Abstract
Antibody-dependent cellular cytotoxicity (ADCC) is dependent on the fucose content of oligosaccharides bound to monoclonal antibodies (MAbs). As MAbs with a low fucose content exhibit high ADCC activity, it is important to control the defucosylation levels (deFuc%) of MAbs and to analyze the factors that affect deFuc%. In this study, we observed that the deFuc% was inversely related to culture medium osmolality for MAbs produced in the rat hybridoma cell line YB2/0, with r2 values as high as 0.92. Moreover, deFuc% exhibited the same correlation irrespective of the type of compound used for regulating osmolality (NaCl, KCl, fucose, fructose, creatine, or mannitol) at a culture scale ranging from 1 to 400 L. We succeeded in controlling MAb deFuc% by maintaining a constant medium osmolality in both perfusion and fed-batch cultures. In agreement with these observations, reverse transcription PCR analyses revealed decreased transcription of genes involved in glycolysis, GDP-fucose supply, and fucose transfer under hypoosmotic conditions.
Keywords: Antibody, Antibody-dependent cellular cytotoxicity, Cell culture, Fucosylation, Glucose, Glycosylation, Hybridoma, Osmolality, YB2/0 cells
Introduction
The contribution of biopharmaceutical industries to general healthcare is rapidly increasing, with over 165 such industries having been approved globally since 1982 (Walsh 2006). Within the therapeutic applications of biopharmaceuticals, monoclonal antibodies (MAbs) are of growing interest (Burnouf 2011; Grillberger et al. 2009; Mori et al. 2007), and are generally administered as active proteins in large amounts and at high protein concentrations, unlike other aqueous proteinaceous biopharmaceuticals such as cytokines.
As protein molecules tend to aggregate at high concentrations, extreme care must be taken during the manufacture of MAb solutions to prevent this from happening, as it could lead to fouling of aseptic filters during manufacture or immunogenic reactions in patients (Ishikawa et al. 2010b). Although MAbs have low side effects compared with other pharmaceuticals because of their high antigen specificities, minimization of their immunogenicity is nevertheless still important (Ishikawa et al. 2010a). Thus, enhancement of MAb activities is desirable in order to reduce the dose requirement. This will not only widely shift manufacturing and supply systems by reducing the amount and solution concentration of MAb, but will also lower MAb immunogenicity.
Antibody efficacies critically depend on the structure of the oligosaccharides attached to the polypeptide as well as the amino acid sequences of the heavy and light chains. IgG1 has two N-linked oligosaccharide chains bound to the Fc region. The oligosaccharides are of the complex biantennary type, composed of a trimannosyl core structure with or without core fucose, bisecting N-acetylglucosamine (GlcNAc), galactose, and terminal sialic acid, all of which give rise to structural heterogeneities. These heterogeneities make comparability control difficult because of differences in pharmacokinetics, pharmacodynamics, and immunogenicity (Putnam et al. 2010). Antibody-dependent cellular cytotoxicity (ADCC) is triggered upon binding of the antibody Fc region to lymphocyte receptors (FcγRs), and is dependent on the content of the fucose attached to the innermost GlcNAc of the N-linked Fc oligosaccharide, so is dramatically enhanced by a reduction in the fucose content (Mori et al. 2007; Shields et al. 2002; Shinkawa et al. 2003).
Non-fucosylated therapeutic antibodies show 50–1,000-fold higher efficacy than their fucosylated counterparts. It has been demonstrated that the MAbs produced in a rat myeloma cell line (YB2/0) exhibit higher ADCC activity than MAbs produced in Chinese hamster ovary (CHO) or NS0 myeloma cells (Kanda et al. 2006). This is because YB2/0 cells produce MAbs with a lower fucose content than those by CHO or NS0 cells. Although YB2/0 appears to be a good candidate for the large-scale production of high activity MAbs, there have been limited reports about the bio-process development in these cells to be employed with confidence (Arathoon and Birch 1986; Keen 1995; Konno et al. 2011; Rhodes and Birch 1988; Teylaert et al. 2011). In addition, we have observed that the defucosylation levels (deFuc%) of oligosaccharides in MAbs produced by YB2/0 vary according to cell culture conditions (unpublished observations).
In this article, we show that osmolality is one of the critical process parameters responsible for controlling the deFuc% and, eventually, the ADCC activity. We also discuss the molecular bases of this phenomenon.
Materials and methods
Cell culture
Rat hybridoma YB2/0 cells (ATCC CRL-1662, Manassas, VA) were used as the host cell line (Shitara et al. 1994). A YB2/0 cell line expressing proprietary recombinant mouse/human chimeric IgG1 antibodies A and B was cultured in suspension in Hybridoma SFM or CD-Hybridoma medium (Life Technologies, Carlsbad, CA) with appropriate supplements in a 250-mL Erlenmeyer flask (Corning, Corning, NY) or various sized bioreactors: 1 L with flat paddle (ABLE, Tokyo, Japan); 30 L with Ruston (Bioengineering AG, Wald, Switzerland); and 5 and 400 L with pitched paddle blade (Marubishi Bioeng., Tokyo, Japan). The medium was provided in powder form and osmolality was adjusted via the amount of powder used.
Cells were inoculated at a density of at least 2 × 105 cells/mL and cultured at 37 °C with occasional feeding with amino acids and vitamins from serum-free Iscove’s Modified Dulbecco’s Medium until the decline phase. In 1-L bioreactor cultures, the culture was maintained at pH 7.1 by a CO2 gas overlay and 1 mM Na2CO3 alkaline solution at 50% dissolved oxygen by sparger O2 gas. The perfusion system equipped with the Sorvall Centritech™ Lab II system (Thermo, Waltham, MA) arbitrarily controlled the medium osmolality at 4-day intervals in a stepwise fashion. The perfusion rate was set at 1 volume of fresh medium per working volume of reactor per day (vvd). For comparison, the anchorage-dependent SP2/0 (ATCC, CRL-1581) and NS0 cell lines (Riken, RCB0213, Ibaraki, Japan) expressing recombinant mouse/human chimeric IgG1 antibody A were cultured in cell culture bags (Medtronic, Minneapolis, MN) and 225 cm2 T-flasks (AGC, Tokyo, Japan).
Cell culture monitoring
The number of viable and dead cells was determined using a CEDEX™ counter (Innovatis AG, Bielefeld, Germany) by the trypan blue dye exclusion method. Off-line measurements of culture glucose and lactate were carried out using a YSI 2700 bioanalyzer (Yellow Springs Inc., Yellow Springs, OH). The concentration of MAbs in the culture supernatant was determined by high performance liquid chromatography (HPLC) using a Protein A column. Medium osmolality was measured by a Vogel osmometer OSMOMAT 030-D (Vogel, Giessen, Germany) using freezing-point depression. Fucosylation levels were measured as the monosaccharide composition of each purified MAb, as previously described (Shinkawa et al. 2003). Total cellular RNA was isolated as described previously (Shinkawa et al. 2003) and reverse transcription polymerase chain reaction (RT-PCR) analyses were carried out as previously reported for the α-1,6-fucosyltransferase gene (FUT8) and GDP-mannose 4,6-dehydratase gene (GMD) (Kanda et al. 2006) and GDP-fucose metabolic pathway gene expression (Yoshisue et al. 2002). Table 1 lists the specific primers used.
Table 1.
