Abstract
Polyphenols are known to exhibit wide spectrum of benefit for brain health and to protect from several neurodegenerative diseases. The present study was sought to determine the neuroprotective effects of Rosmarinus officinalis’ polyphenols (luteolin, carnosic acid, and rosmarinic acid) through the investigation of stress-related proteins. We carried out measurement of the expression of heat-shock protein (Hsp) 47 promoter in heat stressed Chinese hamster ovary transfected cells. We performed proteomic analysis and confirmed gene expression by real time PCR in PC12 cells. Results showed that these compounds modulated significant and different effects on the expression of 4 stress-related proteins: heat shock protein 90 α (Hsp90), Transitional endoplasmic reticulum ATPase (VCP/p97), Nucleoside diphosphate kinase (NDK), and Hypoxia up-regulated protein 1 (HYOU1)) at translational and post translational levels in PC12 cells and they downregulated the expression of Hsp47 activity in Chinese hamster transformed cells. These findings suggest that luteolin, carnosic acid, and rosmarinic acid may modulate the neuroprotective defense system against cellular stress insults and increase neuro-thermotolerance.
Keywords: Rosmarinus officinalis, Hsp90 α, VCP/p97, NDK, HYOU1, Hsp47
Introduction
Neurodegenerative disease manifests in elderly people and most commonly in developed countries where life span is long. However, the World Health Organization has recognized it as a global problem since it is the fourth most common source of death. Neurodegenerative diseases are expected to impose severe impact on our society emotionally, socially and financially in the next coming decades (Park et al. 2010). In this respect understanding, the pathogenesis mechanism and finding potential therapeutic targets for such diseases is becoming a focus point for the scientific community.
In neuronal cells, heat shock proteins (Hsps), provide a fundamental mechanism to defend the cell against diverse external physiological stress (Luo et al. 2007). Induced by several stressors like temperature, hypoxia, inflammation, infections and environmental pollutants, stress proteins (Hsps) play key roles in living systems (Taguchi and Razzaque 2007). It was postulated by several studies that Hsps are working as chaperones together with ubiquitin–proteasome system (UPS) to assist folding/refolding of nonnative protein, to help in the degradation of irreversibly damaged proteins, and other proteins essential for the protection and recovery from cell damages associated with perturbation of protein homeostasis. In neuronal cells, Hsps may have anti-apoptotic effects and keep the homeostasis against stress conditions (Chen and Brown 2007; Luo et al. 2007; Winklhofer et al. 2008; Oza et al. 2008). Recently, Hsps became a therapeutic target in research of neurodegenerative disorder and aging because the pathogenesis mechanism of these diseases is thought to be related to an abnormal increase of Unfolded Protein Response (UPR), failure of UPS and protein misfolding and/or aggregation (Zhao et al. 2010).
Numerous studies in the last decade have shown that dietary polyphenols may have, in vitro and in vivo, a neurorescue impact in aging and neurodegenerative diseases to retard or even reverse the accelerated rate of neuronal degeneration (Ramassamy 2006; Sun et al. 2010; Rajeswari and Sabesan 2008; Ortega 2006; Spencer 2009) and aging (Queen and Tollefsbol 2010; Wilson et al. 2006). However, little is done about their effect on Hsps in relation with neurodegenerative disease.
Rosmarinus officinalis is traditionally used to improve memory, in connection with Alzheimer’s disease (AD) and dementia, for general symptoms of old age, debility and fatigue (El Omri et al. 2010). Recently several studies showed that R. officinalis or its main compounds like carnosic acid (CA), rosmarinic acid (RA) and luteolin (Lut) (Kosaka et al. 2010; El Omri et al. 2010; Lin et al. 2010) can be good candidates to substitute nerve growth factor (NGF). Moreover, R. officinalis phenolic compounds are endowed with anti AD (Liu et al. 2009; Lin et al. 2010; El omri et al. 2010), anti Parkinson’s disease (PD) (Park et al. 2010) and anti amyotrophic lateral sclerosis (ALS) (Shimojo et al. 2010).
This study is the first conducted to determine the effects of R. officinalis’ polyphenols: Lut, CA, and RA on stress-related proteins expression in PC12 cells and to confirm their expression using RT–PCR.
Materials and method
Chemicals
The following reagents were purchased from several manufacturers and were used to prepare the culture medium and the required solutions: Dulbecco’s modified Eagle medium (DMEM) was purchased from Sigma–Aldrich (United kingdom), fetal bovine serum (FBS) was purchased from Bio west (France), horse serum (HS) and Geneticin (G418) were purchased from Invitrogen (Carlsbad, CA, USA), F12 Medium was from Invitrogen (Tokyo, Japan). Penicillin–streptomycin was purchased from Lonza, Walkersville Inc., (MD, USA), DTT and TEMED were purchased from Amersham Bioscience (Sweden). Luteolin, carnosic acid, NGF 7 s, Trizma base, kanamycin solution, trypsin (ethylenediaminetetra-acetic acid [EDTA]), MgCl2 · 6H2O, 4-methylumbelliferyl-β-galactose (MUG), protease inhibitor cocktail, and Ribonuclease A, all were purchased from Sigma–Aldrich (USA). Rosmarinic acid, and Na2HPO4 · 7H2O were purchased from MP Biomedicals LLC (France). Spemine base and acetonitrile were purchased from Sigma–Aldrich (Germany). Thiourea, APS and protein rainbow marker were purchased from GE Healthcare (United Kingdom), urea, acrylamide, Bis, Bromophenol blue, CHAPS, CBB G-250, Glycol, glycine, iodoacetamide, SDS, Tris, urea, IPG buffer and IPG strips were purchased from GE Healthcare (Sweden). Lysis buffer was from (Promega). NaCl, KH2PO4, KCl, NaH2PO4 · 2H2O, bovine serum albumin, NaN3, dimethyl sulfoxide, glycine, NaOH, and Deoxyribonuclease DNAse A were purchased from Wako (Japan).
