Abstract
In this study, the anti-allergy potency of thirteen tannins isolated from the galls on buds of Carpinus tschonoskii (including two tannin derivatives) was investigated. RBL-2H3 (rat basophilic leukemia) cells were incubated with these compounds, and the release of β-hexosaminidase and cytotoxicity were measured. Of the thirteen tannins, tetragalloylglucose (2), pentagalloylglucose (3), casuarictin (4), and casuarinin (9) were the most potent inhibitors, and all the tannins showed no cytotoxic effect after 24 h of incubation. The results obtained suggest that tannins from C. tschonoskii are capable of inhibiting allergic reactions and may be useful for the treatment or prevention of type I allergic diseases.
Keywords: Antiallergic, Carpinus tschonoskii, Hydrolyzable tannins, Rat basophilic leukemia cells, Degranulation
Introduction
Galls result from the pathological development of plant tissue and are induced by the stimulus of a parasitic organism. This stimulus is considered to be a growth-regulating chemical produced by the parasite. Each gall-making species causes an excrescence structurally different from all others. Therefore, study on the constituents of galls is expected to lead to new bioactive compounds. In our previous study, in search for new bioactive compounds from galls, plant growth inhibitory polyacetylenes were isolated from the insect galls of Hedera rhombea Bean (Yamazoe et al. 2006a, b, 2007). Furthermore, two new (carpinerin A and B) and ten known hydrolyzable tannins were isolated from the galls on buds of Carpinus tschonoskii. The twelve compounds were identified as 1,2,6-trigalloylglucose (1), 1,2,3,6-tetragalloylglucose (2), pentagalloylglucose (3), casuarictin (4), pedunculagin (5), tellimagrandin II (6), carpinerin B (7), rhoipteleanin H (8), casuarinin (9), liquidambin (10), hippophaenin (11), and carpinerin A. In addition, their inhibitory effects on the growth of the cress (Lepidium sativum L.) seedling roots were discovered. Carpinus tschonoskii belongs to the Betulaceae family and this gall is formed by a parasitic mite (Eriophyes sp.). In addition, we have found that the constituents of the gall and normal bud were different; hydrolyzable tannins were produced by the gall form (Ono and Shigemori 2009).
Tannins, a large group of polyphenolic compounds widely distributed in plants, are often encountered in our daily life, being present in foods, beverages and medicinal plants (Okuda et al. 1989). Previous studies have reported apoptotic effect on HL-60 cells, anti-inflammatory activity of casuarinin (9) (Yang et al. 2000; Pan et al. 2000), anti-dementia activity of tellimagradin II (6) and pentagallolylglucose (3) extracted from Sanguisorbae Radix (Lee et al. 2005), anti-diabetic activity of pentagallolylglucose (3) extracted from Paeonia lactiflora roots (Baumgartner et al. 2010), anti-oxidation activity of casuarinin (9) extracted from Terminalia arjuna (Chen et al. 2004) and T. chebula (Cheng et al. 2003), anti-oxidation and antitumor activities of pentagallolylglucose (3) (Okuda et al. 2009), antibacterial activity of tellimagradin II (6) extracted from Rosa rugosa (Kamijo et al. 2008), antioxidant activity and DPPH radical-scavenging activity of pedunculagin (5), casuarictin (4) and casuarinin (9) isolated from walnuts (Juglans regia L.), and preventive effect on liver damage induced by carbon tetrachloride of tellimagrandin II (6) and casuarictin (4) isolated from walnuts (J. regia L.) (Fukuda et al. 2003; Shimoda et al. 2008). Inhibitory effects of apple condensed tannins on compound 48/80-induced mast cell degranulation (Tokura et al. 2005) and on histamine release from RBL cells (Kanda et al. 1998) were reported. Inhibitory effects of hydrolyzable tannins on histamine release from KO2 and compound 40/80 induced rat peritoneal mast cells were also shown (Kanoh et al. 2000). However, the possible anti-allergic activities of the obtained hydrolyzable tannins from C. tschonoskii on IgE-sensitized BSA-stimulated RBL-2H3 cells have not been studied.