Oligonucleotide primers for RT-PCR analysis
| Gene symbol | Gene name | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|---|
| ACTB* | Actin, beta | ACAGCTGAGAAGGGAAATCGTG | TCCACACAGAGTACTTGCGCTC |
| HMBS* | Hydroxymethylbilane synthase | TGGAGTCTAGATGGCTCAGATAGC | CCACAAACTACTGAGGGAAAGG |
| Gck | Glucokinase | CAGAAAATGGCGGAAAATACTC | GAGATGATTCCTGCTTGAATAGTG |
| Hk1 | Hexokinase 1 | TCTAAACTCTGGGAAACAAAGG | AAGTTTGTAGAGCGTCCCATC |
| Hk2 | Hexokinase 2 | GGACTCTGTATAAGCTTCATCCTC | GTACAGGAAGTAGAGTGGGGG |
| Hk3 | Hexokinase 3 | AAGTTAAAGTATCTGGCCTTCTCC | GCAGATTTTCTTCTCCACATTC |
| Fpgt | Fucose-1-phosphate guanylyltransferase | TCAAGAGCTAGGCTTACAGTCC | GCTCAGGTTCCTCTTACTTCC |
| Fuca | Fucosidase, alpha-l- 1, tissue | AGTCTGGGAGGCAACTATCTTC | TGTCAGCTTTAGAGTCCAGGC |
| Fuk | Similar to l-fucose kinase | CTCTGGTCTTGGCACTAGC | GCTTCAGCACACTTCTCAGTC |
| Mpi | Mannose phosphate isomerase | AATTCATTGATGTGTCAACCC | AGATTGCACAATAGGGACAGG |
| GMD | Similar to GDP-mannose 4,6-dehydratase | TTTGTCATAGCTACTGGGGAAG | TAGATGCAGGGACAACACAG |
| Pmm1 | Phosphomannomutase 1 | AATGACTTTGAGATCTATGCGG | CTCCAGAGTATAAGTCCCATGC |
| Pmm2 | Phosphomannomutase 2 | GGAGTGGTAGGTGGGTCAG | CCTATGGAGAACGTGAGGC |
| Fut1 | Fucosyltransferase 1 | CCCCAGAGAAACTTCAAAGAC | TATACCTGATGTCAGCCAAATG |
| Fut8 | Fucosyltransferase 8 | TTTAGACCTGTAAGTGAGACATGC | GTGCGAGAAGCTGAAAATG |
* β-actin and hydroxymethylbilane synthase reference genes selected to calculate normalization factor with geNorm normalization (Vandesompele et al. 2002) from 10 genes
Results and discussion
Effects of initial culture medium osmolality on deFuc%
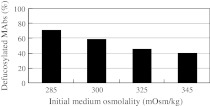
Osmolality is an important parameter in the mammalian cell culture process. The reported effects of osmolality on production yield, glycosylation, site occupancy, and sialylation are summarized in Table 2. At the beginning of YB2/0 cell culture, the MAb deFuc% was increased with the dilution (which lowers the osmolality) of basal medium (Fig. 1). For the fed-batch culture in a 5-L bioreactor, various osmolalities of the initial basal media were obtained by dilution with distilled water (285, 300, 325, and 345 mOsm/kg). The deFuc% of MAbs was found to be inversely related to the osmolality (70% at 285 mOsm/kg vs. 40% at 345 mOsm/kg). Although protein production and specific MAb production rate (SPR) are known to be affected by culture medium osmolality (Lee and Lee 2001; Park and Lee 1995; Zhang et al. 2010), no significant changes were observed in the amount of MAb produced under the conditions employed in the present study. To our knowledge, osmolality has not been previously been used for controlling glycosylation patterns. In fact, Kimura and Miller reported that cell culture osmolality did not significantly affect the monosaccharide content of the tissue plasminogen activator expressed in CHO cells (Kimura and Miller 1997). It is unclear whether the difference in the observed effects of osmolality can be ascribed to differences in cells used or proteins analyzed.
Table 2.
Reported effects of medium osmolality in animal cell culture
| Effect | Range (mOsm/kg) | Product | Cell line | Note | References |
|---|---|---|---|---|---|
| Productivity enhancer of mouse hybridoma | 350 or 400 | Antibody (Ab) | Hybridoma | Increased RNA and protein contents of high-salt cultures. Overall lg levels can be increased by 2.3 times by combining osmotic pressure and butyrate treatment | (Oh et al. 1993) |
| 285–455 | IgG2b anti-idiotype anti Ab | Hybridoma (S3H5/γ2bA2) | The specific Ab production rate was increased by 55% after abrupt increase in culture osmolality from 286 mOsm/kg to 398 mOsm/kg | (Park and Lee 1995) | |
| Productivity enhancer of CHO | 310 | Tissue plasminogen activator (tPA) | rCHO (MT2-1-8) | No changes were observed in the total sialic acid content | (Kimura and Miller 1997) |
| 300, 250, 200, or 150 | Chimeric Ab | rCHO (CS13*-1.0) | When the media osmolality was decreased from 300 mOsm/kg to 150 mOsm/kg, μ was decreased by 68% and qAb was increased by 128% | (Lee and Lee 2001) | |
| 392, 469, 542, or 620 | Chimeric Ab | rCHO with different cloned gene dosage (CS13*-0.02, CS13*-0.08, CS13*-0.32, and CS13*-1.00) | The cell line with a lower cloned gene dosage displayed more significant enhancement in qAb and less reduction in μ at hyperosmolality. However, the cell line with a higher cloned gene dosage still yielded a higher maximum Ab concentration at hyperosmolality up to 469 mOsm/kg | (Ryu et al. 2001) | |
| 300–500 | tPA | rCHO [l-15500 (ATCC CRL-9606)] | The osmotic pressure was shifted up or down during growth and production phases. The μ was higher at a lower osmotic pressure after the shift. The specific tPA production rate was increased by the shift irrespective of the direction (up or down), and cyclical changes in osmolality improved the production 1.13-fold compared with that attained under constant osmotic pressure | (Takagi et al. 2001) | |
| 294–522 | Humanized Ab against the S surface antigen of hepatitis B virus | rCHO (SH2-0.32) | Ab yields increased to 161% | (Kim et al. 2002) | |
| 490 | Anti-Rhesus D IgG | CHO DG44 | Addition of 90 mM NaCl to the medium resulted in qAb up to 4-fold, volumetric yields 50 mg /L in a 6 day batch culture of 3 L, reduced cell growth and increased cell size | (Zhang et al. 2010) | |
| Improvement of culture longevity | 223–540 | IgG2b anti-idiotype anti Ab | Hybridoma (S3H5/γ2bA2, DB9G8) | 5-fold increase in final Ab concentration | (Ryu and Lee 1999) |
| Hypoosmotic stress | 168–329 | IgG2b anti-idiotype Ab against a carcinogen-induced B cell tumor (38C13) | Hybridoma (S3H5/γ2bA2, DB9G8) | The 2 cell lines (S3H5/γ2bA2 and DB9G8) responded similarly to osmotic pressures. The cell growth and Ab production at 276 mOsm/kg were comparable to those at 329 mOsm/kg (standard DMEM). Both cell lines grew well at 219 mOsm/kg, though their growth and Ab production were slightly decreased. Cell growth did not occur at 168 mOsm/kg | (Soo Ryu and Min Lee 1997) |
| 285–425 | Chimeric Ab | Transfectoma (KR12H-2) | Enhanced qAb at higher osmolality appears to be mainly due to enhanced transcription levels of Ig mRNA. | (Lee and Lee 2000) | |
| Increased cell size also observed | 290, 338, 386, 435, or 580 | IgG1 | Mouse hybridoma (167.465.3) | Under conditions of high osmolality (435 mOsm/kg), cells were substantially larger than control (290 mOsm/kg). At 580 mOsm/kg, cells did not grow | (Ozturk and Palsson 1991) |
| 300–520 | IgG1 | Mouse hybridoma (6H11) | A 40% increase in hybridoma cell volume was observed when the growth media osmolality was increased from 300 to 520 mOsm/kg | (Øyaas et al. 1994) | |
