-cultures. a Cell viability and TRAP activity. UAMS-32 cells and BMCs were cultured in the presence of veraguensin and galgravin (1, 3, 10, 30 and 100 μM) for 6 days. Cell viability was determined by MTT assays (white bar). Cells were also fixed and TRAP activity was determined (black bar). b TRAP-positive multinucleated cells containing more than five nuclei were considered to be osteoclasts and were counted. c TRAP staining of cells plated in 96-well plates. *p < 0.05, **p < 0.005. Bar = 200 μm
Veraguensin and galgravin inhibited osteoclast differentiation in RAW264.7 and BMM cell cultures
Next, we determined the effects of veraguensin and galgravin on osteoclast formation from osteoclast progenitor cells using RAW264.7 cells and BMMs. RAW264.7 cells and BMMs were incubated with both compounds in the presence of sRANKL and PD98059 (RAW264.7 cells), or with sRANKL and M-CSF (BMMs), which promoted cell growth and differentiation into osteoclasts. As shown in Fig. 3a–c, veraguensin and galgravin inhibited TRAP activity and osteoclast formation induced by RANKL at concentrations of 3–100 μM in both cell types. However, MTT assays revealed that the viabilities of RAW264.7 cells and BMMs were not affected by veraguensin and galgravin, even at concentrations exceeding 100 μM. These results suggest that veraguensin and galgravin directly act on osteoclast progenitors, and they inhibit osteoclast formation without affecting cell viability.
Fig. 3.
Veraguensin and galgravin inhibit RANKL-induced osteoclast formation in RAW264.7 cells and mouse BMMs. a RAW264.7 cells were cultured in 96-well plates with 100 ng/mL RANKL and 20 μM PD98059 in the presence of veraguensin and galgravin for 72 h. b BMMs were cultured in 96-well plates with 100 ng/mL RANKL and 50 ng/mL M-CSF in the presence of veraguensin and galgravin for 72 h. The cells were fixed and TRAP activity was determined (black bar). Cell viability was determined by MTT assays (white bar). c TRAP staining of RAW264.7 cells plated in 96-well plates. *p < 0.01, **p < 0.05. Bar = 200 μm
Effects of veraguensin and galgravin on the sRANKL-induced signaling cascade
To identify the inhibitory mechanisms and pathway targeted by veraguensin and galgravin, BMMs were treated with both compounds (30 μM) in the presence of sRANKL for 0–60 min. The degradation of IκB and phosphorylation of p38 and JNK in response to sRANKL reached maximal levels within 10 min, and then returned to the basal levels (Fig. 4a). Phosphorylation of p38 MAPK was impaired by veraguensin and galgravin, whereas IκB degradation and JNK phosphorylation were not. Next, we examined the effects of both compounds on c-Fos expression, a pivotal transcription factor for osteoclastogenesis (Fig. 4c). Veraguensin and galgravin strongly impaired sRANKL-stimulated expression of c-Fos. Taken together, these findings indicate that veraguensin and galgravin inhibit osteoclast formation by inhibiting of phosphorylation of p38 MAPK and downregulating c-Fos expression.
Fig. 4.
Effects of veraguensin and galgravin on RANKL-induced signaling pathways. BMMs were preincubated in the presence of 30 μM veraguensin and galgravin for 10, 30 or 60 min, and then treated with 100 ng/mL RANKL for the indicated times. Cell lysates were collected and separated by 10% SDS-PAGE. a Western blotting analysis of phosphorylated or unphosphorylated IκB, p38 and JNK. b Western blotting analysis of c-Fos. The results are representative of three independent experiments
Veraguensin and galgravin inhibited osteoclastic pit formation
To investigate the inhibitory effects of veraguensin and galgravin on bone resorption by mature osteoclast, we examined the effects of both compounds on pit formation by mature osteoclasts prepared in the co-culture system. Mature osteoclasts readily formed resorption pits on dentine slices. Both compounds inhibited pit formation on the dentin slices in a dose-dependent manner; at 30 μM, pit formation was reduced by approximately 70–80% (Figs. 5a, b). Mature osteoclasts were also seeded onto 96-well plates and treated with veraguensin and galgravin for 24 h. As shown in Figs. 5c, d, TRAP activity and the number of osteoclasts were decreased by these compounds. These results suggest that the inhibitory effects veraguensin and galgravin on bone resorption by osteoclasts may be mediated by their suppressive effects on the survival of mature osteoclasts.
Fig. 5.
Effects of veraguensin and galgravin on the pit formation ability of osteoclasts. Mature osteoclasts were collected from co-cultures on a collagen gel matrix. a Mature osteoclasts were cultured on dentin slices in the presence of veraguensin and galgravin (3, 10 and 30 μM). After 24 h, cells were removed and the dentin slices were stained with hematoxylin to identify resorption pits under a microscope. b Resorption activity was quantified by counting the number of resorption pits. Mature osteoclasts were placed in 96-wells in the presence of veraguensin and galgravin for 24 h. c After 24 h, TRAP activity was examined. d TRAP-positive mature osteoclasts were counted under a microscope. *p < 0.05, **p < 0.005. Bar = 200 μm
Discussion
Traditional Chinese herbs are good sources for screening for new drug candidates (Lam 2007; Harvey 2008). In our screening program, we found that veraguensin and galgravin, which were isolated from the traditional Chinese herb Shin-i, were capable of suppressing osteoclastogenesis. Veraguensin and its stereoisomer galgravin are lignan compounds that exhibit various pharmacological effects in vitro and in vivo, including anti-inflammatory (da Silva Filho et al. 2004) and anti-malarial (da Silva Filho et al. 2008) activities. However, the effects of veraguensin and galgravin on osteoclastic bone resorption have not previously been investigated. Therefore, in the present study, we examined the effects of veraguensin and galgravin on osteoclast differentiation, function and survival, and determined the possible mechanisms of action. In the first part of our study, we found that veraguensin and galgravin inhibited osteoclast differentiation and function.