Cell culture
Chinese hamster ovary (CHO) cells, stably transfected with (+) or without (−) Hsp47 promoter, were used for this experiment (Isoda et al. 2004). The cells were provided by S. Yokota (Kaneka), and were grown as adherent monolayer in 75 cm2 tissue culture flasks using F12 Medium supplemented with 10% Fetal Bovine Serum, 200 μg/mL of G418 (Gibco BRL 13075-015) and 0.1 g/L kanamycin solution. The cultures were maintained in a 5% CO2 incubator at 37 °C. Cell passage was carried out at 80% confluence at 1:2 ratio using 0.25% trypsin with 1 mM EDTA.
Hsp47-transfected cells were grown as adherent monolayer in 75 cm2 tissue culture flasks using F12 Medium supplemented with 10% Fetal Bovine Serum, 200 μg/mL of G418 and 0.1 g/L kanamycin solution. The cultures were maintained in a 5% CO2 incubator at 37 °C. Cell passage was carried out at 80% confluence at 1:2 ratio using 0.25% trypsin with 1 mM EDTA. The cells were used between passage 3 and 8 for the reported experiments.
PC12 cells (Riken Tsukuba, Japan) were cultured in 75 cm2 flask (BD Falcon, USA) and maintained in DMEM containing 10% heat inactivated horse serum and 5% fetal bovine serum supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin, in a water-saturated 5% CO2 atmosphere at 37 °C. The cells were used between passage 3 and 8 for the reported experiments.
Heat shock protein 47 assay
Hsp47-transfected cells were trypsinized and plated onto 96-well plates at initial concentrations of 1 × 104 cells per well in 100 μL of culture medium. The cells were allowed to attach for 48 h at 37 °C supplemented with 5% CO2, heat-shocked for 90 min at 42 °C, 5% CO2 and recovered for 2 h in a 5% CO2 incubator. After cell recovery, the medium was removed and changed to fresh one containing R. officinalis extract and polyphenols at desired concentrations. Then, the cells were incubated for 3 h in 5% CO2 incubator at 37 °C.
After incubating cells with samples, the medium was removed and the cells washed twice with PBS. 50 μL lysis buffer (Promega) was then added and the plates incubated for 30 min at room temperature. 20 μL of cell lysate was transferred to a new plate, to which 100 μL of substrate solution (10 mM NaH2PO4 · 2H2O, 100 mM NaCl, 1% BSA, 0.005% NaN3, 1 mM MgCl2 · 6H2O, 1% 4-methylumbelliferyl-β galactosidase (MUG), pH 7) was added in order to trigger the conversion of MUG into galactose and methylumbelliferyl by galactosidase. After allowing the reaction to occur in the dark for 30 min at room temperature, 60 μL of reaction stop buffer (1 M glycine-NaOH, pH 10.3) was added and the fluorescence at 365 nm excitation/450 nm emission was then determined using a multi-detection microplate.
PC12 cells treatment and protein extraction
PC12 cells were seeded at 2 × 106 cells/100-mm poly-l-lysine coated dishes (Wako, Japan). Following overnight incubation in a 5% CO2 humidified incubator at 37 °C. Cells were treated with 50 μM luteolin, 15 μM rosmarinic acid, 20 μM carnosic acid and 50 ng NGF for 48 h. The cells were rinsed three times with ice-cold PBS, scraped gently and collected in PBS. Then, the cell pellet was lysed in 1 mL of lysis buffer containing 7 M urea, 2 M thiourea, 4% w/v CHAPS, 1 mM EDTA, 100 mM DTT, 25 mM spermine base, 1% protease inhibitor cocktail (Han et al. 2010) and 0.1 volume of DNAse I (1 mg/mL)/RNAse (0.25 mg/mL) mixture. DNAse I, RNAse, DTT and Protease inhibitor cocktail were immediately added to the extraction-lysis buffer. The extraction was carried out firstly at 4 °C for 45 min to degrade nucleic acid followed by 1 h shaking at room temperature (Yang et al. 2006). Then the lysate was clarified by ultracentrifugation at 46,000 rpm (79660g) at 15 °C for 60 min. The final protein amount was determined using 2-D Quant kit.