| Intracellular level in osmolyte | 273 or 600 | – | Mouse L-929 cells | The media was made hyperosmotic (600 mOsm/kg) by the addition of NaCl, sorbitol or proline by successively adjusting intracellular levels of different osmolytes: Na+, K+, amino acids and sorbitol | (Libioulle et al. 2001) |
| Omics analysis | 300 or 450 | Chimeric antibody directed Against hepatitis B virus |
rCHO DG44 (CS13*-1.00) | The glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase and pyruvate kinase were found to be up-regulated | (Lee et al. 2003) |
| 290 or 390 | IgG2a directed against CD3 | Mouse hybridoma [OKT3 (ATCC number CRL-8001)] | Within the 215 characterized, differentially expressed genes, many are involved in metabolism/catabolism (induced vs. repressed: 19 vs. 12), cell-cycle regulation (10 vs. 5) and apoptosis (8 vs. 2), regulation of transcription (18 vs. 13) and translation (2 vs. 2), transport and signaling pathways (24 vs.12). Surprisingly, there were very few changes within stress-response genes | (Shen and Sharfstein 2006) | |
| 350–650 | Anti-Rhesus D IgG | CHO (B0) | A set of osmotically regulated genes was generated, and compared them with the ones previously obtained for mouse-hybridoma OKT3 cells (Shen and Sharfstein 2006). The overall transcriptomic responses of the two cell lines differed, although many functional groups (e.g., transcriptional regulator and metabolism) were commonly perturbed | (Shen et al. 2010) | |
| 206 or 498 | – | ES cell (CGR8) | Hypothesis that protein alterations due to osmotic stress contribute to the pathology of neurodegenerative diseases, due to the observed 60% expression overlap with proteins found altered in Alzheimer’s, Huntington’s, or Parkinson’s disease | (Mao et al. 2008) | |
| 290 or 450 | Chimeric B72.3 IgG4 | GS-NS0 | Gene expression analysis by DNA microarray | (Wu et al. 2004) | |
| Metabolism | 280–370 | MAb | NS0 | Metabolic analysis suggested that cells cultured in hypoosmolality demand a higher energy supply for normal maintenance, but in such conditions the lower glutamine metabolic rate was insufficient to support Ab synthesis | (Zhao et al. 2009) |
| Apoptosis | 315–610 with 90 mM-NaCl | EPO, MAb | rCHO | The induction of programmed cell death by hyperosmotic stress occurred independently of nutrient depletion | (Han et al. 2010) |
| Aggregation | 300, 340, 367, or 395 | Anti PSA MAb | Hybridoma | Different values of pH and osmolality were tested: 7.1, 7.5, 8.0, 8.5 for pH, and 300, 340, 367, 395 mOsm/kg H2O for osmolality. By modification of the cell culture medium, a significant decrease of MAb aggregates was obtained | (Franco et al. 1999) |
| 360, 390, 430, 470, or 510 | IFN-β | rCHO | Both hyperosmolality (470 mOsm/kg) and hypothermia (32 °C) increased specific native INF-β productivity. Furthermore, IFN-β molecular aggregation was decreased, although severe IFN-β molecular aggregation could not be avoided in the later phase of culture | (Han et al. 2009) | |
| Cell cycle | From 290 up to 400 | IgG1 directed against phosphorylcholine | Hybridoma [167.4G5.3 (a mouse- mouse hybridoma with plasmacytoma parent P3X63-Ag8.653)] | Intracellular Ab pool sizes increased as cells progressed through the cell cycle for both control and hyperosmotic cultures | (McNeeley et al. 2005) |
| From 290 up to 400 | IgG1 directed against phosphorylcholine | Hybridoma [167.4G5.3 (a mouse- mouse hybridoma with plasmacytoma parent P3X63-Ag8.653)] | Cell size and intracellular Ab pool increased. Hyperosmotic stress altered the cell cycle distribution, increasing the fraction of cells in S-phase | (Sun et al., 2004) | |
| Osmolyte | 335–500 | Moloney mouse leukaemia virus (MoMLV)-derived retroviral vectors | Fly A7, HCT-116 (ATCC CCL-247) | From the conditions tested, sorbitol addition, ensuring osmolalities between 410–450 mOsm/kg, yields the best production conditions; NaCl hampered viral infectious production, while fructose leads to lower cell yields | (Coroadinha et al. 2006) |
| Osmoprotective compounds (osmopurotectant) | 300–520 | Ab | Mouse hybridoma (6H11) | Osmoprotectant: glycine betaine, dimethylglycine, and sarcosine | (Øyaas et al. 1994) |
| 292–573 | hTPO | rCHO (dhfr-B22-4, CS13-0.02*, and CS13-1.00*) | Inclusion of 15 mM glycine betaine in hyperosmolar medium enabled rCHO to grow at 557–573 mOsm/kg; cells could not grow in the absence of glycine | (Ryu et al. 2000) | |
| 335, 350, 400, 480, or 570 | tPA | rCHO (MT2-1-8) | Glycine betaine and l-Proline (10, 20, and 30 mM) partially mitigate the decrease in neural cell adhesion molecule poly sialylation at an osmolality above 435 mOsm/kg | (Schmelzer and Miller 2002a) | |
| Glycosylation and pCO2 | 310–376 | tPA | rCHO (MT2-1-8) | No significant changes were observed in the total sialic acid content. | (Kimura and Miller 1996, Kimura 1997) |
| 337–469 | IgG2a MAb against benzene-arsonate | Hybridoma (AB2-143.2, Sp2/0-derived mouse–mouse hybridoma) | Hybridoma cells exposed to elevated pCO2 in well-plates experienced both growth inhibition and enhanced cell death, with greater detrimental effects without osmolality compensation | (deZengotita et al. 1998) | |
| 105, 76.8, or 52.6 mM NaCl controlled at 320 ± 5 | tPA | CHO (MT2-1-8) | By increasing (NaCl), hyperosmolality alone decreased cell surface poly-sialic acid content, but to a lesser extent than for the same osmolality increase due to elevated (NaHCO3) | (Zanghi et al. 1999) | |
| 320, 375, 435, or 476 | IgG2a MAb | Hybridoma (AB2-143.2, Sp2/0-derived mouse–mouse hybridoma) | Elevated pCO2 (with or without osmolality compensation) inhibited glycolysis. Osmolality had little effect on glycolysis. On the other hand, elevated pCO2 alone had no effect on glutamine metabolism, whereas elevated osmolality increased glutamine uptake. Hybridoma mean pHi was about 0.2 pH units lower than control at 140 mmHg pCO2 (with or without osmolality compensation), but further increases in pCO2 did not further decrease pHi. Osmolality had little effect on pHi | (deZengotita et al. 2002) | |
| 320, 375, 435, or 475 | IgG2a MAb | Hybridoma (AB2-143.2, Sp2/0-derived mouse–mouse hybridoma) | IgG2a site occupancy did not change significantly under any condition, which is consistent with the robust glycosylation of other Abs produced under various environmental stresses. Changes in the isoelectric point were greater under hyperosmotic stress from 7.39 to 7.71 at 435 mOsm/kg | (Schmelzer and Miller 2002b) | |
| 303,313,347, or 336 | Recombinant fusion glycoprotein B1 | rCHO DG44 | Osmolality and pCO2 levels were eliminated as the causes for the change from N-acetylneuraminic acid (Neu5Ac) to N-glycolylneuraminic acid (Neu5Gc) forms of sialic acid levels, as they remained unchanged between the conditions | (Borys et al. 2010) | |
| Optimized cell growth | 40–250 mM NaCl | – | Hela | Optimal NaCl concentration was 100–300 mM for growth and DNA synthesis | (Stubblefield and Mueller 1960) |
Fig. 1.