The discovery of drugs for the treatment of inflammatory allergic diseases such as asthma, allergic rhinitis, and sinusitis is a very important subject in human health. Allergic diseases are immunologic disorders, traditionally referred to as immediate or type I hypersensitivity reactions with IgE playing an important role. Crosslinking of the FcεRI (high-affinity IgE receptor) induced by complex formation of IgE with an antigenic protein is an essential event in the IgE-mediated allergic reaction (Beaven and Metzger 1993), so the inhibition of the binding between IgE and FcεRI has been a target for the development of anti-allergic drugs. The interaction of IgE with allergen on mast cells or basophils leads to allergic reactions causing the release of an array of inflammatory mediators resulting in the inflammation of airway mucus membrane leading to clinical symptoms in the target organ (Novak et al. 2001).
RBL-2H3 cells, a tumor analog of mast cells, display characteristics of mucosal-type mast cells and express several hundred thousand FcεRI on the membrane surface. RBL-2H3 cells have been extensively used for the study of mast cell degranulation through the antigen-induced aggregation of FcεRI (Cheong et al. 1998). Among the various inflammatory mediators produced by mast cells, β-hexosaminidase is usually released along with histamine when mast cells or basophils are immunologically activated, such as during the crosslinking of FcεRIs (Ortega et al. 1988; Schroeder et al. 1995). Therefore, β-hexosaminidase activity in the medium is used as a marker of mast cell degranulation to predict possible anti-allergic activities of either natural or synthetic compounds (Cheong et al. 1998; Schiwartz et al. 1981; Passante and Frankish 2009).
In this study, we focused on the inhibitory effect of thirteen hydrolyzable tannins on type I allergy, and investigated their potential anti-allergic ability using the degranulation model of RBL-2H3 cells. We show herein that the antigen-induced activation of RBL-2H3 cells could be inhibited by these compounds.
Materials and methods
Chemicals
DNP-BSA (dinitrophenylated bovine serum albumin) was purchased from Cosmo Biotechnology Co (Tokyo, Japan). Anti-DNP-IgE, Ketotifen fumarate (99 %), and l-glutamine were purchased from Sigma (Sigma Aldrich Co., Ltd., Tokyo, Japan). FBS (fetal bovine serum) was purchased from Hyclone Co. Ltd. Eagle’s MEM (Minimum Essential Medium) was purchased from Nissui Pharmaceutical Co., Ltd., Tokyo, Japan.
Plant material
Galls of C. tschonoskii induced by infection of Eriophyes sp. were collected at the University of Tsukuba, Japan. A voucher specimen has been deposited at the Graduate School of Life and Environmental Sciences, University of Tsukuba, Japan.
Extraction and isolation
Galls of C. tschonoskii (100 g) were homogenized with a blender, extracted with MeOH (250 mL), and concentrated in vacuo. The MeOH extracts (8.3 g) were partitioned between EtOAc (250 mL × 3) and H2O (250 mL) and the H2O layer was further partitioned with BuOH (250 mL × 3). The EtOAc-soluble portion (1.85 g) was chromatographed on a Sep-Pak C18 cartridge (Waters, H2O/MeOH, 10:0 → 0:10) to give 4 fractions (Fr. 1 ~ Fr. 4). Fr. 2 (274 mg) was subjected to a Sep-Pak C18 cartridge (Waters, H2O/MeOH, 10:0 → 0:10) to give 8 fractions. The fraction (78.4 mg) eluted with H2O/MeOH (8:2) on the Sep-Pak C18 cartridge was further separated by reversed-phase HPLC (TSK-gel ODS-80Ts, φ4.8 × 200 mm, flow rate 1.0 mL/min, A: 3 % AcOH in H2O, B: 80 % CH3CN, A/B, 90:10 → 85:15) to give carpinerin B (8, 26.1 mg, tR = 21 min) and rhoipteleanin H (7, 18.3 mg, tR = 22 min). The fraction (78.7 mg) eluted with H2O/MeOH (7:3) on the Sep-Pak C18 cartridge was further separated by reversed-phase HPLC (Cosmosil 5C18-MS-II, φ10 × 250 mm, flow rate 2.5 mL/min, A: 0.1 % AcOH in H2O, B: 80 % CH3CN, A/B, 84:16 → 77:23 → 72:28 → 65:35) to give casuarictin (4, 22.8 mg). The fraction (50.2 mg) eluted with H2O/MeOH (6:4) on the Sep-Pak C18 cartridge was further separated by reversed-phase HPLC (Cosmosil 5C18-MS-II, φ10 × 250 mm, flow rate 2.5 mL/min, A: 0.1 % AcOH in H2O, B: 80 % CH3CN, A/B, 84:16 → 77:23 → 72:28 → 65:35 → 55:45) to give trigalloylglucose (1, 1.2 mg, tR = 14 min), tellimagrandin II (6, 9.4 mg, tR = 19 min), carpinerin A (3.4 mg, tR = 20 min), and tetragalloylglucose (2, 2.1 mg, tR = 21 min). The fraction (16.9 mg) eluted with H2O/MeOH (55:45) on the Sep-Pak C18 cartridge was pentagalloylglucose (3, 16.9 mg). The BuOH-soluble portion (2.21 g) was chromatographed on ODS (Cosmosil 75 C18-OPN, φ2.2 × 30 cm, H2O/MeOH, 10:0 → 0:10) to give 17 fractions (Fr. 1 ~ Fr. 17). Fr. 6 (317 mg) was subjected to a Sep-Pak C18 cartridge (H2O/MeOH, 10:0 → 0:10) to give 12 fractions. The fraction (27.2 mg) eluted with H2O/MeOH (7:3) on the Sep-Pak C18 cartridge was further separated by reversed-phase HPLC (Cosmosil 5C18-MS-II, φ10 × 250 mm, flow rate 2.5 mL/min, A: 0.1 % AcOH in H2O, B: 80 % CH3CN, A/B, 85:15 → 80:20 → 72:28 → 65:35) to give pedunculagin (5, 6.2 mg, tR = 9, 11 min) and hippophaenin A (11, 3 mg, tR = 14 min). Fr. 7 (216 mg) was subjected to the Sep-Pak C18 cartridge (H2O/MeOH, 10:0 → 0:10) to give 9 fractions. The fraction eluted with H2O/MeOH (7:3) on the Sep-Pak C18 cartridge was further separated by reversed-phase HPLC (Cosmosil 5C18-MS-II, φ10 × 250 mm, flow rate 2.5 mL/min, A: 0.1 % AcOH in H2O, B: 80 % CH3CN, A/B, 85:15 → 75:25 → 70:30 → 65:35) to give liquidambin (10, 35.6 mg, tR = 11 min) and casuarinin (9, 9.8 mg, tR = 12 min) (Ono and Shigemori 2009).
Cells and cell culture
RBL-2H3 cells were purchased from JCRB Cell Bank, Japan. The cells were maintained in MEM supplemented with 10 % FBS and 2 mM l-glutamine and incubated at 37 °C in a 5 % CO2 incubator.
Measurement of degranulation
The β-hexosaminidase release inhibition assay using RBL-2H3 cells was performed as previously described (Yamada et al. 2008). For the β-hexosaminidase inhibition assay, RBL-2H3 cells were seeded onto 96-well plates at 5.0 × 104 cells/well in 100 μL of medium. The cells were incubated for 24 h at 37 °C and sensitized with 0.3 μg/mL anti-DNP-IgE, then washed twice with PBS (−) to eliminate free IgE. After incubating the cells at 37 °C for 10 min in 60 μL per well of releasing mixture containing 5 μL of hydrolyzable tannins (final 3, 10 and 30 μM, respectively), the cells were exposed to 0.3 μg/mL DNP-BSA in PBS (−), followed by incubation at 37 °C for 1 h. 3 mM ketotifen fumarate (Keto.) and PBS (−) were used as positive and negative controls, respectively. The β-hexosaminidase release from RBL-2H3 cells caused by the test sample was calculated using the following equation:
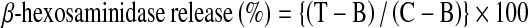 |
Control (C): Cell (+), DNP-BSA (+), test sample (−); Test (T): Cell (+), DNP-BSA (+), test sample (+); Blank (B): Cell (−), DNP-BSA (+), test sample (+).