Two-dimensional gel electrophoresis (2-DE)
The first dimension electrophoresis was carried out on an Ettan IPGphor II (GE Healthcare) apparatus. Immobilized pH gradient (IPG) strips (pH 3–10, 24 cm, GE Healthcare) were rehydrated (7 M Urea, 2 M Thiourea, 2% CHAPS, traces of Bromophenol blue, 50 mM DTT and 0.5% IPG buffer, IPG buffer and DTT were added immediately before use) with 350 μg of sample solution. The total volume loaded by strip was 450 μL. The rehydration and separation programs were processed using the following parameters: step 1: 500 Vh, step 2: 750 Vh, step 3: 16.5 KVh, step 4: 27.5 KVh and step 5 was 500 V for 24 h. The proteins were separated according to their isoelectric points. The isoelectrically focused IPG strips were immediately equilibrated for 2× 15 min using equilibration buffer (6 M urea, 50 mM Tris–HCl, pH 8.8, 30% glycerol (w/w), 2% (w/v) SDS, traces of bromophenol blue). The first equilibration was with 1.0% w/v DTT followed by a second equilibration with 2.5% w/v iodacetamide. Then the strips were immersed in 10 mL of electrophoresis buffer for 5 min.
The strips were subsequently subjected to a second dimension SDS gel (255 mm × 200 mm × 1 mm), the proteins were separated using 12% SDS PAGE, using Ettan DALTSix™ electrophoresis unit (GE Healthcare). The SDS–PAGE was performed at 2 W/gel for 40 min, then 15 W/gel until the dye front reached the bottom of gels. After being fixed with 3% ethanol, 0.5% acetate solution, gels were stained with CBB for 8 h. After being distained by rinsing with fixing solution, gels were scanned at 300 dpi resolution and the image were analyzed with Image Master™ 2D software (ver. 4.9: GE Healthcare). For statistical quantification, three experiments were performed for each experiment. Coomassie blue stained 2-DE gel images were acquired with image scanner and subsequently subjected to visual assessment to detect changes in protein expression level between different treatments. Spots were expressed as percentages (% vol) of relative volumes by integrating the value of each pixel in the spot area as described previously in our study (Han et al. 2010).
In-gel digestion and mass spectrometry
Protein spots of interest were excised from the CBB-stained gel; the excised spots were transferred to Eppendorf tube loaded with 100 μL of 50% ACN/25 mM ammonium bicarbonate solution (1:1). After being decolorized, gel samples were rehydrated with 100 μL of 100% ACN for 5 min and then thoroughly dried in the SpeedVac concentrator (miVac, England) for 5 min. Then, the dried gels were reduced in 100 μL 10 mM DTT/25 mM ammonium bicarbonate with shaking at 56 °C for 1 h, and washed with 100 μL of 25 mM ammonium bicarbonate with shaking at room temperature for 10 min. Reduced gel particles were then alkylated in 100 μL of 55 mM Iocetamide/25 mM ammonium bicarbonate and incubated in the dark for 45 min at room temperature and washed as described previously. After that, gel samples were dehydrated with 100 μL of 100% ACN for 10 min and then thoroughly dried in the SpeedVac concentrator for 5 min. Subsequently the dried gel particles were rehydrated with 2 μL/sample trypsin in 25 mM ammonium bicarbonate (enzyme ratio 1:50) at 4 °C for 30 min, and incubated at 37 °C for 15 h. After trypsin digestion, the supernatant was transferred to another tube. The remaining peptide mixture was extracted twice with 50% ACN/5% formic acid at 37 °C for 30 min using 50 μL for the first extraction and 25 μL for the second extraction. Subsequently the combined solution was concentrated in the SpeedVac to 10 μL and analyzed using MALDI TOF as described in our previous study (Han et al. 2010).
Analysis of gene expression by quantitative real-time PCR
To confirm the activation of stress-related proteins in R. officinalis’polyphenols-treated PC12 cells, the expression of Hsp90α, HYOU1, VCP/p97, and NDK were determined by real-time PCR using glyceraldehydes 3-phosphate dehydrogenase (GAPDH) as an internal positive control. After incubating seeded plates for 6 and 12 h, total RNA was purified using the ISOGEN kit (Nippon GeneCo. Ltd., Japan) following the manufacturer’s instructions. Total RNA was quantified by measurement with Thermo scientific Nanodrop 2000 (USA). Reverse transcription reactions were carried out with the Superscript III reverse transcriptase kit (Invitrogen, Carlsbad/CA, USA) using 1 μg of total RNA. Briefly, RNA was denatured at 65 °C for 5 min and incubated with 1 μL oligo (dT)12–15 primers and chilled at 4 °C. After adding SuperScript II reverse transcriptase (200 U) the reaction mix was incubated at 42 °C for 60 min, then 10 min at 70 °C (Han et al. 2010). Primers and TaqMan probes used for these experiments were purchased from Applied Biosystems. Hsp90α (Rn00822023) and GAPDH (Rn99999916_s1) were inventoried gene expression assays. VCP/p97 (Rn01439521_m1), NDK (Rn01465378-gH), and HYOU1 (Rn02586251_m1) were obtained as ‘Assays-on-demand’ kits.
For the quantification of mRNA, TaqMan real-time quantitative PCR amplification reactions were carried out in an AB 7500 fast real-time system (Applied Biosystems). Amplifications were performed in 20 μL final volume, using 10 μL of TaqMan Universal PCR Master Mix UNG (2X), 1 μL of the corresponding primer/probe mix and 9 μL of template cDNA (70 ng μL−1). Cycling conditions were: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 95 °C for 15 s followed by 60 °C for 1 min.