Dependence of MAb defucosylation levels (deFuc%) produced by YB2/0 cells on initial medium osmolality. The medium was diluted with distilled water to attain the indicated osmolality at the beginning of the culture. Fed-batch method was used in a 5-L bioreactor. Independent experiments under similar conditions exhibited similar results in 1-L bioreactors
Effect of dilution at the mid-phase of fed-batch culture on deFuc%
The inverse relationship between deFuc% and medium osmolality was also observed following dilution (with distilled water) at the mid-phase of YB2/0 cell culture. Osmolality is influenced by various growth environments, including the addition of alkaline solutions (for pH adjustment) and feed solutions (containing glucose and amino acids), as well as by the accumulation of by-products. For fed-batch culture in a 5-L bioreactor, the osmolality of the initial basal medium was set at 290 and 340 mOsm/kg by dilution with distilled water. To mimic changes in osmolality, the 340 mOsm/kg culture was diluted on day 6 with distilled water to reduce its osmolality to 290 mOsm/kg. As a result, deFuc% in MAb glycosylation was increased by 20% and became comparable with that observed in the sample cultured at 290 mOsm/kg throughout. This indicates that the deFuc% depends on the osmolality of the culture medium when the MAbs are produced, but not on the osmolality of the initial culture medium. These data also suggest that the deFuc% can be controlled by varying medium osmolality even during culture periods, irrespective of initial culture conditions.
deFuc% control has previously been obtained by cell line engineering, including FUT8 knock-out (Yamane-Ohnuki et al. 2004) and GMD knock-out (Kanda et al. 2007) of host cell lines, expression of small interfering RNA (siRNA) against FUT8 (Mori et al. 2004) or GDP-fucose transporter gene (GFT) (Omasa et al. 2008), and use of the glycosidase inhibitor Kifunensine (van Berkel et al. 2010; Zhou et al. 2008). The practical control of MAb deFuc% in process development areas, however, has not been reported thus far.
Effects of different cell lines on deFuc%
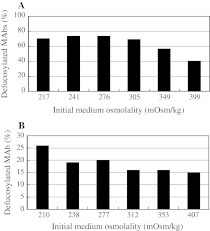
The inverse relationship between deFuc% and culture osmolality was also observed in other cells that are used to produce MAbs, in addition to YB2/0 cells. The number of cell lines that can be employed for the production of MAbs for the market is limited, and includes CHO or mouse myeloma cell lines (NS0 or SP2/0). Since deFuc% in MAb glycosylation must be regulated for ADCC quality control, it is important to understand the effects of culture conditions on deFuc% in each cell line. Thus, we evaluated the effects of osmolality in NS0 and SP2/0 cell lines (Fig. 2). Although the detailed osmolality dependence of deFuc% was somewhat different among the three cell lines studied, we observed a general inverse relationship between deFuc% and osmolality of the culture medium in NS0 and SP2/0 cells as well as in YB2/0 cells.
Fig. 2.
Dependence of defucosylation levels (deFuc%) of IgG1 MAbs produced from NS0 (a) and SP2/0 (b) cells on initial medium osmolality. Two independent experiments under similar conditions exhibited similar results
ADCC activity is greatly dependent on the deFuc% of MAb oligosaccharides, so appropriate selection of host cells is particularly relevant when the ADCC is the intended activity of MAb. This is because different host cell lines exhibit distinct glycosylation patterns (especially fucosylation), and MAbs produced in a particular host will demonstrate a specific ADCC activity (Shields et al. 2002; Shinkawa et al. 2003; Wurm 2004). In the purpose of adopting a quality by design (QbD) policy (FDA 2009), a reasonable choice would be to use the engineered host cell line for robust control, such as the development of a CHO host cell line with FUT8 knock-out (Yamane-Ohnuki et al. 2004). Such a strategy is, however, not always possible in actual development. Biopharmaceutical developments take about 12 years from discovery through registration, and are often carried out in different organizations and in multiple firms depending on the development stage (Steinmeyer and McCormick 2008; Werner 2004). Thus, it is not always practical to change the host cells at a later stage. Not only will clinical data have been accumulated regarding the engineered hosts, but such a change involves altering cell banks and controlling comparability. A method to control deFuc% by culture conditions, irrespective of cell line, would be of great benefit for biopharmaceutical production in which many cell lines are used as hosts: mouse myeloma line NS0, SP2/0, hamster CHO, BHK, dog MDCK, human HEK293, MRC-5, and HT-1080.
Controlling deFuc% in perfusion culture
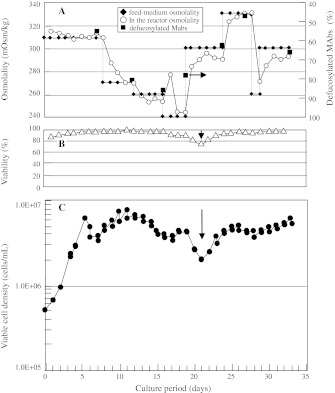
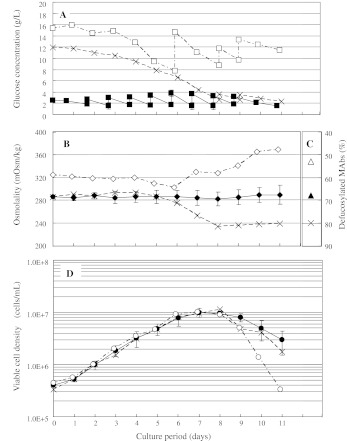
The deFuc% was controlled arbitrarily from 45 to 85% by feeding media with different osmolalities (260–330 mOsm/kg) into perfusion cultures (Fig. 3a). The deFuc% was found to be increased/decreased to intended levels (solid squares) when the reactor osmolality (open circles) reached the targeted value by introducing media of lower/higher osmolalities (filled diamonds). Although deFuc% could be controlled by regulating medium osmolality, cell viability was decreased at the very low osmolality of 240 mOsm/kg (Fig. 3b, c, arrows). Thus, it is important to keep in mind that the regulation of medium osmolality by dilution with distilled water has limitations regarding cell viability and unduly low concentrations of critical components.
Fig. 3.
Defucosylation levels (deFuc%) of MAbs produced by YB2/0 cells cultured under various osmolalities. Cells were cultured by a perfusion method that enabled arbitrary changes to be made to the medium osmolality. aOpen circles observed medium osmolality, closed diamonds osmolality of feeding medium, closed squares observed deFuc%. b Reactor cell viability (open triangles). c Reactor viable cell density (closed circles). Reproducibility is ensured because several conditions were adopted, although reproducibility is just one aspect of perfusion culture
Effects of compounds used to adjust medium osmolality on deFuc%
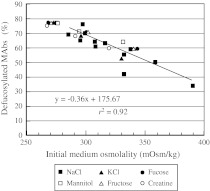
The deFuc% was inversely correlated with osmolality, irrespective of the compounds used to adjust osmolality (ionic vs. non-ionic or substrate vs. non-substrate) (Fig. 4). To determine whether the colligative value of osmolality simply affects the deFuc%, or whether the chemical/biochemical properties of the compound used to adjust osmolality influence the deFuc%, we added either an ionic compound (NaCl or KCl), a substrate (fucose, fructose, or creatine), or a non-substrate (mannitol) to the medium diluted with distilled water to 250 mOsm/kg (from 320 mOsm/kg). Cells were then cultured at 37 °C for 11 days in the 250 mL Erlenmeyer flasks. Surprisingly, the effects of different compounds obeyed the same correlation (with the r2 value being as high as 0.92, Fig. 4), and KCl exhibited the same effect as NaCl, even though the Na+/K+ balance is generally regarded as critical in cell culture. Although it was unexpected that fucose supplementation did not increase fucosylation levels (i.e., decrease the deFuc%), it is known that GDP-fucose, the substrate for fucosylation, is derived mainly (90%) from GDP-mannose (Becker and Lowe 2003). The conversion of free fucose (transported into the cytosol from extracellular medium or from lysosomes after catabolism of fucosylated glycans) to GDP-fucose is a minor salvage pathway, which explains why the addition of fucose resulted only in an increase in osmolality. SPR is also known to depend on medium osmolality. The extent of this effect, however, varies between osmolytes (Coroadinha et al. 2006), in contrast to the effect on deFuc%.
Fig. 4.