Cell viability assay
Cell viability was assessed by MTT assay, which is a measure of the mitochondrial respiration of the cells (Mossman 1983) to test if the compounds used in this study could induce cell death. RBL-2H3 cells were harvested at approximately 60–80 % confluence and seeded onto 96-well plates at 5.0 × 104 cells/well in 100 μL of medium. After overnight incubation, the cells were either left untreated (as positive control) or treated with hydrolyzable tannins at different concentrations (final 3, 10, and 30 μM). The cells were then incubated for 24 h, before 10 μL of 5 mg/mL of MTT was added. After 24 h of incubation, 100 μL of 10 % sodium dodecyl sulfate (SDS) was added, followed by another 24 h of incubation to completely dissolve the formazan produced by the cells. The absorbance was then determined at 570 nm with a microplate reader. Blanks were prepared at the same time to correct for the absorbance caused by sample color and by the inherent ability of a sample to reduce MTT in the absence of cells. The optical density of the formazan produced by the untreated control cells represented 100 % viability. Results represent one trial (n = 6). Two additional trials show similar results.
Statistical analysis
Our results are expressed as means ± SD (n = 4). The statistical evaluation of the results was performed by one-way analysis of variance (ANOVA) followed by Tukey’s HSD post hoc tests. A value of p < 0.05 was judged to be statistically significant.
Result and discussion
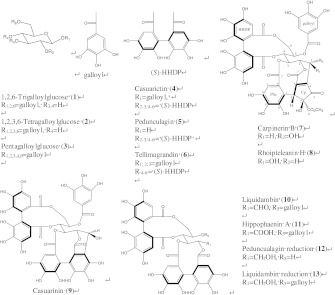
Chemical mediator release is a characteristic feature of activated mast cells or basophils upon stimulation of crosslinking antigens [18]. The effects of tannins 1–11 isolated from C. tschonoskii galls and tannin derivatives 5-O-galloyl-2,3,4,6-di-O-(S)-hexahydroxydiphenoyl-d-glucitol (12) from liquidambin (10) and 2,3,4,6-di-O-(S)-hexahydroxydiphenoyl-d-glucitol (13) from pedunculagin (5) (Ono and Shigemori 2009) (Fig. 1) on IgE-sensitizated BSA-stimulated RBL-2H3 cells were examined. These thirteen compounds were added at various concentrations and incubated with the cells at 10 min periods before stimulation of DNP-BSA. The inhibitory capacity of these compounds was evaluated as the release of β-hexosaminidase in the culture medium as compared with that from untreated cells. As shown in Fig. 2, all the compounds inhibited β-hexosaminidase release from RBL-2H3 cells, with a significant effect at 3.0-30.0 μM, and they also exhibited a higher inhibitory effect than ketotifen (IC50 = 214 μM), a clinically used drug. Compounds 2, 3, 4, and 9 were the most potent inhibitors with IC50 values of 1.9, 2.1, 2.1, and 2.1 μM, respectively, while compounds 10 and 1 were also effective inhibitors, with IC50 values of 3.0 and 3.9 μM, respectively. Compounds 7 and 13 were less potent (IC50 values of 22.6 and 31.8 μM, respectively) in comparison.
Fig. 1.
Chemical structures of hydrolyzable tannins isolated from the galls of C. tschonoskii
Fig. 2.