Results
Luteolin, carnosic acid and rosmarinic acid decrease the expression of Heat shock protein 47
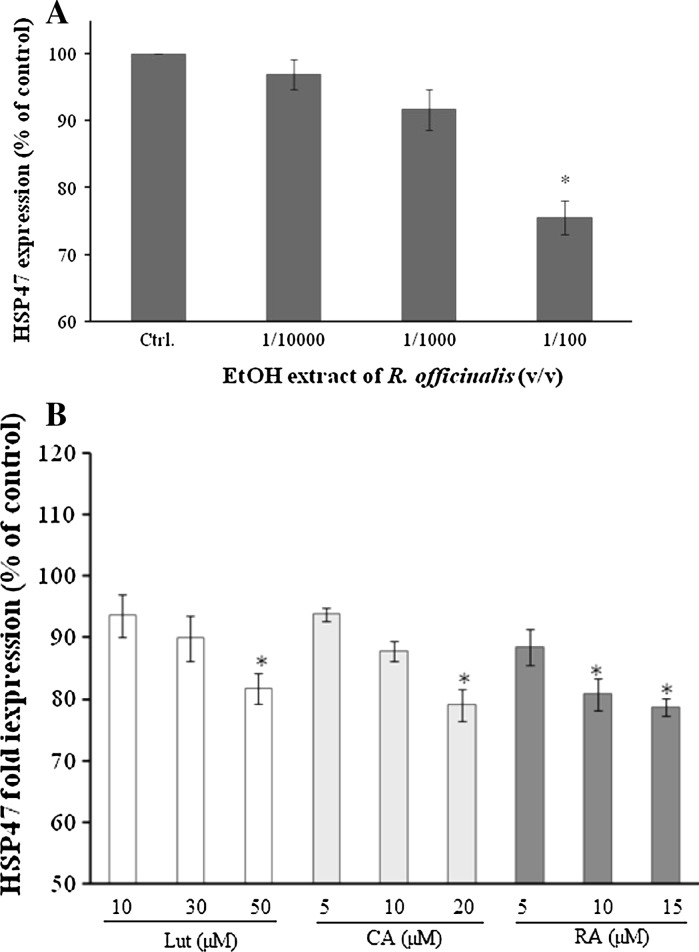
Hsp47-transformed cells were heat-shocked for 90 min at 42 °C, 5%CO2 and recovered for 2 h. Then we proceeded to the screening of R. officinalis’ EtOH extract and the effect of its main polyphenols (lut, CA, and RA) on the recovery of Hsp47. As shown in Fig. 1a, 70% EtOH extract of R. officinalis reduced significantly the expression of Hsp47 at 1/100 dilution v/v, by 25%. Additionally, Lut, CA, and RA expressed the same activities. As indicated in Fig. 1b, all three compounds significantly reduced the expression of Hsp47 in a dose dependent manner. 20% of Hsp47 stress recovery was observed at the doses of Lut (50 μM), CA (20 μM), and RA (15 μM).
Fig. 1.
Effect of R. officinalis polyphenols on Hsp47 expression in Chinese Hamster ovary transfected cells. a The effect of R. officinalis EtOH extract on Hsp47 expression, cells were treated with R. officinalis 70% EtOH at 1/10,000, 1/1,000, 1/100 v/v dilution. b The effect of R. officinalis polyphenols on Hsp47 expression, cells were treated with luteolin (10, 30, 50 μM), carnosic acid (5, 10, 20 μM), rosmarinic acid (5, 10, 15 μM) and NGF 50 ng/mL. The vector containing the plasmid used in this work was constructed by connecting the restriction enzyme avall (−197 to +38 KDa) fragment containing the heat shock factor binding DNA sequence of the mouse Hsp47 promoter to a 3.1-Kb HindIII containing the structural gene for β-galactosidase on the upstream side of HindIII. Hsp47 expression was determined as described in Materials and Methods. Results are expressed as the mean of 12 wells from three independent experiments ± S.D. *p < 0.05 treatment versus control (Student’s t-test)
Effect of luteolin, carnosic acid and rosmarinic acid on stress related protein expression
Since the major phenolic compounds of R. officinalis are Lut, CA, and RA, in subsequent analysis we assumed that this anti-stress activity could be mainly due to the presence of these compounds and tried to elucidate their neuroprotective effect in PC12 cells using proteomics analysis.