Osteoblasts produce RANKL and its decoy receptor OPG, which together regulate differentiation, function and survival of osteoclasts. Although veraguensin and galgravin inhibited osteoclast differentiation in co-cultures, they did not affect the expression of RANKL or OPG. In fact, veraguensin and galgravin inhibited osteoclast differentiation induced by RANKL in both cell types tested (BMM and RAW264.7 cells). Thus, veraguensin and galgravin inhibit osteoclast progenitor cells, not osteoblastic cells. Veraguensin and galgravin inhibited osteoclastic pit formation on bone slices as well as cell survival of mature osteoclasts; both effects occurred at the same concentrations. The inhibitory effects of veraguensin and galgravin on osteoclast function may be due to their inhibitory effects on the survival of mature osteoclasts. These results suggest that veraguensin and galgravin suppress bone resorption by inhibiting osteoclast differentiation and survival of mature osteoclasts.
RANKL binds to its receptor RANK on the surface of osteoclast precursor cells and activates several signaling pathways, which are crucial for the differentiation, function and survival of mature osteoclasts (Lacey et al. 1998). Stimulation of RANKL has been reported to activate three MAPKs: extracellular signal regulating kinase (ERK), JNK and p38. In our study, veraguensin and galgravin specifically suppressed p38 activation, without affecting the degradation of IκB, an inhibitor of NF-κB, and phosphorylation of JNK. The pyridinyl imidazole SB203580, a specific inhibitor of p38 MAPK, was reported to inhibit osteoclast differentiation (Li et al. 2002). Recent studies have also shown that compounds with a lignan structure such as honokiol (Hasegawa et al. 2010) or saucerneol (Kim et al. 2010) limit MAPK phosphorylation in osteoclasts and inhibit osteoclast differentiation. MAPKs are important for the induction of c-Fos and NFATc1 during osteoclast differentiation. Veraguensin and galgravin also blocked the expression of c-Fos, another molecule that plays a critical and essential role in osteoclast differentiation.
Taken together, veraguensin and galgravin inhibit osteoclast differentiation by suppressing RANKL-induced p38 phosphorylation and c-Fos expression. Although RANKL supports the survival of mature osteoclasts, it failed to induce phosphorylation of p38 MAPK (Li et al. 2002). Thus, the inhibitory effects of veraguensin and galgravin on p38 phosphorylation may not be involved in their effects on the survival of mature osteoclasts in the presence of osteoblastic cells. Osteoblasts also produce M-CSF, which supports the survival of mature osteoclasts. To reduce the survival of mature osteoclasts, veraguensin and galgravin may act on signaling pathways independent of p38 phosphorylation or M-CSF. Further studies are necessary to determine the mechanism(s) involved in their anti-survival effects.
Acknowledgments
This study was performed at a laboratory supported by an endowment from ERINA Co., Inc.
Footnotes
Midori Asai and Ji-Won Lee contributed equally to this work.
References
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Chen ZN, Yu PZ, Xu PJ (1993) Anti-platelet activating factor constituents, 2,5-diaryltetrahydrofuran type lignans, from Piper futokadsura Sied. et Zucc. Zhongguo Zhong Yao Za Zhi 18:292–294, 318 [PubMed]
- da Silva Filho AA, Andrade e Silva ML, Carvalho JC, Bastos JK. Evaluation of analgesic and anti-inflammatory activities of Nectandra megapotamica (Lauraceae) in mice and rats. J Pharm Pharmacol. 2004;56:1179–1184. doi: 10.1211/0022357044058. [DOI] [PubMed] [Google Scholar]
- da Silva Filho AA, Costa ES, Cunha WR, e Silva ML, Nanayakkara NP, Bastos JK (2008) In vitro antileishmanial and antimalarial activities of tetrahydrofuran lignans isolated from Nectandra megapotamica (Lauraceae). Phytother Res 22:1307–1310 [DOI] [PubMed]
- Feng X. Regulatory roles and molecular signaling of TNF family members in osteoclasts. Gene. 2005;25:1–13. doi: 10.1016/j.gene.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Harvey AL. Natural products in drug discovery. Drug Discov Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Yonezawa T, Ahn JY, Cha BY, Teruya T, Takami M, Yagasaki K, Nagai K, Woo JT (2010) Honokiol inhibits osteoclast differentiation and function in vitro. Biol Pharm Bull 33:487–492 [DOI] [PubMed]
- Kim SN, Kim MH, Kim YS, Ryu SY, Min YK, Kim SH (2010) Inhibitory effect of (-)-saucerneol on osteoclast differentiation and bone pit formation. Phytother Res 23:185–191 [DOI] [PubMed]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, HSU H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176 [DOI] [PubMed]
- Lam KS. New aspects of natural products in drug discovery. Trends Microbiol. 2007;15:279–289. doi: 10.1016/j.tim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, Suda T, Takahashi N (2002) p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology 143:3105–3113 [DOI] [PubMed]
- Lim H, Son KH, Bae KH, Hung TM, Kim YS, Kim HP (2009) 5-lipoxygenase-inhibitory constituents from Schizandra fructus and Magnolia flos. Phytother Res 23:1489–1492 [DOI] [PubMed]
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- Suda T, Nakamura I, Jimi E, Takahashi N (1997) Regulation of osteoclast function. J Bone Miner Res 12:869–879 [DOI] [PubMed]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357 [DOI] [PubMed]
- Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999;24:449–455. doi: 10.1006/bbrc.1999.0252. [DOI] [PubMed] [Google Scholar]