Relationship between defucosylation levels (deFuc%) of MAbs produced and medium osmolalities attained by supplementing with different compounds: closed squares NaCl, closed triangles KCl, closed circles fucose, open squares mannitol, open triangles fructose, open circles creatine
Although the QbD approach would be conceptually beneficial, its application to the actual control of cell culture processes is not always straightforward. One reason for this is that process parameters involved in culture are complex and inter-dependent, even though they are usually optimized by design of experiment (DOE)-based strategies (Abu-Absi et al. 2010; Horvath et al. 2010). Under these circumstances it would be advantageous to determine the critical parameter that directly affects the quality of the product, such as medium osmolality (on deFuc%) as demonstrated in this study.
Effect of different physical conditions on deFuc%
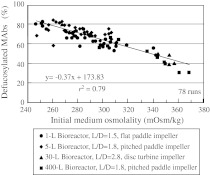
In industrial manufacturing, scale-up processes involve changes to various physical conditions such as aspect ratio, size of reactors, impeller type, and sparger pore-size. Moreover, the increased liquid volume per surface area involved in scale-up leads to accumulation of dissolved carbon dioxide (dCO2) (Matsunaga et al. 2009). This lowers the expression of MAbs in several cell types (deZengotita et al. 1998; Goudar et al. 2007; Zhu et al. 2005) due to altered biochemical conditions within the cells, which in turn alters the deFuc%. We analyzed the relationship between the deFuc% and medium osmolality for four types of culture scales (1, 5, 30, and 400 L). Cells were cultured at 37 °C for 11 or 12 days with various initial medium osmolalities in reactors of different sizes as well as with different blades and aspect ratios. The deFuc% was still found to be inversely correlated with osmolality irrespective of reactor size, with r2 values as high as 0.79 (Fig. 5). Thus, the deFuc% was not affected by different physical conditions other than osmolality in fed-batch cultures.
Fig. 5.
Relationship between defucosylation levels (deFuc%) and initial medium osmolalities analyzed under four culture scale conditions. MAbs were produced under 78 different physical conditions: four reactor sizes (1, 5, 30, and 400 L) each with different blades and aspect ratios (L/D), as well as different osmolalities
Previous reports on the bio-process development of mammalian cell culture discussed site-occupancy, antennary structure, and sialylation of oligosaccharides attached to proteins, and the major physical factors shown to affect these properties included ammonia, pH, dissolved oxygen, temperature, harvest-criteria (Butler 2006; Goochee et al. 1991; Hossler et al. 2009), and dCO2 (Kimura and Miller 1997; Oliveira et al. 2008; Zanghi et al. 1999). However, no reports have yet analyzed the effect of these physical conditions on deFuc%.
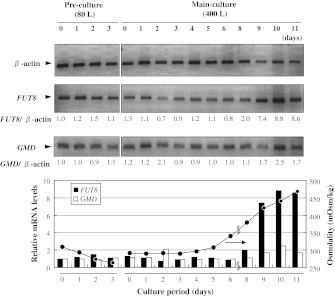
RT-PCR analysis of cultured YB2/0 cells in bioreactors
Semi-quantitative RT-PCR analysis was performed to elucidate changes in mRNA expression levels of FUT8 and GMD in YB2/0 cells during an 11-day fed-batch culture in 400-L reactors (Fig. 6). After day 6, the ratio of FUT8 versus β-actin (control) increased markedly, following the increase in medium osmolality from 300 to 450 mOsm/kg, up to approximately 8-fold (relative to day 0 of pre-culture) at the late-phase of culture; in response to this increase in the medium osmolality, the deFuc% decreased from 55 to 35%. On the other hand, the ratio of GMD versus β-actin exhibited only a small increase. These observations suggest that activities of enzymes involved in MAb fucosylation are correlated with medium osmolality. We therefore next analyzed the effects of medium osmolality (hypo- vs. hyperosmolality) on the gene expression of enzymes involved in fucose metabolism and glycolysis.
Fig. 6.
RT-PCR analysis of cultured YB2/0 cells in 80-L pre-culture and 400-L main fed-batch culture in bioreactors. FUT8 and GMD transcript amounts relative to β-actin, calculated from the fluorescence intensity of each band measured by FluoroImager SI, are shown below the RT-PCR panels. Two independent experiments under similar conditions exhibited similar results
Effects of medium osmolality on gene expression of GDP-fucose metabolism
Under hypoosmotic conditions, gene expression of enzymes involved in the utilization and synthesis of GDP-fucose and in glycolysis was found to be reduced in comparison with hyperosmotic conditions. We compared gene expression under hypoosmotic versus hyperosmotic conditions by microarray followed by RT-PCR analyses. cDNAs were prepared from mRNAs extracted from YB2/0 cells expressing IgG1 B that were sampled on days 6, 9, 11, and 12 during serum-free 1-L fed-batch culture in hypoosmotic (250 mOsm/kg) and hyperosmotic (410 mOsm/kg) media. Microarray analyses indicated that hypoosmotic conditions led to the down-regulation of glycolysis gene expression, particularly pyruvate kinase and aldolase (data not shown), which is in agreement with the proteomic and transcriptomic analyses of the effects of osmotic stress on CHO and hybridoma (Shen and Sharfstein 2006; Shen et al. 2010).
Next, we used semi-quantitative RT-PCR to evaluate gene expression under hypoosmotic versus hyperosmotic conditions and observed three marked responses (Table 3). First, under hypoosmotic conditions, the key enzymes of glycolysis, glucokinase, and hexokinase 1 were strongly down-regulated. The values did not vary significantly with the culture period because the relative (hypoosmotic vs. hyperosmotic) osmolality did not vary with time, in contrast to the case of Fig. 6. Although the glucose consumption and lactate production were lower in hypoosmotic compared with hyperosmotic cultures, there were no significant differences in growth or viabilities (data not shown). These observations suggest that specific glucose consumption and lactate production are decreased under hypoosmotic conditions. Second, expression of the gene group of the GDP-fucose supply pathway is decreased under hypoosmotic conditions. The synthesis of GDP-fucose in mammalian cells involves both the GDP-mannose-dependent de novo pathway and the free fucose-dependent salvage pathway. Quantitative studies of fucose metabolism in HeLa cells indicate that more than 90% of GDP-fucose is derived from the de novo pathway, even in cells fed with fucose (Becker and Lowe 2003). Expression of GMD in the de novo pathway (Table 3; Fig. 6) and l-fucose kinase in the salvage pathway (Table 3) was decreased under hypoosmotic conditions, suggesting that the supply of GDP-fucose is limited under hypoosmotic conditions. Third, the expression of FUT8, which is responsible for the fucosylation of MAb oligosaccharides and requires the substrate GDP-fucose, was markedly decreased under hypoosmotic conditions. These results suggest that the control of medium osmolality is critical for the production of MAbs with a constant deFuc%, and hence ADCC activity in these cell lines. The effects of osmolality on recombinant protein expression and glycosylation have been previously reported (Borys et al. 2010); however, no description was made regarding fucosylation.
Table 3.