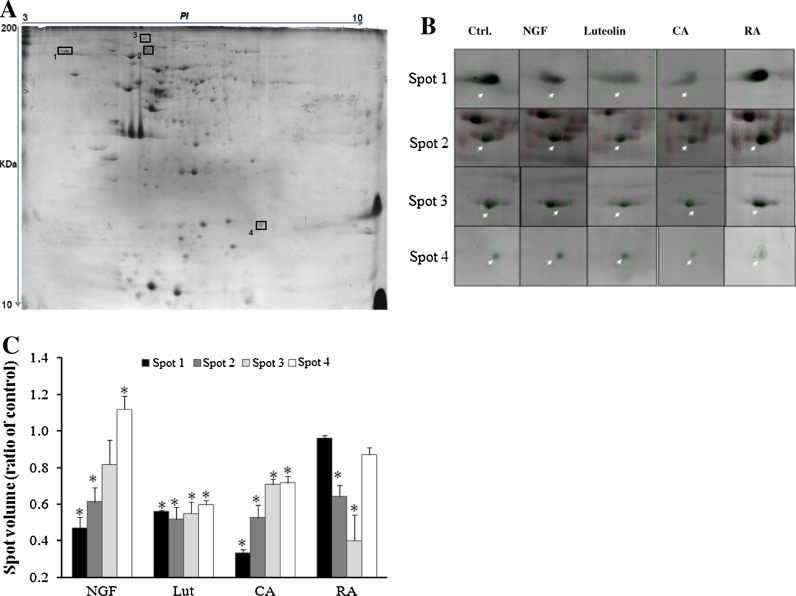
PC12 cells were treated with Lut (50 μM), CA (20 μM), and RA (15 μM) for 48 h, then total proteins were separated by 2D-gel electrophoresis. A protein pattern of PC12 cells is shown in Fig. 2a. Approximately 200 well-resolved spots were detected in each coomassie blue-stained gel, with molecular-mass ranges of 15–225 kDa and a pI ranging from 3 to 10. As shown in Fig. 2b, treatment with R. officinalis polyphenols caused substantial changes in 4 particular spots (p < 0.05). Protein spots were selected and analyzed with MALDI-TOF. The peptide mass fingerprinting (PMF) spectrum was used to search protein sequence using Mascot engine versus Swiss prot database as reported in our previous study (Han et al. 2010). These spots were identified to be stress-related proteins: Hsp90, VCP/p97, HYOU1, and NDK (Table 1). Lut and CA significantly down regulated all 4 proteins: Lut treatment induced a decrease by around 40% of studied proteins, CA induced severe down regulation of Hsp90 and VCP/p97. However, RA significantly decreased the HYOU1 and VCP/p97 protein spot volume to 0.4% and 0.7%, respectively.
Fig. 2.
Two-dimensional gel electrophoresis of PC12 cell proteins (a), the magnified images of the boxed regions (b) and spot volume (c). PC12 cells were treated with 50 μM luteolin, 20 μM carnosic acid, 15 μM rosmarinic acid and 50 ng/mL NGF for 48 h. The 2-DE gel was stained with coomassie brilliant blue. Spot volume was measured by ImageMaster 2D Platinum software. These spots were identified as Hsp90, VCP/p97, HYOU1, and NDK by MALDI-TOF mass spectrometry. Each bar represents the mean ± SD of three independent experiments. *p < 0.05 treatment versus control (Student’s t-test)
Table 1.
Proteins of PC12 cells changed by R. offcinalis’ polyphenols and identified by TOF analysis
| Spot no. | Accession no. | Score | Calculated PI value/observed PI value | Calculated Mw value/observed Mw value (KDa) | Protein sequence | Name of protein |
|---|---|---|---|---|---|---|
| 1 | P82995 | 80 | 4.93/4.0 | 85.161/100 | K.VILHLKEDQTEYLEER.R | Heat shock protein alpha (Hsp90α) |
| 2 | P46462 | 454 | 5.14/5.5 | 89.977/105 | K.MDELQLFR.G K.EMVELPLRHPALFK.A R.RIVSQLLTLMDGLK.Q R.EVDIGIPDATGRLEILQIHTK.N R.ETVVEVPQVTWEDIGGLEDVKR.E R.ELQELVQYPVEHPDKFLK.F K.GPELLTMWFGESEANVR. R.KYEMFAQTLQQSR.G |
Transitional endoplasmic reticulum ATPase Valosin containing protein (Vcp/p97) |
| 3 | Q63617 | 639 | 5.11/5.5 | 111.448/115 | R.SRFPEHELNVDPQR.Q R.SLAEDFAEQPIKDAVITVPAFFNQAER.R K.VLQLINDNTATALSYGVFR.R R.TLGGLEMELR.L R.DAVIYPILVEFTR.E R.YSHDFNFHINYGDLGFLGPEDLR.V K.LYQPEYQEVSTEEQREEISGK.L K.LCQGLFFR.V |
Hypoxia up-regulated protein 1 (HYOU1) |
| 4 | P19804 | 313 | 6.92/7.2 | 17.386/23 | R.TFIAIKPDGVQR.G K.DRPFFPGLVK.Y R.VMLGETNPADSKPGTIR.G R.GDFCIQVGR.N R.NIIHGSDSVESAEKEIGLWFKPEELIDYK.S K.EIGLWFKPEELIDYK.S |
Nucleoside diphosphate kinase B (NDK) |
Validation of differentially expressed stress related protein by quantitative RT–PCR
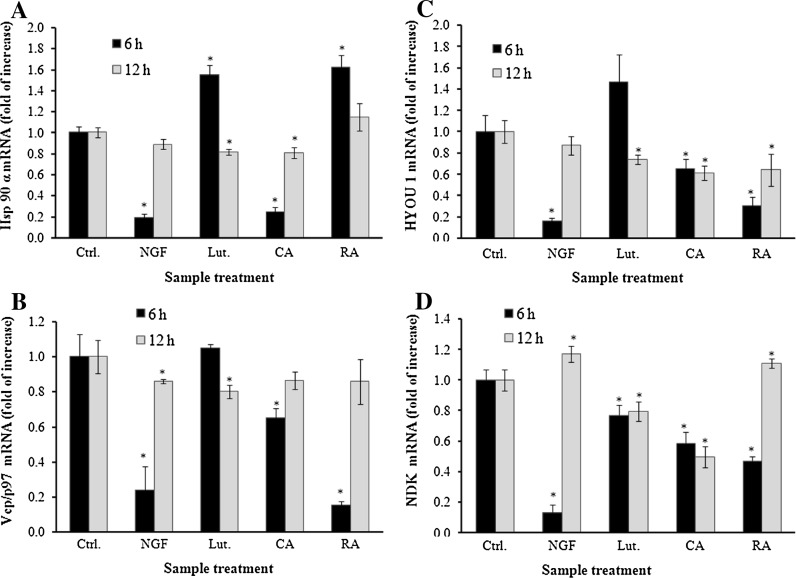
To confirm the protein expression of Hsp90, HYOU1, VCP/p97, and NDK, we evaluated mRNA expression of previously cited proteins after 6 h and 12 h treatment. As shown in Fig. 3a, CA, Lut and NGF treatments respectively significantly reduced Hsp90 mRNA expression to 0.2, 0.84 and 0.25. Lut decreased Hsp90 expression from 1.5 (6 h treatment) to 0.81 (12 h treatment). Meanwhile, CA and NGF increased Hsp90 after 12 h to 0.80 and 0.88, respectively. RA showed a significantly increased expression of Hsp90 at 6 h which was decreased to control levels after 12 h of treatment. VCP/p97 was significantly down regulated by CA, RA, and NGF to 0.23, 0.64, and 0.15, respectively, after 6 h of treatment. However, Lut treatment only showed decrease in mRNA expression after 12 h (Fig. 3b). Similarly, HYOU1 was down regulated by CA, RA, and NGF to 0.16, 0.65, and 0.31, respectively. Lut treatment showed an increase after 6 h to 1.4 fold and a decrease to 0.73-fold after 12 h (Fig. 3c).