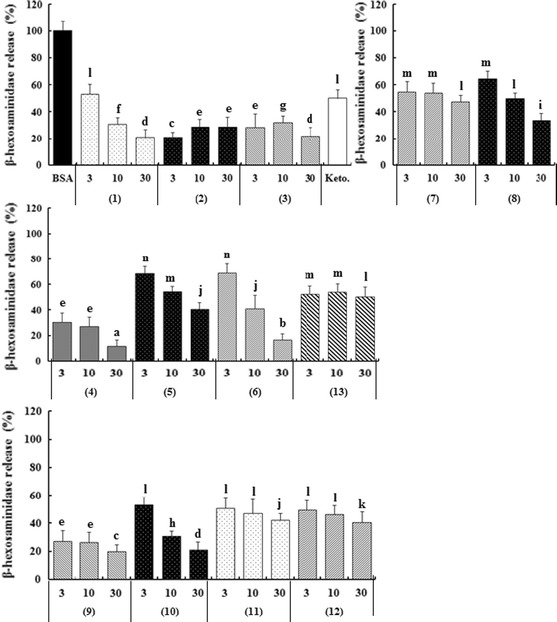
Inhibitory effect of thirteen hydrolyzable tannins on β-hexosaminidase release from antigen-stimulated RBL-2H3 cells. The cells (5.0 × 104 cells/well) were preincubated with different concentrations (μM) of each compound at 37 °C for 10 min prior to their incubation with DNP-BSA. The results, which represent the average of three independent experiments ± SD, are presented as a percentage of control (100 % i.e., DNP-BSA only). Statistically significant results (p < 0.05) were determined by one-way analysis of variance followed by Tukey’s HSD post hoc tests. Means without a common letter within the same graph differ significantly
However, we found evidence suggesting that in some compounds, the anti-allergic activity is related to the structure. For example, 4 and 5 have very similar structures with the only difference being that 4 has one galloyl group, while in 5, the galloyl group was replaced by OH. Therefore, the inhibitory activity is dependent on tannin structures. The six tannins that displayed an inhibitory effect against β-hexosaminidase release showed considerable chemical structural similarities (Fig. 1). In particular, 2, 3, 4, and 9, which possessed a galloyl group at Glc-1, showed the strongest inhibitory activity. Comparison between 4 (IC50 = 2.1 μM) with the galloyl group and 5 (IC50 = 16.4 μM) without the galloyl group at Glc-1 showed that the inhibitory activity was different by almost 8-fold. Casuarinin (9) with a ring-opened glucose and gallic acid itself also have the activity. It is suggested that a galloyl group was important for enhancing the activity, and connecting to Glc-1 in particular was more effective. Moreover, comparison between 3 (IC50 = 2.1 μM) with galloyl groups at Glc-1, 2, 3, 4, 6 and 4 (IC50 = 2.1 μM) with the hexahydroxydiphenoyl (HHDP) group at Glc-2, 3, 4, 6, or 6 (IC50 = 7.7 μM) with the HHDP group at Glc-4, 6 showed that the HHDP group at Glc-2, 3 was important for enhancing the activity. The comparison of highly active tannins (9) with poorly active ones (11) in this study showed that the position of the hydroxyl group with a ring-opened glucose was important for enhancing the activity.
Gallic acid (GA) inhibits mast cell-derived inflammatory allergic reactions by blocking histamine release and proinflammatory cytokine expression, and also decreases histamine release from RBL-2H3 cells (Kim et al. 2006). The triphenol structure plays an important role in the inhibitory activity of tea polyphenols (Matsuo et al. 1997). Furthermore, there was a significant correlation between inhibitions of chemical mediator release and DPPH or superoxide anion radical scavenging activities (Suzuki et al. 2005). The anti-allergic activity of the thirteen hydrolyzable tannins may be related to the inhibitory activity of gallic acid, polyphenols, and free radical scavengers on inflammation allergy reaction. Effect of agrimoniin, the first oligomer of ellagitannin isolated from plants on histamine release by rat peritoneal mast cells was reported (Kanoh et al. 2000). We would like to emphasize the differences in inhibitory effect on histamine release between the KO2 or compound 40/80 induced degranulation and IgE-mediated degranulation (immediate allergy).
Different types of ellagitannins (ETs) are reported to have various biological activities, such as antioxidant, antiviral, and antitumor activities. However, there are few definitive studies on the absorption and metabolism of ETs (Ito 2011). ETs are composed entirely of common acyl units such as galloyl, HHDP, and dehydrohexahydroxydiphenoyl (DHHDP) groups. Previous study has shown that ETs are hydrolyzed to ellagic acid (EA), which is then absorbed into the human body (Seeram et al. 2004) and metabolized by human colonic (Adams et al. 2010) and gut microflora (Clifford and Scalbert 2000) to 3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one (Urolithin A) type derivatives. These metabolites were recently found in the biofluids of rats, mice, and humans in in vivo studies (Ito 2011). Ito (2011) has pointed out about the possibility of a metabolites play an important role in biological effect after the oral administration of intact ETs. In vivo anti-inflammatory and antioxidant properties of ET metabolite Urolithin A and Urolithin B were also reported (Larrosa et al. 2010; Ishimoto et al. 2011). Furthermore, ETs such as geraniin is hydrolyzed to GA by microflora (Ito 2011), also gallotannins are hydrolyzed to GA, the triphenol compounds, pyrogallic acid and GA have been reported to inhibit chemical mediator release from mast cells (Matsuo et al. 1997; Kim et al. 2006). It will be necessary to clarify the in vivo antiallergic activity of hydrolyzable tannins metabolites in the future.