Gene expression ratio (hypoosmotic vs. hyperosmotic conditions) during fed-batch culture as analyzed by RT-PCR
| Gene symbol | Culture periods (days) | 6 | 9 | 11 | 12 |
|---|---|---|---|---|---|
| Gene name | |||||
| Gck | Glucokinase | 0.14 | 0.11 | 0.15 | 0.13 |
| Hk1 | Hexokinase1 | 0.37 | 0.23 | 0.27 | 0.26 |
| Hk2 | Hexokinase2 | 0.88 | 0.72 | 0.94 | 0.44 |
| Hk3 | Hexokinase3 | 0.61 | 0.59 | 0.72 | 0.63 |
| Fpgt | Fucose-1-phosphate guanylyltransferase | 1.16 | 0.68 | 0.92 | 1.04 |
| Fuca | Fucosidase, alpha-L-1 | 0.94 | 0.59 | 0.50 | 0.74 |
| Fuk | L-fucose kinase | 0.51 | 0.33 | 0.32 | 0.44 |
| Mpi | Mannose phosphate isomerase | 0.74 | 0.56 | 0.55 | 1.01 |
| Pmm1 | Phosphomannomutase 1 | 0.83 | 0.45 | 0.77 | 0.33 |
| GMD | GDP-mannose 4,6-dehydratase | 1.05 | 0.74 | 0.78 | 0.89 |
| Fut1 | Fucosyltransferase 1 | 0.46 | 0.18 | 0.36 | 0.27 |
| Fut8 | Fucosyltransferase 8 | 0.29 | 0.04 | 0.18 | 0.12 |
Normalization factors geNorm were 0.91, 1.92, 2.08, and 2.08, for days 6, 9, 11, and 12, respectively
Controlling glycosylation patterns in fed-batch culture
Based on the findings obtained in this study, a new simple method was devised for the industrial-scale production of MAbs with high deFuc%. Although fed-batch is the most common industrial method for MAb production, it is difficult to control the reactor osmolality throughout the culture period because it is affected by many factors, including the addition of alkaline solutions for pH adjustment, accumulation of dCO2 (which affects the buffer property), consumption of nutrients, and accumulation of product and various byproducts. In addition, since the tank size limits the volume of feed solutions, dilution is not practical for industry-level production. Thus, a method of reducing osmolality without dilution would be extremely useful.
We employed custom-made medium containing a low concentration of sodium chloride. We varied and controlled the glucose concentration in order to regulate the osmolality, because glucose is a nutrient contained at a high initial concentration and is consumed and metabolized by cells. In the presence of a high glucose concentration, the osmolality decreased steadily throughout the culture period due to glucose consumption. It was appended on days 8 and 9 to sustain cell viability while achieving hypoosmotic conditions (Fig. 7a, b, cross symbols). An increase in osmolality (hyperosmotic conditions) could be achieved by supplementing with glucose from day 6 and on days 8 and 9 (Fig. 7a, b, open symbols). Constant medium osmolality could be attained by the addition of glucose on days 2–9 in addition to other nutrients (closed symbols). The observed deFuc% values of the MAbs harvested on day 11 were inversely correlated with osmolality, as expected (Fig. 7b, c). Moreover, the viable cell density observed under hypoosmotic conditions, under which the highest deFuc% was attained, was comparable to that observed under constant osmolality (Fig. 7d). These results suggest that osmolality can be regulated, such that MAb deFuc% can also be controlled arbitrarily in the fed-batch culture.
Fig. 7.
Defucosylation levels (deFuc%) of MAbs produced with different methods to regulate medium osmolality. Open symbols hyperosmotic, cross symbols hypoosmotic, closed symbols constant osmotic conditions. Cells were cultured in a 5-L fed-batch bioreactor with custom-made medium containing low NaCl. Osmolality was adjusted by supplementing with NaCl. a glucose concentration in the medium, b medium osmolality, c deFuc% MAbs harvested on day 11, and d viable cell density. Independent experiments under similar conditions exhibited similar initial results in 1-L bioreactors
Approximately 70% of therapeutic proteins and biologic candidates are glycoproteins (Sethuraman and Stadheim 2006). Variation in glycoforms can be seen in site-occupancy (macroheterogeneity) and in the structure of attached glycans (microheterogeneity) (Butler 2006). Glycosylation is a critical protein quality attribute that can modulate the efficacy of a commercial therapeutic glycoprotein (Hossler et al. 2009). One example for this is fucosylation, which affects bioactivities such as heparin binding of antithrombin (Garone et al. 1996) as well as ADCC of MAbs. Currently, it is impossible to control total enzymatic activities in cells by regulating culture conditions. The regulation of medium osmolality with glucose is, however, sufficient for achieving a desirable deFuc% necessary for efficacious ADCC in YB2/0 cell culture. The present method to control deFuc% by medium osmolality opens the way to using mammalian cells that are unsuitable for glycoprotein production because of unwantedly high and/or uncontrollable fucose content in the oligosaccharides attached to the protein.
Conclusion
In this study, we found that osmolality is a critical process parameter in the control of deFuc% for recombinant glycoprotein production in fed-batch and perfusion cultures of YB2/0 and other cell-lines. We showed that low medium osmolality directly results from the suppression of mRNA expression levels of various enzymes involved in glycolysis, GDP-fucose supply, and fucose transfer. Elucidation of these critical conditions is beneficial and complementary to the DOE strategy that comprehensively evaluates the effects of various parameters on the attributes.
Acknowledgments
We would like to thank Mr. Yuya Kawagoe, Mr. Kazutoshi Maki, Mr. Noriyuki Takahashi, and Ms. Chigusa Hijikata for their expert analyses. We also thank Dr. Mitsuo Sato, Dr. Jun Yamaya, Dr. Kazuhisa Uchida, and Mr. Hiroshi Takasugi for their helpful discussions and encouragement.
Glossary
- ADCC
Antibody-dependent cellular cytotoxicity
- CHO
Chinese hamster ovary
- dCO2
Dissolved carbon dioxide
- deFuc
Defucosylation levels, the extent with which the GlcNAc residue at the reducing terminal is free from glycosylation with fucose
- DOE
Design of experiment
- Fut8
Alpha-1,6-fucosyltransferase gene
- GMD
GDP-mannose 4,6-dehydratase gene
- GlcNAc
N-acetylglucosamine
- MAbs
Monoclonal antibodies
- QbD
Quality by design
- RT-PCR
Reverse transcription polymerase chain reaction
- SPR
Specific MAb production rate (pg cells−1 d−1)
- vvd
Volume of fresh medium per working volume of reactor per day
References
- Abu-Absi SF, Yang L, Thompson P, Jiang C, Kandula S, Schilling B, Shukla AA (2010) Defining process design space for monoclonal antibody cell culture. Biotechnol Bioeng 106:894–905 [DOI] [PubMed]
- Arathoon W, Birch J. Large-scale cell culture in biotechnology. Science. 1986;232:1390–1395. doi: 10.1126/science.2424083. [DOI] [PubMed] [Google Scholar]
- Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- Borys MC, Dalal NG, Abu-Absi NR, Khattak SF, Jing Y, Xing Z, Li ZJ (2010) Effects of culture conditions on N-glycolylneuraminic acid (Neu5Gc) content of a recombinant fusion protein produced in CHO cells. Biotechnol Bioeng 105:1048–1057 [DOI] [PubMed]
- Burnouf T. Recombinant plasma proteins. Vox Sang. 2011;100:68–83. doi: 10.1111/j.1423-0410.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- Butler M. Optimisation of the cellular metabolism of glycosylation for recombinant proteins produced by mammalian cell systems. Cytotechnology. 2006;50:57–76. doi: 10.1007/s10616-005-4537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroadinha AS, Silva AC, Pires E, Coelho A, Alves PM, Carrondo MJ (2006) Effect of osmotic pressure on the production of retroviral vectors: Enhancement in vector stability. Biotechnol Bioeng 94:322–329 [DOI] [PubMed]