Fig. 3.
Effect of R. officinalis polyphenols on the expressions of Hsp90, Vcp, HYOU1, NDK mRNAs in PC12 cells. GAPDH was used as reference (housekeeping genes). The mRNA expression of all genes was normalized by GAPDH mRNA expression and expressed as ratio of Ctrl. PC12 cells were treated with 30 μM luteolin, 20 μM carnosic acid, 15 μM rosmarinic acid and 50 ng/mL NGF for 6 and 12 h. Each bar represents the mean ± SD of three independent experiments. *p < 0.05 treatment versus control (Student’s t-test)
NDPK mRNA expression was decreased by all treatments. Lut, CA, RA and NGF showed a significant decrease in NDK expression after 6 h of treatment to 0.77, 0.58, 0.47, and 0.13. This effect was maintained in case of Lut and CA and it was increased to 1.17 and 1.11, respectively, for NGF and RA after 12 h of treatment.
Discussion
Previously, we and others demonstrated that the main polyphenols of R. officinalis: Lut, CA, and RA are able to induce PC12 cell differentiation (El Omri et al. 2010; Lin et al. 2010). In the present study we observed for the first time that Lut, CA, and RA reduced significantly the expression of Hsp47 in heat stressed transfected Chinese hamster ovary cells (Fig. 1). Proteomic analysis and qRT-PCR showed that differentiation of PC12 cells into neuron-like cells is associated with an attenuation of stress related proteins expression. Our result is consistent with previous observations of a reduced induction of Hsp70 (Dwyer et al. 1996), NDPK (Kim et al. 2002), and VCP/p97 (Kobayashi et al. 2002) during neuronal differentiation of PC12 cells (Dwyer et al. 1996).
Hsp47 is a heat stress protein that interacts with procollagen in the lumen of the endoplasmic reticulum (ER) (Taguchi and Razzaque 2007). It is the main chaperone involved in collagen elaboration and maturation (Rocnik et al. 2002). Reducing the activity of Hsp47 means that R. officinalis polyphenols could protect mammalian cells against heat stress and increase thermotolerance. Hsp90 is a molecular chaperone. In neurodegenerative diseases, it is involved in the protection of neuronal cells against the accumulation of toxic aggregates (Luo et al. 2010). VCP/p97 is cytosolic chaperone required for Endoplasmic Reticulum-Associated Protein Degradation (ERAD). It is involved in a variety of cellular processes, including membrane fusion and ubiquitin-dependent protein degradation and it is a chaperone-like protein (Nagahama et al. 2003). Recent studies showed that inhibition or stable complexing of Hsp90 may alleviate and prevent from some neurological disease with motor impairments and tauopathy (Ali et al. 2010). HYOU1 belongs to Hsp70 superfamily. It has been suggested to be a neuroprotective factor against ischemia and excitotoxicity. It was reported to be upregulated under hypoxic or excitotoxicity conditions, that potentially induce ER stress in neurons (Zhao et al. 2010). In our study, we demonstrated that R. officinalis polyphenols downregulated the expression of HYOU1. Subsequently they may alleviate stress insults in PC12 cells through their antioxidant activity. NDK is involved in the proteolytic functions of the proteasome. It may act by catalyzing the activities of ATP hydrolysis when Hsp70 and VCP/p97 are activated (Yano et al. 1999). Meanwhile, (Kimura et al. 2003) reported the involvement of NDP kinases in the regulation of cell growth and differentiation. Particularly in PC12 cells, NDK may control the molecular switch to determine the cell fate toward proliferation or differentiation in response to environmental signals.