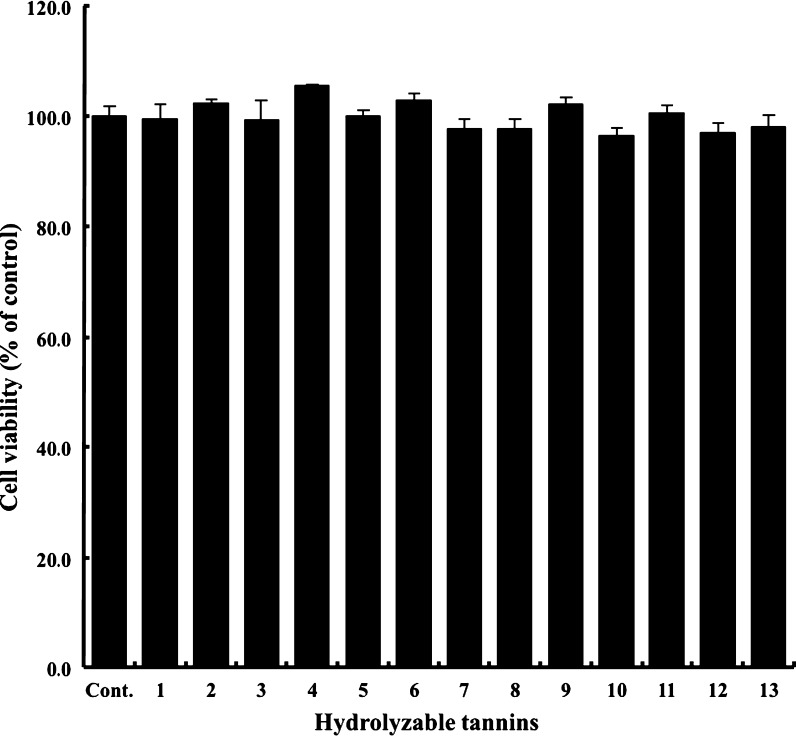
In an attempt to explore the effects of the thirteen hydrolyzable tannins on the inhibition of β-hexosaminidase release from RBL-2H3 cells, their cytotoxicities were evaluated by MTT (3-(4, 5-dimethylthiazolyl)-2, 5-diphenyltetrazolium bromide) assay. Figure 3 shows the inhibitory effects of the thirteen tannins (including two tannin derivatives) isolated from C. tschonoskii on the growth of RBL-2H3 cells. These results indicate that, at physiologically relevant concentrations, these tannin constituents do not cause a significant reduction in cell viability, indicating that they possibly affect signaling pathways leading to the reduced degranulation of RBL-2H3 cells.
Fig. 3.
Cytotoxicity effect of thirteen hydrolyzable tannins on RBL-2H3 cells by MTT assay. The percent survival of RBL-2H3 cells is shown following 24 h of incubation with various hydrolyzable tannins (30 μM)
In conclusion, the present results suggest that thirteen hydrolyzable tannins, particularly, tetragalloylglucose (2), pentagalloylglucose (3), casuarictin (4), and casuarinin (9) from galls of C. tschonoskii can prevent and/or treat BSA-induced allergy. To the best of our knowledge, this is the first report on the degranulation activity of these isolated tannins.
Acknowledgment
The authors are grateful to Ph.D. Fahmi.Ben.Fredj (University of Tsukuba) for the statistical analysis of this research.