- deZengotita V, Kimura R, Miller W. Effects of CO2 and osmolality on hybridoma cells: growth, metabolism and monoclonal antibody production. Cytotechnology. 1998;28:213–227. doi: 10.1023/A:1008010605287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deZengotita VM, Schmelzer AE, Miller WM. Characterization of hybridoma cell responses to elevated pCO2 and osmolality: intracellular pH, cell size, apoptosis, and metabolism. Biotechnol Bioeng. 2002;77:369–380. doi: 10.1002/bit.10176. [DOI] [PubMed] [Google Scholar]
- FDA (2009) Guidance for industry. Q8 (R2) pharmaceutical development
- Franco R, Daniela G, Fabrizio M, Ilaria G, Detlev H (1999) Influence of osmolarity and pH increase to achieve a reduction of monoclonal antibodies aggregates in a production process. Cytotechnology 29:11–25 [DOI] [PMC free article] [PubMed]
- Garone L, Edmunds T, Hanson E, Bernasconi R, Huntington JA, Meagher JL, Fan B, Gettins PG (1996) Antithrombin–heparin affinity reduced by fucosylation of carbohydrate at asparagine 155. Biochemistry 35:8881–8889 [DOI] [PubMed]
- Goochee CF, Gramer MJ, Andersen DC, Bahr JB, Rasmussen JR (1991) The oligosaccharides of glycoproteins: bioprocess factors affecting oligosaccharide structure and their effect on glycoprotein properties. Biotechnology 9:1347–1355 [DOI] [PubMed]
- Goudar CT, Matanguihan R, Long E, Cruz C, Zhang C, Piret JM, Konstantinov KB (2007) Decreased pCO2 accumulation by eliminating bicarbonate addition to high cell-density cultures. Biotechnol Bioeng 96:1107–1117 [DOI] [PubMed]
- Grillberger L, Kreil TR, Nasr S, Reiter M (2009) Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol J 4:186–201 [DOI] [PMC free article] [PubMed]
- Han YK, Koo TY, Lee GM. Enhanced interferon-β production by CHO cells through elevated osmolality and reduced culture temperature. Biotechnol Prog. 2009;25:1440–1447. doi: 10.1002/btpr.234. [DOI] [PubMed] [Google Scholar]
- Han YK, Kim YG, Kim JY, Lee GM (2010) Hyperosmotic stress induces autophagy and apoptosis in recombinant Chinese hamster ovary cell culture. Biotechnol Bioeng 105:1187–1192 [DOI] [PubMed]
- Horvath B, Mun M, Laird M. Characterization of a monoclonal antibody cell culture production process using a quality by design approach. Mol Biotechnol. 2010;45:203–206. doi: 10.1007/s12033-010-9267-4. [DOI] [PubMed] [Google Scholar]
- Hossler P, Khattak SF, Li ZJ. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology. 2009;19:936–949. doi: 10.1093/glycob/cwp079. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Ito T, Endo R, Nakagawa K, Sawa E, Wakamatsu K (2010a) Influence of pH on heat-induced aggregation and degradation of therapeutic monoclonal antibodies. Biol Pharm Bull 33:1413–1417 [DOI] [PubMed]
- Ishikawa T, Kobayashi N, Osawa C, Sawa E, Wakamatsu K (2010b) Prevention of stirring-induced microparticle formation in monoclonal antibody solutions. Biol Pharm Bull 33:1043–1046 [DOI] [PubMed]
- Kanda Y, Yamane-Ohnuki N, Sakai N, Yamano K, Nakano R, Inoue M, Misaka H, Iida S, Wakitani M, Konno Y, Yano K, Shitara K, Hosoi S, Satoh M (2006) Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng 94:680–688 [DOI] [PubMed]
- Kanda Y, Imai-Nishiya H, Kuni-Kamochi R, Mori K, Inoue M, Kitajima-Miyama K, Okazaki A, Iida S, Shitara K, Satoh M (2007) Establishment of a GDP-mannose 4, 6-dehydratase (GMD) knockout host cell line: a new strategy for generating completely non-fucosylated recombinant therapeutics. J Biotechnol 130:300–310 [DOI] [PubMed]
- Keen MJ. The culture of rat myeloma and rat hybridoma cells in a protein-free medium. Cytotechnology. 1995;17:193–202. doi: 10.1007/BF00749657. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim NS, Sung YH, Lee GM (2002) Biphasic culture strategy based on hyperosmotic pressure for improved humanized antibody production in Chinese hamster ovary cell culture. In Vitro Cell Dev Biol Anim 38:314–319 [DOI] [PubMed]
- Kimura R, Miller WM. Effects of elevated pCO2 and/or osmolality on the growth and recombinant tPA production of CHO cells. Biotechnol Bioeng. 1996;52:152–160. doi: 10.1002/(SICI)1097-0290(19961005)52:1<152::AID-BIT15>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kimura R, Miller WM. Glycosylation of CHO-derived recombinant tPA produced under elevated pCO2. Biotechnol Prog. 1997;13:311–317. doi: 10.1021/bp9700162. [DOI] [PubMed] [Google Scholar]
- Konno Y, Aoki M, Takagishi M, Sakai N, Koike M, Wakamatsu K, Hosoi S (2011) Enhancement of antibody production by the addition of Coenzyme-Q10. Cytotechnology 63:163–170 [DOI] [PMC free article] [PubMed]
- Lee MS, Lee GM. Hyperosmotic pressure enhances immunoglobulin transcription rates and secretion rates of KR12H–2 transfectoma. Biotechnol Bioeng. 2000;68:260–268. doi: 10.1002/(SICI)1097-0290(20000505)68:3<260::AID-BIT4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Lee M, Lee G. Effect of hypoosmotic pressure on cell growth and antibody production in recombinant Chinese hamster ovary cell culture. Cytotechnology. 2001;36:61–69. doi: 10.1023/A:1014032701800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kim KW, Kim YH, Lee GM (2003) Proteome analysis of antibody-expressing CHO cells in response to hyperosmotic pressure. Biotechnol Prog 19:1734–1741 [DOI] [PubMed]
- Libioulle C, Corbesier L, Gilles R. Changes in major intracellular osmolytes in L-929 cells following rapid and slow application of hyperosmotic media. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:461–470. doi: 10.1016/S1095-6433(01)00415-9. [DOI] [PubMed] [Google Scholar]
- Mao L, Hartl D, Nolden T, Koppelstätter A, Klose J, Himmelbauer H, Zabel C (2008) Pronounced alterations of cellular metabolism and structure due to hyper- or hypo-osmosis. J Proteome Res 7:3968–3983 [DOI] [PubMed]
- Matsunaga N, Kano K, Maki Y, Dobashi T (2009) Estimation of dissolved carbon dioxide stripping in a large bioreactor using model medium. J Biosci Bioeng 107:419–424 [DOI] [PubMed]
- McNeeley K, Sun Z, Sharfstein S. Techniques for dual staining of DNA and intracellular immunoglobulins in murine hybridoma cells: applications to cell-cycle analysis of hyperosmotic cultures. Cytotechnology. 2005;48:15–26. doi: 10.1007/s10616-005-2926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, Wakitani M, Yamano K, Imai H, Kanda Y, Niwa R, Iida S, Uchida K, Shitara K, Satoh M (2004) Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng 88:901–908 [DOI] [PubMed]
- Mori K, Iida S, Yamane-Ohnuki N, Kanda Y, Kuni-Kamochi R, Nakano R, Imai-Nishiya H, Okazaki A, Shinkawa T, Natsume A, Niwa R, Shitara K, Satoh M (2007) Non-fucosylated therapeutic antibodies: the next generation of therapeutic antibodies. Cytotechnology 55:109–114 [DOI] [PMC free article] [PubMed]
- Oh SK, Vig P, Chua F, Teo WK, Yap MG (1993) Substantial overproduction of antibodies by applying osmotic pressure and sodium butyrate. Biotechnol Bioeng 42:601–610 [DOI] [PubMed]
- Oliveira JE, Damiani R, Vorauer-Uhl K, Bartolini P, Ribela MT (2008) Influence of a reduced CO2 environment on the secretion yield, potency and N-glycan structures of recombinant thyrotropin from CHO cells. Mol Biotechnol 39:159–166 [DOI] [PubMed]