In neuronal cells, physiological and pathological processes that disturb protein folding in the ER cause ER stress and activate a set of signaling pathways termed UPR (Samali et al. 2010). Particularly, in neurons misfolded and/or aggregated proteins cannot be diluted, and accumulate with aging (Chen and Brown 2007), leading to several neurodegenerative disorders, like AD, PD, ALS, Huntington’s disease (HD), and other polyglutamine expansion disorders (Luo et al. 2007; Oza et al. 2008). It was suggested by Taguchi and Razzaque (2007) that targeting Hsp is a promising alternative in the area of neurodegenerative disorder, where protein aggregation and neuron degeneration are the common pathological features. In this respect, it was demonstrated by Wilson et al. (2006) that blue berry polyphenol uptake increased life span of C. elegans by promoting stress resistance. Melatonin was demonstrated by Ozacmak et al. (2009) to protect rats by reducing Hsp70 expression during chronic cerebral hypoperfusion. Resveratrol was reported to protect cells against heat stress through chaperone activation (Putics et al. 2008). Curcumin consumption by Indian reduced AD incidence in comparison to American people (Ali et al. 2010).
As a part of this study, we examined possible mechanisms for the beneficial effects of R. officinalis’ polyphenols treatment in a neuronal cell-like model. As shown stress induced-protein expression by Lut, CA, and RA was correlated with qRT-PCR. However, at mRNA level we observed a decrease after 6 h followed by an increase of mRNA of different genes in case of CA and RA treatment, and the opposite was observed for Lut treatment. This observable fact could be correlated with structure-functions of these three different polyphenols. Apart from being great scavengers of free radicals, R. officinalis’ polyphenols may directly stimulate the cell defense against stress response through cellular chaperone in early time treatment. However, after scavenging free radicals, R. officinalis polyphenols may become themselves pro-oxidant after being oxidized in cell culture media (Halliwell 2008). Oxidation of polyphenols produces peroxide, hydroperoxide a complex mixture of semiquinones and quinones, all of which are potentially cytotoxic (Halliwell 2008). In response to these pro-oxidants, the cell may act to regulate and conserve its stress defense system to maintain ER function and thus protect cells from toxic insults.
Regardless of their effect on downregulation of Hsp47, Hsp90, VCP/p97, HYOU1, and NDK, it is clear from these experiments that natural polyphenols available in rosemary leaves can reduce neuronal stress, and increase thermotolerance. This is a significant finding that lends support to previous experiments on cultured neuronal cells or in vivo studies showing beneficial effects against neurodegenerative-related declines.
Acknowledgments
This work was partially supported by by Mitsui & Co., Ltd. Environment Fund.
Contributor Information
Abdelfatteh E. L. Omri, Email: omriabdel@gmail.com.
Hiroko Isoda, Phone: +81-29-8535775, FAX: +81-29-8535776, Email: isoda@sakura.cc.tsukuba.ac.jp, Email: isoda.hiroko.ga@u.tsukuba.ac.jp.
References
- Ali YO, Kitay BM, Zhai RG. Dealing with misfolded proteins: examining the neuroprotective role of molecular chaperones in neurodegeneration. Molecules. 2010;15:6859–6887. doi: 10.3390/molecules15106859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative diseases. Cell Stress Chaperones. 2007;12:51–58. doi: 10.1379/CSC-236R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DS, Liu Y, Miao S, Bradley RJ. Neuronal differentiation in PC12 cells is accompanied by diminished inducibility of Hsp70 and Hsp 60 in response to heat and ethanol. Neurochem Res. 1996;21:659–666. doi: 10.1007/BF02527722. [DOI] [PubMed] [Google Scholar]
- El Omri A, Han J, Yamada P, Kawada K, Abdrabbah MB, Isoda H. Rosmarinus officinalis polyphenols activate cholinergic activities in PC12 cells through phosphorylation of ERK1/2. J Ethnopharmacol. 2010;131:451–458. doi: 10.1016/j.jep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys. 2008;476:107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Han J, Miyamae Y, Shigemori H, Isoda H. Neuroprotective effect of 3,5-di-Ocaffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience. 2010;169:1039–1045. doi: 10.1016/j.neuroscience.2010.05.049. [DOI] [PubMed] [Google Scholar]
- Isoda H, Koyama T, Tasaki M, Oka S, Sugiura N, Maekawa T, Inamori Y, Yokota S, Kitahara M, Nagata K (2004) High-sensitive detection of environmental pollutants. US Patent 6740521 B2
- Kim SH, Fountoulakis M, Cairns NJ, Lubec G. Human brain nucleoside diphosphate kinase activity is decreased in Alzheimer’s disease and Down syndrome. Biochem Biophys Res Commun. 2002;296:970–975. doi: 10.1016/S0006-291X(02)02035-1. [DOI] [PubMed] [Google Scholar]