References
- Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res. 2010;3:108–113. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner RR, Steinmann D, Heiss EH, Atanasov AG, Ganzera M, Stuppner H, Dirsc VM. Bioactivity-guided isolation of 1,2,3,4,6-Penta-O-galloyl-d-glucopyranose from Paeonia lactiflora roots as a PTP1B inhibitor. J Nat Prod. 2010;73:1578–1581. doi: 10.1021/np100258e. [DOI] [PubMed] [Google Scholar]
- Beaven MA, Metzger H. Signal transduction by Fc receptors: the FcεRI case. Immunol Today. 1993;14:222–226. doi: 10.1016/0167-5699(93)90167-J. [DOI] [PubMed] [Google Scholar]
- Chen CH, Liu TZ, Kuo TC, Lu FJ, Chen YC, Chang-Chien YW, Lin CC. Casuarinin protects cultured MDCK cells from hydrogen peroxide-induced oxidative stress and DNA oxidative damage. Planta Med. 2004;70:1022–1026. doi: 10.1055/s-2004-832641. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Lin TC, Yu KH, Yang CM, Lin CC. Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharm Bull. 2003;26:1331–1335. doi: 10.1248/bpb.26.1331. [DOI] [PubMed] [Google Scholar]
- Cheong H, Choi EJ, Yoo GS, Kim KM, Ryu SY. Desacetylmatricarin, an anti-allergic compound from Taraxacum platycarpum. Planta Med. 1998;64:577–578. doi: 10.1055/s-2006-957520. [DOI] [PubMed] [Google Scholar]
- Clifford MN, Scalbert A. Ellagitannins—nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1118–1125. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1118::AID-JSFA570>3.0.CO;2-9. [DOI] [Google Scholar]
- Fukuda T, Ito H, Yoshida T. Antioxidative polyphenols from walnuts (Juglans regia L.) Phytochemistry. 2003;63:795–801. doi: 10.1016/S0031-9422(03)00333-9. [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Shibata M, Myojin Y, Ito H, Sugimoto Y, Tai A, Hatano T. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg Med Chem Lett. 2011;21:5901–5904. doi: 10.1016/j.bmcl.2011.07.086. [DOI] [PubMed] [Google Scholar]
- Ito H. Metaboloties of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011;77:1110–1115. doi: 10.1055/s-0030-1270749. [DOI] [PubMed] [Google Scholar]
- Kamijo M, Kanazawa T, Funaki M, Nishizawa M, Yamagishi T. Effects of rosa rugosa petals on intestinal bacteria. Biosci Biotechnol Biochem. 2008;72:773–777. doi: 10.1271/bbb.70645. [DOI] [PubMed] [Google Scholar]
- Kanda T, Akiyama H, Yanagida A, Tanabe M, Goda Y, Toyoda M, Teshima R, Saito Y. Inhibitory effects of apple polyphenol on induced histamine release from RBL-2H3 cells and rat mast cells. Biosci Biotechnol Biochem. 1998;62:1284–1289. doi: 10.1271/bbb.62.1284. [DOI] [PubMed] [Google Scholar]
- Kanoh R, Hatano T, Ito H, Yoshida T, Akagi M. Effects of tannins and related polyphenols on superoxide-induced histamine release from rat peritoneal mast cells. Phytomedicine. 2000;7:297–302. doi: 10.1016/S0944-7113(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Kim SH, Jun CD, Suk K, Choi BJ, Lim H, Park SS, Lee H, Shin HY, Kim DK, Shin TY. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol Sci. 2006;91:123–131. doi: 10.1093/toxsci/kfj063. [DOI] [PubMed] [Google Scholar]
- Larrosa M, Gonzalez-Sarrias A, Yanez-Gascon MJ, Selma MV, Azorin-Ortuno M, Toti S, Tomas-Barberan F, Dolara P, Espin JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Seong YH, Bae KH, Kwon SH, Kwak HM, Nho SK, Kim KA, Hur JM, Lee KB, Kang YH, Song KS. Beta-secretase (BACE1) inhibitors from Sanguisorbae Radix. Arch Pharm Res. 2005;2:799–803. doi: 10.1007/BF02977345. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Yamada K, Shoji K, Mori M, Sugano M. Effect of tea polyphenols on histamine release from rat basophilic leukemia (RBL-2H3) cells: The structure-inhibitory activity relationship. Allergy. 1997;52:58–64. doi: 10.1111/j.1398-9995.1997.tb02546.x. [DOI] [PubMed] [Google Scholar]
- Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Novak N, Kraft S, Bieber T. IgE receptors. Curr Opin Immunol. 2001;13:721–726. doi: 10.1016/S0952-7915(01)00285-0. [DOI] [PubMed] [Google Scholar]
- Okuda T, Yoshida T, Hatano T. New methods of analyzing tannins. J Nat Prod. 1989;52:1–31. doi: 10.1021/np50061a001. [DOI] [Google Scholar]
- Okuda T, Yoshida T, Hatano T, Ito H. Ellagitannins renewed the concept of tannins. In: Quideau S, editor. Chemistry and biology of ellagitannins. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2009. pp. 40–54. [Google Scholar]
- Ono T, Shigemori H. Two new hydrolyzable tannins, carpinerins A and B, from galls of Carpinus tschonoskii. Heterocycles. 2009;78:1993–2001. doi: 10.3987/COM-09-11683. [DOI] [Google Scholar]
- Ortega E, Schweitzer-Stenner R, Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J. 1988;7:4101–4109. doi: 10.1002/j.1460-2075.1988.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MH, Lin-Shiau SY, Ho CT, Lin JH, Lin JK. Suppression of lipopo-lysaccharide-induced nuclear factor-kappaB activity by theaflavin-3, 3′-digallate from black tea and other polyphenols through down-regulation of IkappaB kinase activity in macrophages. Biochem Pharmacol. 2000;59:357–367. doi: 10.1016/S0006-2952(99)00335-4. [DOI] [PubMed] [Google Scholar]
- Passante E, Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm Res. 2009;58:737–745. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- Schiwartz LB, Lewis RA, Seldin D, Austen KF. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981;126:1290–1294. [PubMed] [Google Scholar]
- Schroeder JT, Kagey-Sobotka A, Lichtenstein LM. The role of the basophil in allergic inflammation. Allergy. 1995;50:463–472. doi: 10.1111/j.1398-9995.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348:63–68. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Shimoda H, Tanaka J, Kikuchi M, Fukuda T, Ito H, Hatano T, Yoshida T. Walnut polyphenols prevent liver damage induced by carbon tetrachloride and D-galactosamine: hepatoprotective hydrolyzable tannins in the kernel pllicles of walnut. J Agric Food Chem. 2008;56:4444–4449. doi: 10.1021/jf8002174. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nakamura T, Iyoki S, Fujiwara A, Watanabe Y, Mohri K, Isobe K, Ono K, Yano S. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol Pharm Bull. 2005;28:1438–1443. doi: 10.1248/bpb.28.1438. [DOI] [PubMed] [Google Scholar]
- Tokura T, Nakano N, Ito T, Matsuda H, Nagasako-Akazome Y, Kanda T, Ikeda M, Okumura K, Ogawa H, Nishiyama C. Inhibitory effect of polyphenol-enriched apple extracts on mast cell degranulation in vitro targeting the binding between IgE and Fcε RI. Biosci Biotechnol Biochem. 2005;69:1974–1977. doi: 10.1271/bbb.69.1974. [DOI] [PubMed] [Google Scholar]
- Yamada P, Zarrouk M, Kawasaki K, Isoda H. Inhibitory effect of various Tunisian olive oils on chemical mediator release and cytokine production by basophilic cells. J Ethnopharmacol. 2008;116:279–287. doi: 10.1016/j.jep.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Yamazoe S, Hasegawa K, Shigemori HZ. Structure-activity relationship of acetylenes from galls of Hedera rhombea as plant growth inhibitors. Naturforsch C. 2006;61:536–540. doi: 10.1515/znc-2006-7-811. [DOI] [PubMed] [Google Scholar]
- Yamazoe S, Hasegawa K, Suenaga K, Shigemori H. Growth inhibitory polyacetylenes from galls of Hedera rhombea Bean. Nat Prod Commun. 2006;1:87–94. [Google Scholar]
- Yamazoe S, Hasegawa K, Ito J, Mikami Y, Shigemori H. Hederyne A, a new antimicrobial polyacetylene from galls of Hedera rhombea Bean. J Asian Nat Prod Res. 2007;9:537–540. doi: 10.1080/10286020600882379. [DOI] [PubMed] [Google Scholar]
- Yang LL, Lee CY, Yen KY. Induction of apoptosis by hydrolyzable tannins from Eugenia jambos L. on human leukemia cells. Cancer Lett. 2000;157:65–75. doi: 10.1016/S0304-3835(00)00477-8. [DOI] [PubMed] [Google Scholar]