- Omasa T, Tanaka R, Doi T, Ando M, Kitamoto Y, Honda K, Kishimoto M, Ohtake H (2008) Decrease in antithrombin III fucosylation by expressing GDP-fucose transporter siRNA in Chinese hamster ovary cells. J Biosci Bioeng 106:168–173 [DOI] [PubMed]
- Øyaas K, Ellingsen TE, Dyrset N, Levine DW (1994) Utilization of osmoprotective compounds by hybridoma cells exposed to hyperosmotic stress. Biotechnol Bioeng 43:77–89 [DOI] [PubMed]
- Ozturk SS, Palsson BO. Effect of medium osmolarity on hybridoma growth, metabolism, and antibody production. Biotechnol Bioeng. 1991;37:989–993. doi: 10.1002/bit.260371015. [DOI] [PubMed] [Google Scholar]
- Park SY, Lee GM. Enhancement of monoclonal antibody production by immobilized hybridoma cell culture with hyperosmolar medium. Biotechnol Bioeng. 1995;48:699–705. doi: 10.1002/bit.260480618. [DOI] [PubMed] [Google Scholar]
- Putnam WS, Prabhu S, Zheng Y, Subramanyam M, Wang YM (2010) Pharmacokinetic, pharmacodynamic and immunogenicity comparability assessment strategies for monoclonal antibodies. Trends Biotechnol 28:509–516 [DOI] [PubMed]
- Rhodes M, Birch J. Large-scale production of proteins from mammalian cells. Nat Biotech. 1988;6:518–523. doi: 10.1038/nbt0588-518. [DOI] [Google Scholar]
- Ryu JS, Lee GM. Application of hypoosmolar medium to fed-batch culture of hybridoma cells for improvement of culture longevity. Biotechnol Bioeng. 1999;62:120–123. doi: 10.1002/(SICI)1097-0290(19990105)62:1<120::AID-BIT14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Ryu JS, Kim TK, Chung JY, et al. Osmoprotective effect of glycine betaine on foreign protein production in hyperosmotic recombinant Chinese hamster ovary cell cultures differs among cell lines. Biotechnol Bioeng. 2000;70:167–175. doi: 10.1002/1097-0290(20001020)70:2<167::AID-BIT6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ryu JS, Lee MS, Lee GM. Effects of cloned gene dosage on the response of recombinant CHO cells to hyperosmotic pressure in regard to cell growth and antibody production. Biotechnol Prog. 2001;17:993–999. doi: 10.1021/bp010116e. [DOI] [PubMed] [Google Scholar]
- Schmelzer AE, Miller WM. Effects of osmoprotectant compounds on NCAM polysialylation under hyperosmotic stress and elevated pCO2. Biotechnol Bioeng. 2002;77:359–368. doi: 10.1002/bit.10175. [DOI] [PubMed] [Google Scholar]
- Schmelzer AE, Miller WM. Hyperosmotic Stress and Elevated pCO2 Alter Monoclonal Antibody Charge Distribution and Monosaccharide Content. Biotechnol Prog. 2002;18:346–353. doi: 10.1021/bp010187d. [DOI] [PubMed] [Google Scholar]
- Sethuraman N, Stadheim TA. Challenges in therapeutic glycoprotein production. Curr Opin Biotechnol. 2006;17:341–346. doi: 10.1016/j.copbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Shen D, Sharfstein ST. Genome-wide analysis of the transcriptional response of murine hybridomas to osmotic shock. Biotechnol Bioeng. 2006;93:132–145. doi: 10.1002/bit.20691. [DOI] [PubMed] [Google Scholar]
- Shen D, Kiehl TR, Khattak SF, Li ZJ, He A, Kayne PS, Patel V, Neuhaus IM, Sharfstein ST (2010) Transcriptomic responses to sodium chloride-induced osmotic stress: a study of industrial fed-batch CHO cell cultures. Biotechnol Prog 26:1104–1115 [DOI] [PubMed]
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 277:26733–26740 [DOI] [PubMed]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 278:3466–3473 [DOI] [PubMed]
- Shitara K, Nakamura K, Tokutake-Tanaka Y, Fukushima M, Hanai N (1994) A new vector for the high level expression of chimeric antibodies in myeloma cells. J Immunol Methods 167:271–278 [DOI] [PubMed]
- Soo Ryu J, Min Lee G. Effect of hypoosmotic stress on hybridoma cell growth and antibody production. Biotechnol Bioeng. 1997;55:565–570. doi: 10.1002/(SICI)1097-0290(19970805)55:3<565::AID-BIT14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Steinmeyer DE, McCormick EL. The art of antibody process development. Drug Discov Today. 2008;13:613–618. doi: 10.1016/j.drudis.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Stubblefield E, Mueller GC. Effects of sodium chloride concentration on growth, biochemical composition, and metabolism of HeLa cells. Cancer Res. 1960;20:1646–1655. [Google Scholar]
- Sun Z, Zhou R, Liang S, McNeeley KM, Sharfstein ST (2004) Hyperosmotic stress in murine hybridoma cells: effects on antibody transcription, translation, posttranslational processing, and the cell cycle. Biotechnol Prog 20:576–589 [DOI] [PubMed]
- Takagi M, Moriyama T, Yoshida T. Effects of shifts up and down in osmotic pressure on production of tissue plasminogen activator by Chinese hamster ovary cells in suspension. J Biosci Bioeng. 2001;91:509–514. doi: 10.1263/jbb.91.509. [DOI] [PubMed] [Google Scholar]
- Teylaert B, Meurice E, Bobowski M, Harduin-Lepers A, Gaucher C, Fontayne A, Jorieux S, Delannoy P (2011) Molecular cloning, characterization, genomic organization and promoter analysis of the alpha1, 6-fucosyltransferase gene (fut8) expressed in the rat hybridoma cell line YB2/0. BMC Biotechnol 11:1 [DOI] [PMC free article] [PubMed]
- van Berkel PH, Gerritsen J, van Voskuilen E, Perdok G, Vink T, van de Winkel JG, Parren PW (2010) Rapid production of recombinant human IgG With improved ADCC effector function in a transient expression system. Biotechnol Bioeng 105:350–357 [DOI] [PubMed]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–11 research0034 [DOI] [PMC free article] [PubMed]
- Walsh G. Biopharmaceutical benchmarks 2006. Nat Biotech. 2006;24:769–776. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- Werner RG. Economic aspects of commercial manufacture of biopharmaceuticals. J Biotechnol. 2004;113:171–182. doi: 10.1016/j.jbiotec.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Wu MH, Dimopoulos G, Mantalaris A, Varley J (2004) The effect of hyperosmotic pressure on antibody production and gene expression in the GS-NS0 cell line. Biotechnol Appl Biochem 40:41–46 [DOI] [PubMed]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, Shitara K, Satoh M (2004) Establishment of FUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 87:614–622 [DOI] [PubMed]
- Yoshisue H, Suzuki K, Kawabata A, Ohya T, Zhao H, Sakurada K, Taba Y, Sasaguri T, Sakai N, Yamashita S, Matsuzawa Y, Nojima H (2002) Large scale isolation of non-uniform shear stress-responsive genes from cultured human endothelial cells through the preparation of a subtracted cDNA library. Atherosclerosis 162:323–334 [DOI] [PubMed]
- Zanghi JA, Schmelzer AE, Mendoza TP, Knop RH, Miller WM (1999) Bicarbonate concentration and osmolality are key determinants in the inhibition of CHO cell polysialylation under elevated pCO2 or pH. Biotechnol Bioeng 65:182–191 [DOI] [PubMed]
- Zhang X, Garcia IF, Baldi L, Hacker DL, Wurm FM (2010) Hyperosmolarity enhances transient recombinant protein yield in Chinese hamster ovary cells. Biotechnol Lett 32:1587–1592 [DOI] [PubMed]
- Zhao L, Wang J, Niu H, Tan W-S (2009) Responses of GS-NS0 myeloma cells to osmolality: cell growth, intracellular mass metabolism, energy metabolism, and antibody production. Biotechnol Bioprocess Eng 14:625–632
- Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, Culm-Merdek K, Park A, Pan C, Edmunds T (2008) Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng 99:652–665 [DOI] [PubMed]
- Zhu MM, Goyal A, Rank DL, Gupta SK, Vanden Boom T, Lee SS (2005) Effects of elevated pCO2 and osmolality on growth of CHO cells and production of antibody-fusion protein B1: a case study. Biotechnol Prog 21:70–77 [DOI] [PubMed]