- Kimura N, Shimada N, Ishijima Y, Fukuda M, Takagi Y, Ishikawa N. Nucleoside diphosphate kinases in mammalian signal transduction systems: recent development and perspective. J Bioenerg Biomembr. 2003;35:41–47. doi: 10.1023/A:1023489722460. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tanaka K, Inoue K, Kakizuka A. Functional ATPase activity of p97/valosin-containing protein (VCP) is required for the quality control of endoplasmic reticulum in neuronally differentiated mammalian PC12 cells. J Biol Chem. 2002;277:47358–47365. doi: 10.1074/jbc.M207783200. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Mimura J, Itoh K, Satoh T, Shimojo Y, Kitajima C, Maruyama A, Yamamoto M, Shirasawa T. Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J Biochem. 2010;147:73–81. doi: 10.1093/jb/mvp149. [DOI] [PubMed] [Google Scholar]
- Lin CW, Wu MJ, Liu IY, Su JD, Yen JH. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 expression. J Agric Food Chem. 2010;58:4477–4486. doi: 10.1021/jf904061x. [DOI] [PubMed] [Google Scholar]
- Liu R, Gao M, Qiang GF, Zhang TT, Lan X, Ying J, Du GH. The anti-amnesic effect of luteolin against amyloid β25–35 peptide induced toxicity in mice involve the protection of the neurovascular unit. Neuroscience. 2009;162:1232–1243. doi: 10.1016/j.neuroscience.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Luo W, Dou F, Rodina A, Chip S, Kim J, Zhao Q, Moulick K, Aguirre J, Wu N, Greengard P, Chiosis G (2007) Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci USA. 104:9511–9516 [DOI] [PMC free article] [PubMed]
- Luo W, Sun W, Taldone T, Rodina A, Chiosis G. Heat shock protein 90 in neurodegenerative diseases. Mol Neurodegener. 2010;5:24. doi: 10.1186/1750-1326-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama M, Suzuki M, Hamada Y, Hatsuzawa K, Tani K, Yamamoto A, Tagaya M. SVIP is a novel VCP/p97-interacting protein whose expression causes cell vacuolation. Mol Biol Cell. 2003;14:262–273. doi: 10.1091/mbc.02-07-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega RM. Importance of functional foods in the Mediterranean diet. Public Health Nutr. 2006;9:1136–1140. doi: 10.1017/S1368980007668530. [DOI] [PubMed] [Google Scholar]
- Oza J, Yang J, Chen KY, Liu AY. Changes in the regulation of heat shock gene expression in neuronal cell differentiation. Cell Stress Chaperones. 2008;13:73–84. doi: 10.1007/s12192-008-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozacmak VH, Barut F, Ozacmak HS. Melatonin provides neuroprotection by reducing oxidative stress and HSP70 expression during chronic cerebral hypoperfusion in ovariectomized rats. J Pineal Res. 2009;47:156–163. doi: 10.1111/j.1600-079X.2009.00695.x. [DOI] [PubMed] [Google Scholar]
- Park SE, Kim S, Sapkota K, Kim SJ. Neuroprotective effect of Rosmarinus officinalis extract on Human dopaminergic cell line, SH-SY5Y. Cell Mol Neurobiol. 2010;30:759–767. doi: 10.1007/s10571-010-9502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putics A, Végh EM, Csermely P, Soti C. Resveratrol induces the heat-shock response and protects human cells from severe heat stress. Antioxid Redox Signal. 2008;10:65–75. doi: 10.1089/ars.2007.1866. [DOI] [PubMed] [Google Scholar]
- Queen BL, Tollefsbol TO. Polyphenols and aging. Curr Aging Sci. 2010;3:34–42. doi: 10.2174/1874609811003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeswari A, Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacol. 2008;16:96–99. doi: 10.1007/s10787-007-1614-0. [DOI] [PubMed] [Google Scholar]
- Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Rocnik EF, Veer E, Cao H, Hegele RA, Pickering JG. Functional linkage between the endoplasmic reticulum protein Hsp47 and procollagen expression in human vascular smooth muscle cells. J Biol Chem. 2002;277:38571–38578. doi: 10.1074/jbc.M206689200. [DOI] [PubMed] [Google Scholar]
- Samali A, Fitzgerald U, Deegan S, Gupta S (2010) Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol. doi:10.1155/2010/830307 [DOI] [PMC free article] [PubMed]
- Shimojo Y, Kosaka K, Noda Y, Shimizu T, Shirasawa T. Effect of rosmarinic acid in motor dysfunction and life span in a mouse model of familial amyotrophic lateral sclerosis. J Neurosci Res. 2010;88:896–904. doi: 10.1002/jnr.22242. [DOI] [PubMed] [Google Scholar]
- Spencer JPE. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4:243–250. doi: 10.1007/s12263-009-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T, Razzaque MS. The collagen-specific molecular chaperone Hsp47: is there a role in fibrosis? Trends Mol Med. 2007;13:45–53. doi: 10.1016/j.molmed.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Liu P, Liu Y, Wang Q, Tong Y, Ji J (2006) Proteomic analysis of rat pheochromocytoma PC12 cells. Proteomics 6:2982–2990 [DOI] [PubMed]
- Yano M, Mori S, Kido H. Intrinsic nucleoside diphosphate kinase-like activity is a novel function of the 20 S proteasome. J Biol Chem. 1999;274:34375–34382. doi: 10.1074/jbc.274.48.34375. [DOI] [PubMed] [Google Scholar]
- Zhao L, Rosales C, Seburn K, Ron D, Ackerman SL. Alteration of the unfolded protein response modifies neurodegeneration in a mouse model of Marinesco-Sjögren syndrome. Hum Mol Genet. 2010;19:25–35. doi: 10.1093/hmg/ddp464. [DOI] [PMC free article] [PubMed] [Google Scholar]